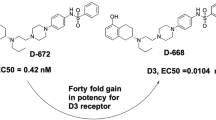
Abstract
Dopamine (1) is a key neurotransmitter whose impact on pharmacological processes is mediated by a family of dopamine receptors designated D1, D2, D3, D4, and D5. Various diseases and conditions such as schizophrenia, drug abuse, depression, restless leg syndrome, Parkinson’s disease (PD), and inflammatory diseases have been linked to aberrant D3 activity. Herein, we report a series of novel D3 ligands with improved solubility over our previous lead compound, MC25-41 (2).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The key neurotransmitter known as dopamine (1) was prepared synthetically for the first time in 1910 by George Barger and James Ewens [1] long before its pharmacological role was recognized. Over 45 years later, Katharine Montagu determined that dopamine was present in the human brain [2], and in 1958 Arvid Carlsson and Nils-Åke Hillarp demonstrated that this chemical acts as a neurotransmitter [3]. Over the next several decades, the pharmacological function and the means through which dopamine exerts its impact on biological systems has been elucidated. It is known, for example, that dopamine (1) is synthesized in the brain and the periphery, and it has been conclusively linked to a wide range of physiological functions. These functions include vasodilation, modulation of renal sodium excretion, altering urine output, learning, movement, and behavioral motivations [4].
At the cellular level, dopamine signaling is mediated by a family of G-protein coupled receptors (GPCRs) that are designated as D1, D2, D3, D4, and D5. The D1-like (D1 and D5) and D2-like (D2, D3 and D4) sub-families are based on genetic organization, amino acid homology, and pharmacological properties of the individual family members [5]. The D3 receptor has been the subject of intense interest as a potential therapeutic target, as it has been linked to various disease states and conditions such as schizophrenia, drug abuse, depression, restless leg syndrome [6], Parkinson’s disease (PD) [7, 8], and various inflammatory diseases [9]. We recently described our effort to identify novel, selective D3 ligands with potential utility for the treatment of cocaine use disorder. These studies led to the identification of MC25-41 (2, Fig. 1) as a potent D3 ligand that (1) possesses a high level of selectivity for D3 over other dopamine receptors and a range of other key CNS targets, (2) has a pharmacokinetic profile suitable for in vivo studies [10], and (3) attenuates motivation for cocaine in Sprague-Dawley rats [11]. While MC25-41 (2) has proven to be an effective tool molecule, its limited solubility (2 µM) may be lead to future problems in formulation and dosing [12]. As part of an effort to address this issue, we have developed a new series of novel, selective D3 ligands whose solubility is significantly improved over our original lead compound (2). Specifically, we have examined the impact of inserting an ether moiety in the linker chain. This provides additional opportunities to form hydrogen bonds with water and increases aqueous solubility. In addition, we have explored replacing the piperazine ring with two bioisosteres, homopiperazine and 2,6-diazaspiro[3.3]heptane. These alternative rings systems oriented their embedded nitrogen atoms in manners significantly different from the orientation of the corresponding piperazine nitrogen atoms. which leads to increased basicity and increased capability to form hydrogen bonds with water. Both of these factors led to increased aqueous solubility for the homopiperazine and 2,6-diazaspiro[3.3]heptane analogs.
Results and discussion
Our effort to improve the solubility of our lead series began with the incorporation of an oxygen atom in the central linker region (3a–3k). This addition adds an additional hydrogen bond acceptor, which could improve the solubility of our compounds, but also increases the length of the linker, which could alter D3 binding affinity and selectivity. Target compounds were prepared as outlined in Scheme 1. DMT-MM mediated coupling of biaryl acid (4) with amino-alcohol (5) provided the corresponding amide, which was converted to the corresponding bromide (6) with carbon tetrabromide and triphenylphosphine. The bromine was displaced with an aryl piperazine (7) to provide the target compounds (3a–3k).
Table 1 includes the in vitro binding (Ki at D3 and D2) as well as the physicochemical properties (MW, TPSA, LogP) of target compounds (3a–3k). Table 2 provides a comparison of the solubility of the target compounds (3a–3k) with the corresponding MC25-41 analogs (8a–8k) The compounds prepared and tested have MW, TPSA, and cLogP values that are consistent with drug like properties, but all examples have either TPSA (3a, 3c) or cLogP (3b, 3d–3k) value outside of the range suggested of BBB penetration (TPSA < 90, cLogP = 2–4) [13]. It is noteworthy, however, that we have previously demonstrated that MC25-41 (2) is efficacious in rat models of cocaine addiction (10 mg/kg IP) [11] and a marmoset model of Parkinson’s disease (10 mg/kg PO) [14]. This indicates that MC25-41 (2) is able to penetrate the BBB despite the fact that its clogP (4.9) value are outside of the range suggestive of BBB penetration and its and TPSA (87.6) is on the boarderline. It further suggests that related compounds may also be able to penetrate the BBB despite out-of-range values for cLogP and TPSA.
The structure-activity relationship analysis began with the 3-cyano analog (3a), which provided a direct comparison with our previous lead compound MC25-41 (2). A decrease in both D3 binding affinity (Ki = 128 nM) and selectivity over D2 (13-fold) were observed. Similar D3 potency was observed when the 3-CN (3a) was replaced with a 3-CF3 (3b, D3 Ki = 175 nM), but selectivity decreased (3.2-fold). Relocation of the cyano substituent to the 2-position (3c) once again produced a compound with similar D3 potency (Ki = 140 nM) and selectivity (3.5-fold). Replacing the 2-CN (3c) with either a 2-Cl (3d) or 2-CF3 (3e) led to an increase in both D3 potency (Ki = 27 nM and 37 nM respectively) and selectivity (11-fold and 17-fold respectively versus D2). Addition of a second chlorine atom in either the 3-position (3f) or the 4-position (3g) caused a drop in D3 potency (Ki = 127 nM and 1653 nM respectively) and selectivity (3.6-fold and 1.3-fold respectively versus D2). D3 binding affinity was restored when the 2 chlorine atoms were relocated to the 3- and 5-positions (3h, D3 Ki = 22 nM), and selectivity over D2 improved (D2 Ki = 332 nM, 14.9-fold). Incorporation of a methoxy group produced mixed results depending on the positioning of the substituent. The 2-methoxy analog (3i) is a potent D3 ligand (Ki = 24 nM), but selectivity was marginal (3.1-fold). The 4-methoxy analog (3j), on the other hand, showed little binding affinity for both D3 (Ki = 15265 nM) and D2 (Ki = 25435 nM). Finally, the 1-napthyl analog (3k) had moderate D3 binding affinity (Ki = 79 nM) and low D2 selectivity (4-fold).
A comparison of the kinetic solubility of the ether-linked compounds (3a–3k) with the corresponding MC25-41 analogs (8a–8k) indicated that this change produced mixed results. A 10-fold increase in solubility, for example, was observed with the 2-OMe analog (3i versus 8i), but when the methoxy substituent was in the 4-position (3j versus 8j), solubility did not increase. Significant increases in solubility over the MC25-41 analogs were also observed for the 3-CN (3a, 29-fold), 2-Cl (3d, 19-fold) and 2,4-di-Cl (3g, 10-fold) analogs, but the impact on solubility was smaller in the 2-CF3 (3e, 6-fold), 2,3-di-Cl (3f, 5-fold), 2-CN (3c, 3-fold), and 3-CF3 (3b, 2-fold) analogs. Interestingly, no improvement in solubility was observed for the 3,5-di-Cl (3h) and 1-napthyl (3k) analogs, both of which were poorly soluble (sol <10 µM).
We next turned our attention to replacing the piperazine ring of our lead compound MC25-41 (2) with either a homopiperazine (9a–9i) or a 2,6-diazaspiro[3.3]heptane (9j, 9k). Both of these moieties have been effectively used as piperazine bioisosteres [15], but there are differences that could impact D3 binding and selectivity (Fig. 2). While the distance between the nitrogen atoms in a piperazine ring (10) and homopiperazine ring (11) is similar (2.86 Å versus 2.89 Å), this is not the case for 2,6-diazaspiro[3.3]heptane (12). The distance between nitrogen atoms is substantially larger (4.17 Å). In addition, the 3-dimensional shapes of the three rings systems are not the same. The shape of the piperazine ring (10) and homopiperazine (11) are similar in that the first exists in a standard chair confirmation, while the second exists in pseudo-chair conformation. The chair confirmation of the piperazine ring (10) allows for significant cross-ring interaction of the loan pairs of electrons of the two nitrogen atoms, but this interaction is substantially decreased in the homopiperazine ring (11) due to its pseudo-chair conformation. This difference makes the homopiperazine nitrogen atoms more basic and more available to participate in hydrogen bonding interactions that could increase solubility. In a similar but more pronounced manner, the nitrogen atoms of 2,6-diazaspiro[3.3]heptane ring system (12) are oriented very differently from the piperazine ring (10). In this instance, the nitrogen atoms are oriented so that their lone pairs of electrons are perpendicular to each other and therefore incapable of undergoing the interaction seen in the chair configuration of the piperazine ring (10). As a result the 2,6-diazaspiro[3.3]heptane (12) nitrogen atoms are more basic and more available to participate in hydrogen bonding interactions that could increase solubility.
The homopiperazine (9a–9i) and the 2,6-diazaspiro[3.3]heptane (9j, 9k) analogs were prepared by the methods described in Schemes 2 and 3. Buchwald coupling of homopiperazine (11) with an aryl bromide (13) provided the requisite aryl homopiperzines (14a–14i), which were reacted with alkyl bromide (15) under basic conditions to provide target compounds (9a–9g). The synthesis of pyridine analogs (9h) and (9i), on the other hand, began with a displacement reaction of homopiperazine (11) with the corresponding chloropyridine (16a or 16b) to provide aryl homopiperzines (14h) and (14i), This was followed by reaction with alkyl bromide (15) under basic conditions to provide target compounds (9j) and (9k). In a similar manner, the synthesis of 2,6-diazaspiro[3.3]heptane (9j) and (9k) began with the reaction of Boc-2,6-diazaspiro[3.3]heptane (17) with a chloropyridine (16a or 16b) followed by TFA mediated deprotection to provide (18a) and (18b). Reaction with alkyl bromide (15) under basic conditions to provide target compounds (9j) and (9k).
Table 3 includes the in vitro binding (Ki at D3 and D2) as well as the physicochemical properties (MW, TPSA, LogP) of target compounds (9a–9k). Table 4 provides a comparison of the kinetic solubility of the target compounds (9a–9k) with the corresponding analogs of MC25-41 (8a–8c, 8e, 8g, 8h, 8k–8o) The majority compounds prepared and tested have MW and TPSA values that are consistent with drug-like properties and suggestive of BBB penetration. The cyano-pyridine analogs (9h) and (9j) are notable exceptions, as their TPSA are 101. cLogP values of the majority of compounds are above the range suggested for orally delivered compounds. Notable exceptions include (9h), (9j), and (9k), and only one compound, (9j), has a cLogP value within the targeted range that suggests BBB penetration. Overall, replacing the piperazine ring with a homopiperazine ring produced better results than those observed with 2,6-diazaspiro[3.3]heptane with respect to D3 binding and selectivity over D2. Incorporation of a cyano substituents in either the 2-position (9a) or 3-positions (9c) produced compounds that were highly potent (Ki = 5.7 nM and 6.3 nM) and highly selective (139-fold and 114-fold versus D2). Replacing the cyano substituents with a CF3 group in either the 2-postion (9b) or 3-position (9d) led to a small decrease in D3 binding potency (Ki = 30.2 nM and 18.6 nM), but selectivity over D2 was substantially diminished (43-fold and 47-fold). The 2,4-di-chloro (9e) and 3,5-di-chloro (9f) analogs were also less potent D3 ligands (Ki = 16.7 nM ad 33.1 nM) and less selective over D2 (80-fold and 27-fold) than the 3-CN analog (9a). Replacing the benzene rings of (9a) and (9b) with a pyridine ring led to a further reduction of D3 binding potency (9h D3 Ki = 64.7 nM, 9i Ki = 70.2 nM), but selectivity over D2 increased (197-fold and 131-fold). Lastly, incorporation of the 2,6-diazaspiro[3.3]heptane ring system (9j and 9k) led to a significant decrease in D3 binding affinity (Ki = 1093 nM ad 464 nM respectively).

A comparison of the solubility of the homopiperazine (9a–9i) and the 2,6-diazaspiro[3.3]heptane based compounds with the corresponding MC25-41 analogs (8a–8c, 8e, 8g, 8h, 8k–8o) (Table 4) demonstrated improvements in all but one of the homopiperazine analogs (9f, sol = 2 µM). Solubility improved by a 2- to 13-fold in all other examples. The 2,6-diazaspiro[3.3]heptane analogs (9j and 9k) had the highest aqueous solubility (sol = 148 µM and 109 µM), but as noted above, these compounds also demonstrated low affinity for D3 (Ki = 1093 nM ad 464 nM respectively). The 2-CN homopiperazine analog (9c) is notable as it is highly soluble (sol = 103 µM), has high affinity for D3 (Ki = 6.3 nM) and is highly selective for D3 over D2 (114-fold). Follow-up studies have demonstrated that this compound is highly selective for D3 over D4 (D4 Ki = 1077 nM). Additional studies on this compound (9c) to assess its affinity for D1 and D5, as well as full in vitro ADME profiling (e.g., Cyp450 inhibition, mouse and human liver microsome stability, permeability) are on-going.
Conclusion
In summary, we have identified analogs of our initial lead compound MC25-41 (2) that have high affinity for D3, are selective for this receptor over D2, and whose aqueous solubility is improved over the corresponding MC25-41 analogs (8a–8o). In addition, we have identified (9c) as a potential next-generation lead compound, given its high D3 affinity, selectivity over D2 and D4, and improved solubility. Future efforts will include follow-up studies on (9c) and close analogs thereof, as well as assessment of our current collection of compounds at the remaining members of the dopamine receptor family (D1, D4, and D5) and in battery of in vitro ADME screens (e.g., Cyp450 inhibition, mouse and human liver microsome stability, permeability).
Experimental methods and materials
Reagents were purchased from Fisher Scientific, VWR International, Sigma Aldrich, and Combi-Blocks, Inc. Chromatographic purification of compounds (normal phase and reverse phase) was carried out on a Teledyne Isco Combiflash RF system. H-NMR spectra were obtained on a Bruker 400-MHz NMR. Chemical shift values (δ values) were reported in ppm relative to TMS. For multiplicity, s = singlet, d = doublet, t = triplet, m = multiplet. Purity (%) and mass spectral data were determined with a Waters Agilent 1200 HPLC/MS (Zorbax SB-C18, 2.1 × 30 mm, 3.5 μm, 100% water/0.1% formic acid to 100% acetonitrile/0.1% formic acid over 4.0 min, 1.0 mL/min.) with a diode array detector from 210–400 nm and Agilent 6130 quadrupole MS. All compounds were purified to 95% purity or greater as determined by HPLC/MS and 1H-NMR. Melting points were recorded on a capillary melting point apparatus.

Preparation of N-(2-(2-hydroxyethoxy)ethyl)-4-(thiophen-3-yl)benzamide: A solution of 4-(thiophen-3-yl)benzoic acid (6 g, 29.27 mmol) and 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (8.88 g, 32.09 mmol) in ethanol (200 ml) was stirred at room temperature for 1 h. After 1 h, 2-(2-aminoethoxy)-ethanol (3.66 ml, 32.2 mmol) was added into the reaction. The reaction was further stirred for 2 days. After 2 days, the reaction was filtered to give a filtrate. The filtrate was purified by reverse phase chromatography (50% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-hydroxyethoxy)ethyl)-4-(thiophen-3-yl)benzamide (6.68, 78% yield): 1H NMR (400 MHz, MeOD-d4) δ 7.90–7.88 (d, J = 8.52 Hz, 2H), 7.79–7.77 (m, 3H), 7.53 (d, J = 2.16 Hz, 2H), 3.72–3.67 (quint, J = 4.84, 4.68, 4.28, 1.92, 1.68, 1.4, 0.72 Hz, 4H), 3.63–3.59 (quart, 4H); MS (LC/MS, M + H+): 292.75.

Preparation of N-(2-(2-bromoethoxy)ethyl)-4-(thiophen-3-yl)benzamide (6): N-(2-(2-hydroxyethoxy)ethyl)-4-(thiophen-3-yl)benzamide (1.67 g, 5.71 mmol) and carbon tetrabromide (2.84 g, 8.58 mmol) were dissolved in dichloromethane. Triphenylphosphine (2.27 g, 8.58 mmol, 1.5 eq) was slowly added into the reaction in an ice bath. The reaction was warmed up to room temperature and was further stirred for 48 h. After 48 h, the reaction mixture was concentrated to provide a solid-oil residue. The resulting material was purified by normal phase chromatography (Ethyl acetate/hexane 0 to 100% gradient) to provide N-(2-(2-bromoethoxy)ethyl)-4-(thiophen-3-yl)benzamide (1.28 g, 63% yield. 1H NMR (400 MHz, MeOD-d4) δ 7.92–7.90 (d, J = 8.43 Hz, 2H), 7.75–7.71 (m, 3H), 7.56 (d, J = 2.26 Hz, 2H), 3.75 (m, 4H), 3.57 (m, 2H), 3.48 (m, 2H), MS (LC/MS, M + H+): 354.70.

Preparation of N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3d): A solution of N-(2-(2-bromoethoxy)-ethyl)-4-(thiophen-3-yl)benzamide (0.14 g, 0.3 mmol), 1-(2-chlorophenyl)piperazine (0.06 g, 0.3 mmol) and N,N-diisopropylethylamine (0.12 ml, 1.2 mmol, 4.0 eq) in anhydrous acetonitrile (6 ml) at room temperature for 48 h. After 48 h, the reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl) benzamide (38.2 mg, 48% yield): 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.36 Hz, 2H), 7.63 (d, J = 8.8 Hz, 2H), 7.47–7.46 (m, 1H), 7.40–7.35 (m, 2H), 7.28–7.25 (m, 1H), 6.93–6.87 (m, 2H), 6.74 (m, 1H), 3.85 (t, J = 4.84 Hz, 2H), 3.73–3.68 (m, 4H), 3.17 (broad s, 4H), 3.13–3.09 (broad s, 6H); MS (LC/MS, M + H+): 470.60.

Preparation of N-(2-(2-(4-(3-cyanophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3a): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 3-(piperazin-1-yl)benzonitrile was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(3-cyanophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (32% yield): 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8 Hz, 2H), 7.63 (d, J = 8 Hz, 2H), 7.58 (s, 1H), 7.49 (s, 1H), 7.48 (s, 1H), 7.42 (s, 1H), 7.13–6.89 (m, 3H), 6.87 (s, 1H), 3.79 (broad s, 2H), 3.70 (broad s, 4H), 3.22 (broad s, 4H), 2.98 (broad s, 6H); MS (LC/MS, M + H+): 461.65.

Preparation of 4-(thiophen-3-yl)-N-(2-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethoxy) ethyl)benzamide (3b): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(3-(trifluoromethyl)phenyl)-piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide 4-(thiophen-3-yl)-N-(2-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethoxy)ethyl)benzamide (61% yield): 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.28 Hz, 2H), 7.65 (d, J = 8.32 Hz, 2H), 7.49–7.48 (m, 2H), 7.42–7.7.40 (m, 1H), 7.38–7.28 (m, 1H), 7.17 (t, J = 8, 7.84 Hz, 1H), 7.09–7.05 (m, 2H), 6.85 (d, J = 8.08 Hz, 1H), 3.81 (t, J = 4.84 Hz, 2H), 3.72 (s, 4H), 3.27 (t, J = 4.76, 4.36 Hz, 4H), 3.00 (t, J = 4.6 Hz, 4H), 2.95 (t, J = 4.84 Hz, 2H); MS (LC/MS, M + H+): 504.60.

Preparation of N-(2-(2-(4-(2-cyanophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3c): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 2-(piperazin-1-yl)benzonitrile was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(2-cyanophenyl)-piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (27% yield): 1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 8.07 (d, J = 8.32 Hz, 2H), 7.61 (d, J = 8.32 Hz, 2H), 7.44–7.43 (t, J = 2.28, 1.64 Hz, 1H), 7.41–7.39 (dd, J = 6.16, 1.44, 1.36 Hz, 1H), 7.35–7.32 (m, 2H), 7.00–6.96 (t, J = 6.32 Hz, 1H), 6.93–6.89 (t, J = 7.32 Hz, 1H), 6.59 (d, J = 8.08 Hz, 1H), 3.91 (t, J = 4.76 Hz, 2H), 3.74 (t, J = 4.72, 4.56 Hz, 2H), 3.69–3.65 (m, 2H), 3.30 (broad s, 7H), 3.19 (m, 3H); MS (LC/MS, M + H+): 461.70.

Preparation of 4-(thiophen-3-yl)-N-(2-(2-(4-(2-(trifluoromethyl)phenyl)piperazin-1-yl)ethoxy) ethyl)benzamide (3e): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(2-(trifluoromethyl)phenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide 4-(thiophen-3-yl)-N-(2-(2-(4-(2-(trifluoromethyl)phenyl)piperazin-1-yl)ethoxy)ethyl)benzamide (57% yield): 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.24 Hz, 2H), 7.65 (d, J = 8.24 Hz, 2H), 7.61 (broad s, 1H), 7.49 (broad s, 1H), 7.49–7.41 (m, 1H), 7.38–7.37 (m, 1H), 7.15 (t, J = 7.96, 7.84 Hz, 1H), 7.08 (d, J = 7.72 Hz, 1H), 7.04 (broad s, 1H), 6.83 (d, J = 7.88 Hz, 1H), 3.81 (t, J = 4.68, 4.6 Hz, 2H), 3.72 (broad s, 4H), 3.25 (t, J = 4.68 Hz, 4H), 3.05 (t, J = 4.44 Hz, 4H), 2.99 (t, J = 4.64, 4.56 Hz, 2H); MS (LC/MS, M + H+): 504.60.

Preparation of N-(2-(2-(4-(2,3-dichlorophenyl)piperazin–1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3f): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(2,3-dichlorophenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(2,3-dichlorophenyl)-piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (37% yield): 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 8.36 Hz, 2H), 7.66 (d, J = 8.44 Hz, 2H), 7.53–7.52 (m, 1H), 7.44–7.40 (m, 2H), 7.15–7.13 (dd, J = 1.4 Hz, 1H), 7.01 (t, J = 8.04 Hz, 2H), 6.82 (dd, J = 6.8, 1.32,1.25 Hz, 1H), 3.71 (broad s, 6H), 3.03 (broad s, 4H), 2.75 (m, 6H); MS (LC/MS, M + H+): 506.10.

Preparation of N-(2-(2-(4-(2,4-dichlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benz-amide (3g): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(2,4-dichlorophenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(2,4-dichlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (45% yield): 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.2 Hz, 2H), 7.48 (d, J = 8.28 Hz, 2H), 7.35 (s, 1H), 7.28–7.26 (m, 1H), 7.23 (m, 1H), 7.19 (s, 1H), 6.92–6.90 (dd, J = 6.32, 2.32, 2.24 Hz, 1H), 6.68 (d, J = 8.64 Hz, 1H), 3.53 (m, 6H), 2.83 (broad s, 4H), 2.54 (broad s, 6H); MS (LC/MS, M + H+): 505.50.

Preparation of N-(2-(2-(4-(3,5-dichlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3h): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(3,5-dichlorophenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(3,5-dichlorophenyl)-piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (49% yield): 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.28 Hz, 2H), 7.64 (d, J = 8.24 Hz, 2H), 7.50 (s, 2H), 7.41–7.37 (m, 2H), 6.80 (s, 1H), 6.64 (s, 2H), 3.77 (t, J = 4.8, 4.72 Hz, 2H), 3.70 (broad s, 4H), 3.21 (t, J = 4.8, 4.32 Hz, 4H), 2.97 (t, J = 4.2, 4.68 Hz, 4H), 2.93 (t, J = 4.88, 4.72 Hz, 2H); MS (LC/MS, M + H+): 504.50.

Preparation of N-(2-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3i): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(2-methoxyphenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile /0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(2-methoxyphenyl)-piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (55% yield): 1H NMR (400 MHz, CDCl3) δ 8.10 (broad s, 1H), 8.00–7.97 (d, J = 9.72 Hz, 2H), 7.59–7.57 (d, J = 8.28 Hz, 2H), 7.40–7.39 (m, 1H), 7.34–7.30 (m, 2H), 6.91–6.86 (m, 1H), 6.72–6.70 (d, J = 8.04 Hz, 1H), 6.58–6.56 (m, 2H), 3.82–3.78 (m, 2H), 3.70 (s, 3H), 3.65–3.61 (m, 4H), 3.15 (broad s, 4H), 3.04 (broad s, 6H); MS (LC/MS, M + H+): 466.70.

Preparation of N-(2-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3j): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(4-methoxyphenyl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (68% yield): 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.49–7.48 (m, 1H), 7.43–7.41 (m, 1H), 7.39–7.28 (m, 1H), 6.97 (broad s, 1H), 6.83 (d, J = 9.2 Hz, 2H), 6.78 (d, J = 9.16 Hz, 2H), 3.77 (s, 3H), 3.71–3.68 (m, 6H), 3.06 (t, J = 5.12, 4.68 Hz, 4H), 2.71–2.68 (m, 6H); MS (LC/MS, M + H+): 466.60.

Preparation of N-(2-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (3k): The title compound was prepared according to the procedure for N-(2-(2-(4-(2-chlorophenyl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide, except 1-(naphthalen-1-yl)piperazine was substituted for 1-(2-chlorophenyl)piperazine. The reaction was purified by normal phase chromatography (100% dichloromethane to 10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (10% acetonitrile/0.1% formic acid in water to 40% acetonitrile/0.1% formic acid in water) to provide N-(2-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethoxy)ethyl)-4-(thiophen-3-yl)benzamide (34% yield): 1H NMR (400 MHz, CDCl3) δ 8.21-8.17 (m, 1H), 7.88 (d, J = 1.6 Hz, 2H), 7.87–7.82 (m, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.55 (d, J = 8.2 Hz, 1H), 7.48–7.45 (m, 3H), 7.42–7.40 (m, 1H), 7.37–7.32 (m, 1H), 7.30–7.28 (d, J = 8.28 Hz, 1H), 7.01 (d, J = 6.88 Hz, 1H), 6.96 (broad s, 1H), 3.74 (m, 6H), 3.13 (broad s, 4H), 2.84 (broad s, 3H), 2.78 (t, J = 5.52, 5.48 Hz, 2H), 2.07 (broad s, 2H); MS (LC/MS, M + H+): 487.15.

Preparation of 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl) benzamide (9b): A solution of 1-(3-(trifluoromethyl)-phenyl)-1,4-diazepane (0.11 g, 0.466 mmol), N-(4-bromobutyl)-4-(thiophen-3-yl)-benzamide (0.16 g, 0.466 mmol) and N,N-diisopropylethylamine (0.25 ml, 1.4 mmol, 3.0 eq) in dry tetrahydrofuran (6 ml) was refluxed at 66 °C for 48 h. After 48 h, the mixture was cooled and filtered to obtain a filtrate. The filtrate was purified by normal phase chromatography (10% methanol in dichloromethane) to provide 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl) benzamide (132.8 mg, 56% yield): 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.36 Hz, 2H), 7.57 (d, J = 8.36 Hz, 2H), 7.45 (t, J = 2.16 Hz, 1H), 1.59–1.52 (m, 4H), 1.90 (pent, J = 5.16 Hz, 2H), 2.48 (t, J = 7 Hz, 2H), 2.57 (t, J = 5.52 Hz, 2H), 7.33 (d, J = 2.16 Hz, 2H), 7.19 (t, J = 8.16 Hz, 2H), 6.80 (d, J = 7.64 Hz, 1H), 6.77 (broad s, 1H), 6.72 (dd, J = 8.4, 2.48 Hz, 2H), 6.47 (broad s, 1H), 3.48 (t, J = 3.88 Hz, 2H), 3.37–3.43 (m, 4H), 2.72 (t, J = 4.16 Hz, 2H); MS (LC/MS, M + H+): 502.60.

Preparation of N-(4-(4-(3-cyanophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (9a): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 3-(1,4-diazepan-1-yl)benzonitrile was substituted for 1-(3-(trifluoromethyl)phenyl)-1,4-diazepane. The reaction mixture was first purified by normal phase chromatography (10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by reverse phase chromatography (50% acetonitrile/0.1% formic acid in water) to provide N-(4-(4-(3-cyanophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (48% yield): 1H NMR (400 MHz, CDCl3) δ 7.72–7.7.70 (d, J = 8.38 Hz, 2H), 7.56–7.54 (d, J = 8.36 Hz, 2H), 7.44–7.43 (t, J = 2.24, 2 Hz, 1H), 7.32 (d, J = 1.88 Hz, 1H), 7.16–7.12 (m, 1H), 6.81–6.79 (d, J = 7.56 Hz, 1H), 6.76–675 (m, 2H), 6.57–6.54 (t, J = 5.32, 5.28 Hz, 1H), 3.42–3.38 (m, 2H), 3.37–3.34 (m, 4H), 2.67–2.65 (t, J = 4.92, 4.88 Hz, 2H), 2.52–2.49 (t, J = 5.56, 5.4 Hz, 2H), 2.44–2.41 (t, J = 6.96, 6.84 Hz, 2H), 1.96–1.82 (m, 2H). 1.59–1.45 (m, 4H); MS (LC/MS, M + H+): 459.

Preparation of N-(4-(4-(2-cyanophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (9c): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 2-(1,4-diazepan-1-yl)benzonitrile was substituted for 1-(3-(trifluoromethyl)-phenyl)-1,4-diazepane to provide N-(4-(4-(2-cyanophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (33% yield): 1H NMR (400 MHz, CDCl3) δ 8.02–8.00 (m, 2H), 7.64–7.63 (m, 3H), 7.55–7.45 (m, 3H), 7.38 (broad s, 2H), 6.99–6.96 (m, 2H), 3.85 (broad s, 2H), 3.52–3.42 (m, 8H), 3.20 (broad s, 2H), 2.54 (broad s, 2H), 2.04 (broad s, 2H), 1.76 (broad s, 2H); MS (LC/MS, M + H+): 459.

Preparation of 4-(thiophen-3-yl)-N-(4-(4-(2-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)-benzamide (9d): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 1-(2-(trifluoromethyl)phenyl)-1,4-diazepane was substituted for 1-(3-(trifluoromethyl)-phenyl)-1,4-diazepane to provide 4-(thiophen-3-yl)-N-(4-(4-(2-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide (59% yield): 1H NMR (400 MHz, CDCl3) δ 7.91–7.90 (d, J = 7.52 Hz, 3H), 7.60–7.59 (d, J = 4.92 Hz, 3H), 7.52–7.48 (t, J = 7.64, 6.88 Hz, 2H), 7.35–7.23 (m, 4H), 3.47 (broad s, 2H), 3.40 (broad s, 2H), 3.31 (broad s, 2H), 3.23 (broad s, 2H) 3.06 (broad s, 4H), 3.17–2.13 (m, 2H), 1.85 (broad s, 2H), 1.67 (broad s, 2H); MS (LC/MS, M + H+): 502.

Preparation of N-(4-(4-(2,4-dichlorophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl) benzamide (9e): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 1-(2,4-dichlorophenyl)-1,4-diazepane was substituted for 1-(3-(trifluoromethyl)-phenyl)-1,4-diazepane to provide N-(4-(4-(2,4-dichlorophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (24% yield): 1H NMR (400 MHz, CDCl3) δ 7.97–7.95 (d, J = 7.92 Hz, 2H), 7.67–7.65 (d, J = 7.88 Hz, 2H), 7.54–7.53 (t, J = 2, 1.96 Hz, 2H), 7.42 (t J = 1.84 Hz, 2H), 7.38–7.37 (d, J = 2.4 Hz, 1H), 7.20–7.17 (dd, J = 6.24, 2.4, 2.36 Hz, 2H), 7.02–7.00 (d, J = 8.64 Hz, 2H), 3.54 (broad s, 2H), 3.43 (broad s, 2H), 3.36 (broad s, 4H), 3.24 (broad s, 2H), 3.09 (broad s, 2H), 2.29 (broad s, 2H), 1.93 (broad s, 2H), 1.75 (broad s, 2H); MS (LC/MS, M + H+): 503.

Preparation of N-(4-(4-(3,5-dichlorophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl) benzamide (9f): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 1-(3,5-dichlorophenyl)-1,4-diazepane was substituted for 1-(3-(trifluoromethyl)-phenyl)-1,4-diazepane to provide N-(4-(4-(3,5-dichlorophenyl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (35% yield): 1H NMR (400 MHz, CDCl3) δ 7.90–7.89 (d, J = 7.72 Hz, 2H), 7.62–7.60 (d, J = 7.84 Hz, 2H), 7.50 (broad s, 1H), 7.38 (broad s, 2H), 6.70 (broad s, 2H), 6.48 (s, 2H), 3.69 (broad s, 2H), 3.43 (broad s, 4H), 3.12 (broad s, 2H), 3.04 (broad s, 2H), 2.93 (broad s, 2H), 2.28 (broad s, 2H), 1.81 (broad s, 2H), 1.65 (broad s, 2H); MS (LC/MS, M + H+): 503.

Preparation of N-(4-(4-(naphthalen-1-yl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (9g): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(3-(trifluoromethyl)phenyl)-1,4-diazepan-1-yl)butyl)benzamide, except 1-(naphthalen-1-yl)-1,4-diazepane was substituted for 1-(3-(trifluoromethyl)phenyl)-1,4-diazepane. The reaction mixture was first purified by normal phase chromatography (10% methanol in dichloromethane) to give a partially pure desired product. The impure product was further purified by normal amine phase (RediSep Rf Gold® Amine #: 69-2203-507) chromatography (100% isopropanol) to provide N-(4-(4-(naphthalen-1-yl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (57% yield): 1H NMR (400 MHz, CDCl3) δ 7.77–7.72 (m, 3H), 7.57 (d, J = 8 Hz, 2H), 7.45–7.43 (m, 1H), 7.39–7.36 (m, 2H), 7.33 (d, J = 1.88 Hz, 2H), 7.28 (t, J = 7.64 Hz, 1H), 7.03 (dd, J = 6.68, 0.72, 0.64 Hz, 1H), 6.82 (broad s, 1H), 3.46 (quart, J = 6.16, 6.12, 5.68 Hz, 2H), 3.30 (m, 2H), 3.26 (t, J = 5.92 Hz, 2H), 2.91 (m, 4H), 2.63 (m, 2H), 1.98 (pent, 2H), 1.66 (m, 4H); MS (LC/MS, M + H+): 484.65.

Preparation of N-(4-(4-(4-cyanopyridin-2-yl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl) benzamide (9h): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(4-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepan-1-yl)butyl)benzamide, except 2-(1,4-diazepan-1-yl)isonicotinonitrile was substituted for 1-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepane. The reaction mixture was first purified by normal phase chromatography (10% methanol in dichloromethane) to give a partial pure desired product. The impure product was further purified by normal amine phase (RediSep Rf Gold® Amine #: 69-2203-507) chromatography (100% isopropanol) to provide N-(4-(4-(4-cyanopyridin-2-yl)-1,4-diazepan-1-yl)butyl)-4-(thiophen-3-yl)benzamide (34% yield): 1H NMR (400 MHz, CDCl3) δ 8.15 (dd, J = 4.44, 0.56, 0.48 Hz, 1H), 7.78 (d, J = 8.36 Hz, 2H) 7.57 (d, J = 8.44 Hz, 2H), 7.45 (t, J = 2.16 Hz, 1H), 7.33 (d, J = 2.12 Hz, 2H), 6.77 (broad s, 1H), 6.62 (dd, J = 4, 1.04, 1 Hz, 1H), 6.57 (s, 1H), 3.82 (broad s, 2H), 3.51 (t, J = 6.2 Hz, 2H), 3.41 (quart, J = 6.24, 5.92 Hz, 2H), 2.84 (t, J = 4.28 Hz, 2H), 2.73 (broad s, 2H), 2.62 (t, J = 6.6 Hz, 2H), 2.06 (broad s, 2H), 1.62 (broad s, 4H), 1.42 (d, J = 6.6 Hz, 2H); MS (LC/MS, M + H+): 460.70.

Preparation of 4-(thiophen-3-yl)-N-(4-(4-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepan-1-yl) butyl)benzamide (9i): A solution of n-butyllithium, 1.6 M in hexane (0.43 ml, 0.69 mmol) was added dropwise into a solution of 1-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepane (0.17 g, 0.69 mmol) in anhydrous tetrahydrofuran (6 ml) at −78 °C for 1 h. After 1 h, N-(4-bromobutyl)-4-(thiophen-3-yl)benzamide (0.23 g, 0.69 mmol) was added into the reaction and was stirred at 0 °C for another 1 h. The reaction mixture was then refluxed at 66 °C for 48 h. After 48 h, the reaction mixture was cooled and purified by normal phase chromatography (100% ethyl acetate to 10% methanol in dichloromethane) to give a partial pure desired product. The impure product was further purified by reverse phase chromatography (100% acetonitrile with 0.1% formic acid) to provide 4-(thiophen-3-yl)-N-(4-(4-(4-(trifluoromethyl)pyridine-2-yl)-1,4-diazepan-1-yl)butyl)benzamide (279 mg, 41%): 1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 3.36 Hz, 1H), 7.82 (d, J = 8.32 Hz, 2H), 7.58 (d, J = 8.32 Hz, 2H), 7.46 (t, J = 2.44 Hz, 1H), 7.34 (m, 2H), 7.16 (s, 1H), 6.72 (d, J = 5.12 Hz, 1H), 6.58 (s, 1H), 4.02 (broad s, 2H), 3.54 (t, J = 6.28 Hz, 2H), 3.44 (t, J = 1 Hz, 2H), 3.08 (broad s, 2H), 2.98 (broad s, 2H), 2.86 (t, J = 7.4 Hz, 2H), 2.29–2.25 (m, 2H), 1.79–1.75 (m, 2H), 1.64–1.61 (m, 2H); MS (LC/MS, M + H+): 503.60.

1-(3-cyanophenyl)-1,4-diazepane (14a) was prepared as described in WO2018089493 [16].

Preparation of 1-(naphthalen-1-yl)-1,4-diazepane (14g): 1-Bromonaphthalene (2.89 ml, 20.65 mmol) was stirred with homopiperazine (2.08 g, 20.65 mmol) in the presence of tris(dibenzylideneacetone)dipalladium(0) (0.05 g), sodium tert-butoxide (2.77 g, 28.92 mmol, 1.4 eq), and (±)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene (0.05 g) in anhydrous toluene (100 ml). The reaction mixture was refluxed at 110 °C for 48 h. After 48 h, the reaction mixture was cooled and filtered with a celite pad to obtain a filtrate. The filtrate was purified by normal phase chromatography (20% methanol in ethyl acetate) to provide 1-(naphthalen-1-yl)-1,4-diazepane (2.91 g, 62% yield): 1H NMR (400 MHz, CDCl3) δ 8.23–8.25 (d, J = 8 Hz, 1H), 7.88–7.85 (d, J = 7.76 Hz, 1H), 7.66–7.64 (d, J = 8.2 Hz, 1H), 7.58–7.50 (m, 2H), 7.45–7.41 (t, J = 8.08, 7.52 Hz, 1H), 7.24–7.22 (dd, J = 6.56, 0.88, 0.84 Hz, 1H), 3.68–3.64 (m, 4H), 3.59–3.57 (m, 2H), 3.41–3.38 (t, J = 6, 5.92 Hz, 2H), 2..44–2.39 (quint, J = 5.88, 5.72, 5.68 Hz, 2H); MS (LC/MS, M + H+): 227.90.

Preparation of 1-(3-(trifluoromethyl)phenyl)-1,4-diazepane (14b): The title compound was prepared according to the procedure for 1-(naphthalen-1-yl)-1,4-diazepane, except 1-bromo-3-(trifluoromethyl)benzene was substituted for 1-bromonaphthalene. The reaction was purified by normal phase chromatography (20% methanol in ethyl acetate) to provide 1-(3-(trifluoromethyl)phenyl)-1,4-diazepane (53% yield): 1H NMR (400 MHz, CDCl3) δ 7.17 (td, J = 7.96, 0.68, 0.64, 0.44 Hz, 1H), 6.75 (m, 3H), 3.47 (t, J = 6.12 Hz, 2H), 3.43 (t, J = 5.48 Hz, 2H), 2.91 (t, J = 5.44 Hz, 2H), 2.71 (t, J = 5.68 Hz, 2H), 1.78 (m, 3H); MS (LC/MS, M + H+): 245.85.

Preparation of 2-(1,4-diazepan-1-yl)benzonitrile (14c): The title compound was prepared according to the procedure for 1-(naphthalen-1-yl)-1,4-diazepane, except 2-bromobenzonitrile was substituted for 1-bromonaphthalene. The reaction was purified by normal phase chromatography (10% methanol in dichloromethane) to provide 2-(1,4-diazepan-1-yl)benzonitrile (47% yield): 1H NMR (400 MHz, CDCl3) δ 7.49 (dd, J = 6.08, 1.68 Hz, 1H), 7.37 (tdtd, J = 7.04, 1.76, 1.6 Hz, 1H), 6.90 (d, J = 8.6 Hz, 1H), 6.78 (tdd, J = 7.28, 0.88 Hz, 1H), 3.66 (m, 4H), 3.15 (m, 2H), 2.99 (t, J = 5.64 Hz, 2H), 2.01 (quint 2H); MS (LC/MS, M + H+): 202.90

Preparation of 1-(2-(trifluoromethyl)phenyl)-1,4-diazepane (14d): The title compound was prepared according to the procedure for 1-(naphthalen-1-yl)-1,4-diazepane, except 1-bromo-2-(trifluoromethyl)benzene was substituted for 1-bromonaphthalene. The reaction was purified by normal phase chromatography (20% methanol in ethyl acetate) to provide 1-(2-(trifluoromethyl)phenyl)-1,4-diazepane (61% yield): 1H NMR (400 MHz, CDCl3) δ 7.66 (broad s, 1H), 7.55–7.53 (d, J = 6.96 Hz, 1H), 7.50–7.46 (t, J = 7.76, 7.6 Hz, 1H), 7.33–7.31 (d, J = 7.96 Hz, 1H), 7.22–7.18 (t, J = 7.64 Hz, 1H), 3.46–3.43 (t, J = 5.68, 5.56 Hz, 2H), 3.39–3.35 (m, 2H), 3.30–3.27 (m, 2H), 3.15–3.12 (t, J = 6.36 Hz, 2H), 2.21–2.15 (quint, J = 6.32, 6.24, 5.72, 5.64 Hz, 2H); MS (LC/MS, M + H+): 246.10

Preparation of 1-(2,4-dichlorophenyl)-1,4-diazepane (14e): The title compound was prepared according to the procedure for 1-(naphthalen-1-yl)-1,4-diazepane, except 1-bromo-2,4-dichlorobenzene was substituted for 1-bromonaphthalene. The reaction was purified by normal phase chromatography (20% methanol in ethyl acetate) to provide 1-(2,4-dichlorophenyl)-1,4-diazepane (38% yield): 1H NMR (400 MHz, CDCl3) δ 7.33 (broad s, 1H), 7.14–7.11 (m, 1H), 7.02–7.00 (d, J = 7.8 Hz, 1H), 3.27–3.22 (quart, J = 6.44, 6 Hz, 4H), 3.09–3.05 (quart, J = 6.24, 5.56 Hz, 4H), 1.98–1.92 (quint, J = 6, 5.84, 5.76 Hz, 2H); MS (LC/MS, M + H+): 245.90.

Preparation of 1-(3,5-dichlorophenyl)-1,4-diazepane (14f): The title compound was prepared according to the procedure for 1-(naphthalen-1-yl)-1,4-diazepane, except 1-bromo-3,5-dichlorobenzene was substituted for 1-bromonaphthalene. The reaction was purified by normal phase chromatography (20% methanol in ethyl acetate) to provide 1-(3,5-dichlorophenyl)-1,4-diazepane (35% yield): 1H NMR (400 MHz, MeOD-d4) δ 6.68–6.66 (d, J = 6.92 Hz, 3H), 3.75 (broad s, 2H), 3.55–3.52 (t, J = 5.92 Hz, 2H), 3.33 (borad s, 2H), 3.22 (broad s, 2H), 2.21 (broad s, 2H); MS (LC/MS, M + H+): 245.90

Preparation of 1-(4-(trifluoromethyl)pyridin–2-yl)-1,4-diazepane (14i): A solution of homopiperazine (0.1 g, 0.998 mmol, 1.1 eq) and 2-chloro-4-(trifluoro-methyl)pyridine (0.23 ml, 0.908 mmol) in 1-butanol was refluxed at 117 °C for 16 h. After 16 h, the reaction mixture was cooled and basified and was stirred with concentrated sodium hydroxide (20 ml) overnight. After that, the mixture was further purified by solid loading in normal phase chromatography (10% methanol with 1% ammonia hydroxide in dichloromethane) to give 1-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepane (169 mg, 69%): 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J = 5.16 Hz, 1H), 6.63 (d, J = 5.12 Hz, 1H), 6.57 (s, 1H), 3.76 (t, J = 6.28 Hz, 2H), 3.66 (t, J = 6.16 Hz, 2H), 3.45 (s, 3H), 3.01 (t, 8 Hz, 2H), 2.84 (t, J = 5.6 Hz, 2H), 1.92 (pent, J = 6.12, 5.88, 5.84 Hz, 2H); MS (LC/MS, M + H+): 245.85.

Preparation of 2-(1,4-diazepan-1-yl)isonicotinonitrile (14h): The title compound was prepared according to the procedure for 1-(4-(trifluoromethyl)pyridin-2-yl)-1,4-diazepane, except 2-bromoisonicotinonitrile was substituted for 2-chloro-4-(trifluoromethyl)pyridine. The mixture was purified by normal phase chromatography (10% methanol with 1% ammonia hydroxide in dichloromethane) to provide 2-(1,4-diazepan-1-yl)isonicotinonitrile (72% yield): 1H NMR (400 MHz, MeOD-d4) δ 6.99 (d, J = 4 Hz, 1H), 5.78 (s, 1H), 5.58 (dd, J = 4.08, 0.96 Hz, 1H), 2.76 (t, J = 5.4 Hz, 2H), 2.47 (t, J = 6.16 Hz, 2H) 2.09–1.98 (m, 4H), 0.91 (pent, J = 5.68 Hz, 2H); MS (LC/MS, M + H+): 203.

Preparation of 2-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane (18b): A solution of 2-chloro-4-(trifluoromethyl)pyridine (0.08 g. 0.411 mmol), tert-butyl 2,6-diazaspiro[3.3]heptane-2-carboxylate (0.1 g, 0.411 mmol) and triethylamine (0.1 ml) in 1-butanol (6 ml) was refluxed at 117 °C for 16 h. After 16 h, the reaction was cooled and purified by normal phase chromatography (10% methanol in dichloromethane) to provide an intermediate, tert-butyl 6-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane-2-carboxylate: MS (LC/MS, M + H+): 344.10. The intermediate was then dissolved in trifluoroacetic acid and stirred for 16 h. After 16 h, the reaction was concentrated to give an oil residue. The residue was dissolved in methanol (20 ml) and stirred with Amberlite™ IRN-78 ion-exchange resin, OH-form at room temperature for 16 h. The basified reaction was then filtered to remove the resin concentrated to give a product, 2-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane, used for the next step without chromatographic purification. (94.5 mg, 77% yield): 1H NMR (400 MHz, MeOD) δ 8.18 (d, J = 5.16 Hz, 1H), 6.63 (d, J = 5.12 Hz, 1H), 6.57 (s, 1H), 3.62 (s, 4H), 3.35 (s, 4H). (LC/MS, M + H+): 244.

Preparation of 2-(2,6-diazaspiro[3.3]heptan-2-yl)isonicotinonitrile (18a): The title compound was prepared according to the procedure for 2-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane, except 2-chloroisonicotinonitrile was substituted for 2-chloro-4-(trifluoromethyl)pyridine. tert-Butyl 6-(4-cyanopyridin-2-yl)-2,6-diazaspiro[3.3]heptane-2-carboxylate: MS (LC/MS, M + H+): 302.10. The intermediate was then deprotected with trifluoroacetic acid and basified with Amberlite™ IRN-78 ion-exchange resin to give 2-(2,6-diazaspiro[3.3]heptan-2-yl)isonicotinonitrile (68% yield). 1H NMR (400 MHz, MeOD) δ 8.12 (d, J = 5.04 Hz, 1H), 6.78 (d, J = 5.18 Hz, 1H), 6.49 (s, 1H), 3.72 (s, 4H), 3.41 (s, 4H), (LC/MS, M + H+): 201.

Preparation of N-(4-(6-(4-cyanopyridin-2-yl)-2,6-diazaspiro[3.3]heptan-2-yl)butyl)-4-(thiophen-3-yl)benzamide (9j): The title compound was prepared according to the procedure for 4-(thiophen-3-yl)-N-(4-(6-(4-(trifluoro-methyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptan-2-yl)butyl) benzamide, except 2-(2,6-diazaspiro[3.3]heptan-2-yl)isonicotinonitrile was substituted for 2-(4-(trifluoro-methyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane. The reaction mixture was first purified by normal phase chromatography (10% methanol in dichloromethane) and reverse phase chromatography (10% acetonitrile/0.1% formic acid in water) to provide N-(4-(6-(4-cyanopyridin-2-yl)-2,6-diazaspiro[3.3]heptan-2-yl)butyl)-4-(thiophen-3-yl)benzamide (47% yield): 1H NMR (400 MHz, CDCl3) δ 8.16-8.15 (dd, J = 4.48, 0.6 Hz, 1H), 7.81-7.79 (d, J = 8.32 Hz, 2H), 7.59-7.57 (d, J = 8.32 Hz, 2H), 7.47-7.46 (t, J = 2.12 Hz, 1H), 7.34 (d, J = 2.16 Hz, 2H), 7.09 (broad s, 1H), 6.72-6.71 (dd, J = 3.96, 1.2, 1.16 Hz, 1H), 6.37 (s, 1H), 4.12 (s, 4H), 3.96 (s, 4H), 3.40 (broad s, 2H), 2..93 (broad s, 2H), 1.62 (broad s, 4H); MS (LC/MS, M + H+): 458.

Preparation of 4-(thiophen-3-yl)-N-(4-(6-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diaza-spiro[3.3] heptan-2-yl)butyl)benzamide (9k): A solution of 2-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptane (0.1 g, 0.411 mmol), N-(4-bromobutyl)-4-(thiophen-3-yl)benzamide (0.14 g, 0.411 mmol) and triethylamine (0.3 ml, 2.12 mmol) in tetrahydrofuran (6 ml) was refluxed at 66 °C for 48 h. After 48 h, the reaction was cooled and purified by normal phase chromatography (10% methanol in dichloromethane) and reverse phase chromatography (40% acetonitrile/0.1% formic acid in water) to provide 4-(thiophen-3-yl)-N-(4-(6-(4-(trifluoromethyl)pyridin-2-yl)-2,6-diazaspiro[3.3]heptan-2-yl)butyl)benzamide (89.1 mg, 43% yield): 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 5.16 Hz, J = 1H), 7.88 (d, J = 7.92 Hz, 2H), 7.64 (d, J = 8 Hz, 2H), 7.53 (s, 1H), 7.40-7.37 (s, 2H), 6.82-6.81 (d, J = 5.08 Hz, 1H), 6.44 (s, 1H), 4.21 (broad s, 4H), 4.07 (broad s, 4H), 3.47 (broad s, 2H), 3.03 (broad s, 2H), 1.68 (broad s, 4H); MS (LC/MS, M + H+): 501.
Computational values: TPSA and cLogP values were calculated using the Dotmatics software suite (Dotmatics LLC The Old Monastery, Windhill Bishops, Stortford Herts, CW23 2ND UK).
Competitive radioligand-binding studies
For competitive binding studies, transfected HEK293 cell homogenates were suspended in homogenization buffer and incubated with radioligand [125I]IABN, in the presence or absence of inhibitor at 37 °C for 60 min with [125I]IABN (total volume = 150 ul) as previously described [17]. Competitive radioligand studies were performed to determine the concentration of inhibitor that inhibits 50% of the specific binding of the radioligand (IC50 value). The final radioligand concentration was approximately equal to the Kd value for the binding of the radioligand. For each competition curve, triplicates were performed using two concentrations of inhibitor per decade over five orders of magnitude. Binding was terminated by the addition of cold wash buffer (10 mM Tris–HCl/150 mM NaCl, pH = 7.5) and filtration over a glass-fiber filter (Pall A/B filters, #66198). A Packard Cobra Gamma Counter was used to measure the radioactivity of [125I]IABN.
The competition curves were modeled for a single binding site using
where Bs is the amount of ligand bound to receptor and Bo is the amount of ligand bound to receptor in the absence of competitive inhibitor. L is the concentration of the competitive inhibitor. The IC50 value is the concentration of competitive inhibitor that inhibits 50% of the total specific binding. IC50 values were determined using non-linear regression analysis with Table Curve 2D v 5.01 (Jandel, SYSTAT, Systat Software, Inc., San Jose, CA, USA). The values for Bs and Bo were constrained using experimentally derived values. The IC50 values were converted to equilibrium dissociation constants (Ki) using the Cheng and Prusoff (1973) correction. Mean Ki values are reported for at least three independent experiments.
Aqueous solubility assay
Compounds were assessed for their solubility at pH 7.4 using the commercially available Millipore MultiScreenTM Solubility filter system (Millipore, Billerica, MA). Analysis was performed by liquid chromatography tandem mass spectrometry (LC/MS/MS).
References
Fahn S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord. 2008;23:S497–508. https://doi.org/10.1002/mds.22028.
Björklund A, Dunnett SB. Fifty years of dopamine research. Trends Neurosci. 2007;30:185–7. https://doi.org/10.1016/j.tins.2007.03.004.
Benes FM. Carlsson and the discovery of dopamine. Trends Pharmacol Sci. 2001;22:46–47. https://doi.org/10.1016/S0165-6147(00)01607-2.
Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–42.
Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharm. 1996;35:1503–19. https://doi.org/10.1016/s0028-3908(96)00100-1.
Kiss B, Laszlovszky I, Krámos B, Visegrády A, Bobok A, Lévay G, et al. Neuronal dopamine D3 receptors: translational implications for preclinical research and CNS disorders. Biomolecules. 2021;11:104 https://doi.org/10.3390/biom11010104.
Bono F, Mutti V, Fiorentini C, Missale C. Dopamine D3 receptor heteromerization: implications for neuroplasticity and neuroprotection. Biomolecules. 2020a;10:1016 https://doi.org/10.3390/biom10071016.
Yang P, Perlmutter JS, Benzinger TLS, Morris JC, Xu J. Dopamine D3 receptor: a neglected participant in Parkinson Disease pathogenesis and treatment? Ageing Res Rev. 2020b;57:100994 https://doi.org/10.1016/j.arr.2019.100994.
Feng Y, Lu Y. Immunomodulatory effects of dopamine in inflammatory diseases. Front Immunol. 2021;12:663102–0. https://doi.org/10.3389/fimmu.2021.663102.
Chen PJ, Taylor M, Griffin SA, Amani A, Hayatshahi H, Korzekwa K, et al. Design, synthesis, and evaluation of N-(4-(4-phenyl piperazin-1-yl)butyl)-4-(thiophen-3-yl)benzamides as selective dopamine D3 receptor ligands. Bioorg Med Chem Lett 2019;29:2690–4. https://doi.org/10.1016/j.bmcl.2019.07.020.
Powell GL, Namba MD, Vannan A, Bonadonna JP, Carlson A, Mendoza R, et al. The long-acting D3 partial agonist MC-25-41 attenuates motivation for cocaine in Sprague-Dawley rats. Biomolecules. 2020;10:1076 https://doi.org/10.3390/biom10071076.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413–20. https://doi.org/10.1023/a:1016212804288.
Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–53. https://doi.org/10.1602/neurorx.2.4.541.
Oh T, Daadi, ES, Kim J, Daadi, EW, Chen PJ, Roy-Choudhury G, et al., Dopamine D3 receptor ligand suppresses the expression of levodopa-induced dyskinesia in nonhuman primate model of Parkinson’s Disease. Exp Neurol. https://doi.org/10.1101/2021.08.10.455884.
Blass BE, Basic principles of drug discovery and development 2nd edn, Academic Press: Cambridge Massachusetts (Printed in the United States) 2021. 5, 257–303, https://doi.org/10.1016/B978-0-12-817214-8.00005-1.
O’neill, DJ, Eddine S, Kang, SWA, Andrew Brearley, A, Jonathan Bentley J, Purrole m TORC inhibitors and uses thereof, WO2018089493, 2018.
Lee B, Taylor M, Griffin SA, McInnis T, Sumien N, Mach RH, et al. Evaluation of substituted N-phenylpiperazine analogs as D3 vs. D2 dopamine receptor subtype selective ligands. Molecules. 2021;26:3182 https://doi.org/10.3390/molecules26113182.
Acknowledgements
The research reported in this publication was supported by the National Institute on Drug Abuse (NIDA)/National Institutes of Health (NIH) under award numbers DA029840-06A1 and DA023957.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blass, B.E., Chen, PJ., Taylor, M. et al. Synthesis and evaluation of cyclic diamino benzamide based D3 receptor ligands. Med Chem Res 31, 446–461 (2022). https://doi.org/10.1007/s00044-021-02845-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02845-z