Abstract
Cancer immunotherapy has achieved a leap from the laboratory to the clinic, especially for therapeutic applications based on programmed cell death-1 (PD-1) and its ligand (PD-L1) that target tumour immune escape and growth. At present, 13 PD-1/PD-L1 monoclonal antibodies (mAbs) have been approved as PD-1/PD-L1 inhibitors by the United States Food and Drug Administration (FDA). However, inherent limitations of mAbs, including poor bioavailability and immunogenicity, have led researchers to pursue alternatives and develop small-molecule inhibitors with low molecular weight. Biphenyl derivatives are small-molecule inhibitors of PD-1/PD-L1 with advantages of oral bioavailability, high tumour penetration and better pharmacokinetic properties. In this work, we review progress and structure-activity relationship analysis of biphenyl derivatives as PD-1/PD-L1 inhibitors. The conclusions could contribute to the design of PD-1/PD-L1 inhibitor candidates for cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer immunotherapy is a highly promising new therapeutic modality to defeat cancer [1, 2]. Unlike traditional cancer treatments such as chemotherapy and radiotherapy, cancer immunotherapy kills tumour cells by activating or mobilising the body’s immune system, rather than targeting the tumour itself [3]. Cancer immunotherapies, including cancer vaccines [4], tumour-infiltrating lymphocyte adoptive cell therapy [5], chimeric antigen receptor T cell (CAR-T) therapy [6] and immune checkpoint suppression [7] have achieved great clinical success and brought new hope to cancer patients.
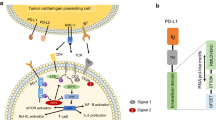
Immune checkpoints act as regulators of the immune system, ensuring appropriate responses by stimulating or inhibiting checkpoint molecules to maintain balance. Several immune checkpoints have been identified including cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1) and its ligand programmed cell death-ligand 1 (PD-L1), T cell membrane protein 3 (TIM-3), lymphocyte activation gene 3 (LAG3), V-domain immunoglobulin suppressor of T cell activation (VISTA) and galectin-9 (Gal-9) [8,9,10]. Among these, CTLA-4 has made rapid progress in clinical studies, and its monoclonal antibody (mAb) ipilimumab was authorised for treating metastatic melanoma by the US Food and Drug Administration (FDA) in 2011 [11]. Encouraged by this, development of immune checkpoint inhibitors accelerated, especially PD-1/PD-L1 immune checkpoint inhibition [12, 13].
PD-1, also known as CD279, belongs to the CD28 family and is expressed in T cells, regulatory T cells (Tregs), depletion T cells, B cells, natural killer cells, natural killer T cells (NKT), dendritic cells (DCs) and tumour-associated macrophages (TAMs) [14, 15]. Human PD-1 (hPD-1) consists of 288 amino acids and is a type I monomeric surface transmembrane glycoprotein composed of a single Ig variable-type (IgV) extracellular domain, a transmembrane domain and a cytoplasmic domain. The cytoplasmic domain contains two structural motifs: an immune tyrosine-based inhibitory motif (ITIM) and an immune receptor inhibitory tyrosine-based switch motif (ITSM) [16]. Two ligands of the PD-1 molecule, PD-L1 (CD274) and PD-L2 (CD273), belong to the B7 family and share 37% sequence homology. PD-L1 is widely expressed and has been detected in antigen-presenting cells, non-lymphoid organs and non-hematopoietic cells [17, 18], while PD-L2 expression is limited mainly to DCs and a few tumour lines [19]. PD-L1 is a type I transmembrane protein possessing extracellular IgV and IgC domains, transmembrane domains and intracellular domains. When PD-1 engages with PD-L1, the intracellular ITIM and ITSM motifs of PD-1 are phosphorylated. Phosphorylated ITIM and ITSM recruit Src homology region 2 domain-containing phosphatase-1 (SHP-1) and SHP-2 into the intracellular domain of PD-1 [20]. SHP-1 and SHP-2 can inhibit downstream T cell receptor signalling pathways (TCRs) including phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and RAS/mitogen activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) pathway [21,22,23]. These actions decrease T cell activation, proliferation and survival, and reduce the production of cytokines such as interferon-γ (IFN-γ) and interleukin 2 (IL-2). The PD-1/PD-L1 interaction releases co-inhibitory signals to activated T cells, inducing apoptosis and inactivating T cells [24, 25].
However, all marketed drugs targeting PD-1/PD-L1 are mAbs. Targeting immune checkpoints using mAbs is a novel approach to cancer therapy, for which James P. Allison and Tasuku Honjo won the 2018 Nobel Prize in Medicine [26, 27]. Despite the clinical efficacy of mAbs, inherent shortcomings, including low oral bioavailability, poor tumour penetration and severe immune-related adverse events limit their clinical application [28, 29]. Low molecular weight PD-1/PD-L1 inhibitors are receiving much attention due to their high oral bioavailability, high tumour penetration and better pharmacokinetic properties [30]. Initial studies on low molecular weight (LMW) PD-1/PD-L1 inhibitors were based on polypeptides and macrocyclic peptides. These inhibitors mimic key antibody residues and are capable of antagonising PD-1/PD-L1 pathway signalling and restoring the functions of T-cells. However, they have mostly been reported in patents and no more information has been disclosed, except for CA-170. This first-in-class oral inhibitor inhibited VISTA and PD-L1 with half maximal inhibitory concentration (IC50) values of 17 nM and 37 nM, respectively, but there was no direct binding between CA-170 and PD-L1 according to nuclear magnetic resonance (NMR) binding, homogenous time-resolved fluorescence (HTRF) and cell-based activation assays [31].
Other LMW PD-1/PD-L1 inhibitors include oxadiazole and thiadiazine [32, 33], sulfamonomethoxine and sulfamethizole [34], and 2-methyl-biphenyl derivatives [35, 36]. There have been no further advances except for biphenyl derivatives, for which several compounds have entered clinical trials in recent years (Table 1). Although many biphenyl derivatives have been studied as PD-1/PD-L1 inhibitors, progress and structure-activity relationship (SAR) analysis has not been reviewed. Herein, we focus on progress and SAR analysis of biphenyl derivatives as PD-1/PD-L1 inhibitors. The findings could contribute to the design of PD-1/PD-L1 inhibitors as candidates for cancer immunotherapy.
Biphenyl derivatives
In 2015, Bristol-Myers Squibb (BMS) reported PD-1/PD-L1 small-molecule inhibitors with a biphenyl scaffold [35]. X-ray crystallography was subsequently used to determine complexes of PD-L1 protein and BMS inhibitors, including BMS-202, BMS-8, BMS-37, BMS-200, BMS-1001 and BMS-1166 (Fig. 1). Based on the results, the general formula of biphenyl derivatives was deduced (Fig. 1), which includes biaryl core, linker, aryl group, tail, extension and ether group junction regions. SAR analysis showed that the biaryl core, aryl group and linker were responsible for core activity and hydrophobicity. The tail is exposed to solvent, engages in hydrogen bonding interactions with PD-L1, and is required for high potency. Extensions and ether bond junctions are not key structural elements but can improve pharmacokinetic characteristics. Design strategies and SAR analysis of biphenyl derivatives are discussed below.
The biaryl core of biphenyl derivatives
Since scientists from BMS first reported (2-methyl-3-biphenylyl) methanol scaffold inhibitors targeting PD-L1 in 2015, biphenyl derivatives have proved to be promising lead compounds for further modification. In 2016, the Holak group determined cocrystal structures of BMS-202 and BMS-8 as PD-L1 antagonists, and found that these inhibitors prevented the PD-1/PD-L1 interaction by causing PD-L1 to dimerise [37]. After revealing the binding mode of BMS compounds, the Holak group also identified the biphenyl core as the minimal fragment responsible for PD-L1 binding using the 1H-15N heteronuclear multiple-quantum coherence (HMQC) NMR method [38]. Therefore, the biphenyl core is the most important pharmacophore for inhibiting PD-1/PD-L1 interaction. Besides the 2-methyl-3-bipheny moiety, studies from BMS incorporated the 2-cyano-3-biphenyl and 2-methyl-3-(thiophen-3-yl)benzyl moiety in the biphenyl core [35]. Compounds 7 and 8 displayed IC50 values of 0.006–0.10 μM and 0.11–1.0 μM, respectively, in HTRF binding assays. The HTRF assay is a highly sensitive, robust approach for measuring activity and probing molecular interactions in vitro using europium cryptate-labelled anti-Ig molecules [39]. SAR analysis showed that the proximal phenyl ring of the biphenyl group favoured substitution of the cyano group, while the distal phenyl ring can be substituted by thiophene. Moreover, activity data showed that replacement of 1,4-benzodioxane in the distal phenyl ring increased activity. A series of biphenyl-based small molecules as PD-L1 inhibitors were prepared with bromine substituted at the proximal phenyl ring, and compound 9 impeded PD-1/PD-L1 interactions in the femtomolar range (IC50 = 0.08 pM) in HTRF binding assays [40] (Table 2).
In 2020, two classes of novel immunomodulatory inhibitors of PD-1/PD-L1 interaction were reported in which 5-methypyrrole was introduced to replace the proximal phenyl ring of the biphenyl [41]. Additionally, small substituents such as halogens and methyl groups were tolerated in the distal phenyl ring. Compounds 10 and 11 exhibited IC50 values of ~2.9 µm in HTRF binding assays. As a new starting point, this scaffold can be further optimised. Another group developed a series of benzoheterocyclic compounds in 2021 [42]. Via ring expansion, the proximal phenyl ring was converted into a quinazoline moiety to give lead compound 12, for which the IC50 value was at the micromolar level in HTRF binding assays. Further SAR studies showed that replacement of 1,4-benzodioxane in the distal phenyl ring and linking the benzonitrile or cyanopyridine to the central phenyl through an ether bond could significantly increase activity. The optimised compound 13 inhibited the PD-1/PD-L1 interaction with an IC50 value of 5 nM in HTRF binding assays, compared with 83 nM for the BMS-37 control. In conclusion, the biphenyl scaffold is the core structural element inhibiting PD-1/PD-L1 interaction, and it can only be partially replaced by aromatic rings. SAR analysis showed that the proximal aromatic ring of the biaryl group tolerates substitution of small group (e.g., methyl, Br, Cl, CN) while the distal phenyl ring favours the replacement of 1,4-benzodioxane.
The linker region of biphenyl derivatives
In patents from BMS, the linker is composed of a methylene ether, which is used to connect the biphenyl and aromatic ring, increasing the flexibility of the structure. SAR analysis showed that the methylene ether is not a key pharmacophore [43]. Patents were published by Guangzhou Maxinovel Pharmaceuticals in which the aromatic benzyl ether linker of biphenyl derivatives was converted to aromatic acetylene, aromatic ethylene, aromatic ethyl or aromatic heterocyclic. Compounds 14, 15, 16 and 17 displayed IC50 values of 18 nM, 44 nM, 1.34 mM and >10 mM, respectively, as measured by HRTF assay [44, 45]. In addition, Syntron Corporation reported a class of heterocyclic amino compounds with a five-membered linker [46]. The highly active compound 18 had an IC50 value of 56 nM measured by HTRF binding assay. The above indicated that reducing the flexibility of the linker decreases the activity of the compound. Li et al. developed a series of aliphatic amine-linked triaryl derivatives by changing the linker, and compound 19 was the most active (IC50 = 12 nM) according to HRTF binding assay [43]. SAR analysis revealed an interaction between aliphatic amines and the Ala121 residue, which improved structural stability and activity. In 2018 and 2019, Incyte Company published several novel scaffolds with modification of the linker [47,48,49], including removing the linker and secondary amine and amide group connection, with compounds 20, 21 and 22 having IC50 values < 10 nM, <10 nM and <100 nM, respectively, measured by HRTF binding assay. These results showed that modifying flexibility of the linker can increase activity (Table 3).
A significant number of new structures has been developed by incorporating bioisosterism principles. In 2019, Chemocentryx combined the methylene in the methylene ether linkage chain with the biphenyl to form a ninhydrin [50], and compound 23 exhibited an IC50 value < 100 nM in enzyme-linked immunosorbent assay (ELISA). The oxygen in the linked chain was then replaced with nitrogen via bioisosterism to generate inden-amine compounds [51], and compound 24 showed an IC50 value of 5–500 nM in ELISA. Guangzhou Wellhealth Bio-pharmaceutical Co. LTD reported new structures in which the linker is combined with the proximal phenyl ring or the aromatic ring to form a five-membered or six-membered ring [52], and compounds 25 and 26 displayed IC50 values of 1.4 nM and 0.75 nM, respectively, in HRTF binding assays. Compared with BMS-202, these compounds showed better metabolic stability in vitro and higher oral utilisation by liver microsomes. Betta Company produced dihydroindole compounds by combining amide link chains with the biphenyl [53], and compound 27 showed an IC50 value < 25 nM in HRTF binding assays. Qin et al. (year) combined the amide link chain with benzene on both sides to form dihydroindoles via the collage strategy [54], and the activity was higher than for the aromatic ring; compound 28 (A13) strongly inhibited the PD-1/PD-L1 interaction and increase IFN-γ secretion in in vitro immune experiments, and compound 13 showed low toxicity. Molecular docking experiments showed that the indole portion of compound 28 not only had hydrophobic effects through Met115 and Ala121, like the 2-methylphenyl group in BMS-37, but also interacted with Try123 to stabilise the inhibitor conformation [54]. Qin et al. (year) then optimised the 4-phenyldihydroindole to generate compound 29 (A30) with ultra-high activity [55]; the IC50 value was 11.2 nM in HTRF binding assays. Chen et al. (year) also designed a class of benzoisothiazole scaffold compounds via collation and bioelectron isoarrangement strategies, and compound 30 (CH20) had an IC50 value of 8.5 nM in HRTF binding assays [56]. Moreover, by removing the linking chains to improve structural rigidity, good activity and drugability was achieved [57,58,59], mainly because the central triphenyl was stabilised by extensive hydrophobic contacts with BTyr56, AMet115, BMet115, AAla121 and BAla121 [57].
The aryl group of biphenyl derivatives
Since BMS reported the terphenyl scaffold PD-1/PD-L1 inhibitor in 2015, the aryl group was known to be an important pharmacophore due to hydrophobic and π-π interactions with Try56. Patents reported by BMS showed that, besides the phenyl moiety, the aromatic ring can include pyridine, 1,2,3,4-tetrahydronaphthalene, hydrindene, thiophene and 1,2,3,4-tetrahydroisoquinoline [35], and compounds 31, 32, 33, 34 and 35 displayed IC50 values of 0.11–1.00 μM in HRTF binding assays. The 2-position of the central pyridine of compound 31 can tolerate methoxy substituents, and BMS-202 displayed an IC50 value of 18 nM in HTRF binding assays. Moreover, activity data showed that the 1,2,3,4-tetrahydronaphthalene substituent at the 5-position was more potent than at the 6-position. In 2019, BMS optimised isoquinoline derivatives by adding an extension, tail and ether group junction, significantly improving the activity of compounds [60]; compound 36 exhibited an IC50 value of 2.0 nM in HRTF binding assays. In 2018 and 2019, Incyte Corporation reported several novel series of biphenyl-based small-molecule inhibitors of PD-1/PD-L1 interaction. Besides the modified linker, Incyte Corporation also reported fused heterocyclic rings in aryl groups [47,48,49], and compounds 37, 38 and 39 respectively showed IC50 values < 10 nM, <10 nM and <100 nM in HRTF binding assays (Table 4).
Coumarins possess antitumor activity, and Ma et al. from Huaqiao University designed biphenyl-based coumarin derivatives in 2019 [61], among which compound 40 achieved 70–100% inhibition of the PD-1/PD-L1 interaction in vitro at a concentration of 5 µM in HTRF binding assays. Moreover, using Sorafenib as a positive control, the compounds were tested for antitumor activity in MCF-7 human breast cancer cells and A549 human non-small cell carcinoma cells. The IC50 values of compound 40 for MCF-7 and A549 cells were 6.27 µM and 10.937 µM, respectively, compared with 10.87 µM and 11.24 µM for sorafenib. In the same year, Southern Medical University successively published three new lead structure patents [62,63,64] based on structures of natural compounds including flavonoids, naphthalene and chalcone. The IC50 values of representative structures 41, 42 and 43 were 1.428 µM, 1–10 µM and 5.387 µM, respectively, in HTRF binding assays. Two months later, Sun et al. from China Pharmaceutical University reported benzodiazole compounds [65] for which the IC50 value of compound 44 was 1.787 nM in HTRF binding assays. Experiments showed that the compounds could significantly block inhibition of PD-L1 in human PBMC cells and tumour cells, and secretion of IFN-γ by human T cells. Moreover, they could promote the proliferation of T cells and enhance the immune function of T cells. In 2021, Peng et al. from China Pharmaceutical University proposed a class of o-benzoylimide compounds [66], among which compound 45 displayed an excellent IC50 value of 6.1 nM in HTRF binding assays.
Shanghai Longwood Biopharmaceuticals Co., Ltd. also explored the aromatic ring. In 2019, the company published a series of fused-ring compounds, including five-membered heterocyclic fused six-membered heterocyclic, six-membered heterocyclic fused six-membered heterocyclic, and dihydrogen five-membered or tetrahydrogen six-membered heterocyclic fused six-membered heterocyclic aromatic compounds [67]. The representative compound 46 achieved 29.5% inhibition of the PD-1/PD-L1 interaction in vitro at a concentration of 8 nM in HTRF binding assays. SAR analysis reveals that replacement of chromane in the aromatic position with 1,4-benzodioxane could decrease activity. In the following year, a class of five-membered diheterocyclic pyridinone derivatives was reported [68], among which compound 47 exhibited an IC50 value < 100 nM in HTRF binding assays. SAR analysis revealed tolerance of thiazole, oxazole, imidazole, furan and thiophene in place of the five-membered heterocyclic part of the aromatic group. Meanwhile, activity data showed that the pyridione part in the aromatic group could be displaced by dihydro-2H-pyridine-2-one. Furthermore, changing the extension part, Shanghai Longwood Biopharmaceuticals developed a significant number of compounds in which the aromatic group was a pentadiheterocyclic pyridinone structure [69, 70].
In 2020, Jubilant Prodel LLC reported a new class of biphenyl inhibitors with pyrimidine as the aromatic part [71], and compound 48 showed an IC50 value < 100 nM in HTRF binding assays. In December of the same year, the company reported a novel class of compounds whose aromatic part was indene or tetrahydronaphthalene [72], differing from BMS compounds because the benzene ring counterpoint is substituted with indene or tetrahydronaphthalene. Compound 49 displayed an IC50 value < 100 nM in HTRF binding assays. In 2021, Chemocentryx reported a class of biphenyl inhibitors with pyrazine as the aromatic part [73, 74], and compound 50 showed an IC50 value ≤ 10 nM in ELISA.
The tail of biphenyl derivatives
The tail of biphenyl derivatives directly interacts with solvent and plays an important electrostatic role. This part is composed of the main chains and side chains of residues LAsp122 to LArg125, and the side chain of LAsp26 flanks a shallow groove in which there are multiple hydrogen bond donors and receptors. Therefore, the tail is composed of residues with hydrogen bond donors and receptors. BMS have extensively investigated the tail structure. However, the tail of biphenyl compounds is protected as a general feature in most patents. Only a class of urea compounds reported by Shenzhen Chipscreen Biosciences Co. Ltd. directly protected the structure of the tail [75]. Representative compound 51 showed an IC50 value of 15.78 nM in HTRF binding assays, indicating these compounds possessed good PD-1/PD-L1 binding activity in vitro. Further SAR studies showed that compounds with a 1-carboxy-2-hydroxythan-1-yl tail displayed higher activity. The 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay was used as a routine cytotoxicity detection approach to evaluate the growth activity of the Jurkat T-cell leukaemia cell line, and the results showed that all 14 compounds could maintain more than 90% cell activity. When measuring the bioactivity of PD-1 signalling inhibition at the cellular level, blocking or inhibiting the PD-1/PD-L1 interaction can enhance T cell activity and IL-2 secretion. ELISA showed that the representative compound had obvious activity for promoting IL-2 secretion by human T cells (>50%) (Table 5).
PROTACs is a novel strategy for removing unwanted proteins by hijacking the ubiquitin-proteasome system [76]. Recent studies showed that the PD-L1 protein is subject to ubiquitin-proteasome-mediated protein degradation [77]. The Chen group from Southern Medical University discovered a class of m-resorcinol diphenyl ether analogues using the PROTAC strategy that can be used as both inhibitors and depressants of PD-L1 [78]. By analysing the cocrystal structure of the dimeric PD-L1 protein and the BMS-8 complex, it was found that the piperidine 2-carboxylate tail group of BMS-8 was exposed to solvent, making it a suitable site for E3 ligase to bind to linkers and ligands. Furthermore, pomalidomide was selected as the ligand of CRBN (cereblon) E3 ligase due to its appropriate molecular weight [79] and immunomodulatory activity [80]. Combining pomalidomide and PD-1/PD-L1 inhibitors could achieve a synergistic effect, and this hypothesis was tested using a series of novel chlorophenol diphenyl ether PROTAC molecules targeting the PD-1/PD-L1 pathway. Compound 52 (P22) was one of the best-performing compounds, with an IC50 value of 39.2 nM in HTRF binding assays. Further SAR studies showed that introducing piperazine into the tail linking chain increased the activity of P22 5-fold. In addition, P22 significantly restored immunosuppression in co-culture models of Hep3B/OS-8/hPD-L1 and CD3 T cells. Flow cytometry and western blotting results also proved that P22 could moderately reduce the PD-L1 protein abundance in a lysosome-dependent manner. These results indicated that PD-1/PD-L1 small-molecule inhibitors were likely to serve as PD-L1-targeted PROTAC molecules. Compound P22 can be used as the starting point for PROTAC-like degradation of PD-L1. In addition, the Yang group from Nankai University reported new PROTAC molecules, among which compound 53 (21a) showed the best performance, with an IC50 value of 21 µM in HTRF binding assays [81]. Moreover, compound 21a could significantly reduce PD-L1 protein abundance, promote the invasion of CD8 + T cells, and inhibit the growth of MC-38 cells in vivo.
The ether group junction of biphenyl derivatives
In 2017, BMS also reported compounds with a scaffold based on the biphenyl core as inhibitors of PD-1/PD-L1 and CD80/PD-L1 with an aliphatic linker or aromatic groups at the ether group junction [82]. Addition of cyanopyridine or benzonitrile at the ether group junction had a positive impact on SAR. Furthermore, replacement of the distal phenyl ring with 1,4-benzodioxane significantly improved binding affinity. The IC50 values of the most potent compounds were in the range of 0.6–10 nM according to HTRF assays. The SAR-modified compound 54 displayed an IC50 value of 0.95 nM as determined by HTRF assay. Holak’s research showed that the benzonitrile portion of BMS-1001 occupies pockets composed of BArg113, BTyr123, BArg125 and AAsp61, providing additional hydrophobic π-π and hydrogen bonding interactions [38]. Hydrophobic interactions occurred between the phenyl group of benzonitrile and ATyr123, and hydrogen bonding occurred between the cyanogen group of benzonitrile and Asp61, which increased the activity of these compounds by more than an order of magnitude in vitro (Table 6).
Based on docking experiments and SAR analysis, the cyano group on the pyridine ring in the ether junction of biphenyl derivatives results in high activity. However, pharmaceutical company scientists are still exploring the ether group junction for novel compounds. The Institute of Materia Medica, Chinese Academy of Medical Science reported a series of 2-site brominated biphenyl compounds for which the ether junction was optimised, and compounds 55 and 56 were the most active with IC50 values of 10.2 nM and 18.2 nM, respectively, according to HTRF binding assays [83]. In 2019, Chemocentryx reported a series of compounds with optimised ether junctions [50]. The ether junction part consisted of a pyridine-acetonitrile scaffold with good activity (IC50 < 100 nM). Other scaffolds included pyrimidine and diazine, for which compounds 57 and 58 had IC50 values < 100 nM and 100–500 nM, respectively, based on ELISA.
In 2020, Guangzhou Wellhealth Bio-pharmaceutical Co. LTD. reported biphenyl small-molecule inhibitors with a dimethyl oxyphosphorus group, dimethyl sulphonimide or morpholino-1,1-oxide group [84] at the ether junction, among which compounds 59 and 60 had IC50 values of 6.6 nM and 16 nM, respectively, by HTRF binding assay, compared with 42 nM for the BMS-202 control. Biological activity evaluation experiments revealed very good tumour immunotherapy in vitro, promotion of the secretion of IFN-γ by T cells in a co-culture system, and significantly stimulated production of IFN-γ by T cells. In a pharmacokinetic study, compound 60 exhibited good pharmacokinetic characteristics as well as good stability and drug properties in terms of particle stability and plasma protein binding rate.
In the same year, Sun et al. from China Pharmaceutical University reported compounds in which the ether junction was replaced with 2,1,3-benzodiazole or thiadiazole [85], and compounds 61 and 62 inhibited PD-1 and PD-L1 protein abundance by 99% at a concentration of 10 nM, with IC50 values of 1.97 nM and 4.64 nM, respectively, in HTRF binding assays, compared with, 64% inhibition and an IC50 value of 34.31 nM for the BMS-1016 control. Moreover, the compounds performed well at blocking the hPD-L1 protein, inhibiting IFN-γ secretion from human peripheral blood mononuclear cells (PBMCs), inhibiting cell proliferation, inhibiting IFN-γ secretion from human T cells, and inhibiting IL-2 secretion from Jurkat cells. A Biacore molecular interaction instrument was used to determine that the combination and hPD-L1 protein bound strongly with a KD value of 0.5 nM. Nuclear factor of activated T-cells (NFAT) reporter gene PD-1/PD-L1 blocking experiments showed that the compounds could significantly interrupt PD-1/PD-L1 interaction in cells, with EC50 values between 0.21 and 3.64 μM. NFAT is a high-activity test method involving Bio-Glo Luciferase reagent and the chemiluminescence technique to probe the influence of antibodies or small-molecule compounds on the PD-1/PD-L1 interaction.
Extension of biphenyl derivatives
Scientists from BMS expanded on the first biphenyl patent [35]. Katarzyna Guzik et al. found that, in contrast to BMS-202, BMS-200 is perpendicular to the C, F and G chains of PD-L1, creating an interface similar to the PD-1-interacting surface [86]. The linear arrangement of the compound leads to the formation of a 16 Å long cylindrical hydrophobic channel between the two hPD-L1 monomers. The 2,3-dihydro-1,4-phenyldioxybenzyl group of the inhibitor BMS-200 causes ATyr56 C(β)−C(γ) to move 77° around the C(α)−C(β) axis, making this part of the compound accessible to solvent, thus turning the deep hydrophobic crack into a deep hydrophobic tunnel. This suggests that the ligand-binding sites of PD-L1 are more flexible than previously believed, and redefines the pharmacophore model previously described, creating new possibilities for further design of PD-L1 inhibitors (Table 7).
In 2018, BMS disclosed a patent related to extending biphenyl derivatives [36]. Representative compounds 63 and 64 had IC50 values in the range of 0.21 to10 nM based on HTRF binding assays. In July the following year, BMS reported another class of compounds with a fused heterocyclic structure at the distal phenyl ring in the biphenyl moiety, and elaborated on the extension [87]. Compounds 65 and 66 displayed IC50 values of 0.67 nM and 1.7 nM, respectively, in HTRF binding assays. Other compounds had IC50 values between 0.18 and 10.0 nM in HTRF binding assays. In 2020, Shenzhen Chipscreen Biosciences designed a series of biphenyl derivatives with an olefin moiety at the extension [88]. Representative compounds 67 and 68 had the ability to inhibit the PD-1/PD-L1 interaction at the nanomolar level in HTRF binding assays. In October of the same year, HEC Pharm reported biphenyl derivatives with a spiral ring structure added at the extension [89, 90], and compounds 69 and 70 exhibited the IC50 values of 1.1 and 0.76 nM, respectively, in HTRF binding assays.
The extension structures designed by Gilead Sciences were mainly C2-symmetric or pseudo-symmetric, hence the molecular weight is large, ranging from 500 to 1100 Da, as described below. In addition, Gilead Sciences designed a series of 9-, 10- or 11-fused-ring heterocyclic or heteraryl compounds at the extension in 2021 [91]. Compounds 71 and 72 showed IC50 values of 0.064 nM based on binding of protein pairs using the microbead amplification luminescence adjacent homogeneity assay (ALPHA) platform. In the same year, Xu et al. from China Pharmaceutical University reported a new biphenyl derivative with a triazole ring in the extension [92]. Representative compound 73 had an IC50 value of 0.1–10 nM in HTRF binding assays, and the affinity between the compound and hPD-L1 was 0.1–10 nM. The compounds in the patent were found to enhance the expression of INF-γ in a dose-dependent manner, significantly more effectively than BMS-202 but slightly lower than Keytruda (5 µM/mL), thereby enhancing the anti-tumour efficacy of T cells. In August of the same year, Zhang et al. from China Pharmaceutical University reported heterocyclic biphenyl compounds [93], and compounds 74 and 75 showed IC50 values between 0.1 and 20 nM in time-resolved fluorescence resonance energy transfer (TR-FRET) experiments, compared with an IC50 value of 18.7 nM for the BMS-202 positive control.
C2-symmetric and pseudo-symmetric structures of biphenyl derivatives
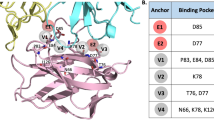
In 2019, BMS published a series of C2-symmetric and pseudo-symmetric compounds [82], among which compounds 76 and 77 showed IC50 values of 0.04 nM and 0.04–20 nM, respectively, in HTRF binding assays. Meanwhile, Basu et al. revealed the binding pattern of C2-symmetric compounds and proteins, among which LH1306 and LH1307 showed strong inhibition of the PD-1/PD-L1 interaction [94]. The cocrystal structure of LH1307 and the PD-L1 protein revealed a molecular arrangement similar to that of the PD-L1/BMS-200 complex previously reported by Guzik et al. (Fig. 2). The symmetrical structure of compound LH1307 facilitates the expansion of the channel, which is generated by the overall movement of a PD-L1 molecule in the dimer. All β chains (ABCDEFG), but not C' chains, participate in this transfer, resulting in an overall rotation of 15° with respect to the BMS-202/PD-L1 structure. Furthermore, the C2-symmetric structure enables the dimer to exhibit greater symmetry than previously described (Table 8).
Incyte Corporation again reported a class of pseudo-symmetric tetrahydroimidazolium[4,5-C]pyridine derivatives in 2019 [95], During T cell activation using artificial antigen-presenting cells (Aapcs), compounds 79 and 80 displayed EC50 values for IFNg secretion <10 nM and between 10 and to 100 nM, respectively. In 2020, Gilead Sciences reported a C2-symmetrical structure containing ninhydrin [96], and compound 81 had an IC50 value of 0.051 nM in AlphaLISA. Shanghai Longwood Biopharmaceuticals published a class of nitrogen-containing heterocyclic symmetric and pseudo-symmetric compounds [68], among which compounds 82 and 83 had IC50 values < 100 nM in HTRF binding assays.
In addition, Betta reported C2-symmetric biphenyl derivatives [97], among which compounds 84 and 85 had IC50 values of 0.21 nM and 0.37 nM, respectively, in HTRF binding assays. The IC50 values of most compounds in the patent were <25 nM. In September the following year, Betta reported another biphenyl symmetrical structure [98], representative compounds 86 and 87 showed IC50 values of 0.13 nM and 33 nM, respectively, in HTRF binding assays, and pseudosymmetric compound 86 showed better activity. In addition, the EC50 value of compound 86 was 24 nM in NFAT experiments. Chemocentryx also reported C2-symmetric or pseudo-symmetric compounds with ninhydrin [99], and compounds 88 and 89 had IC50 values < 100 nM in the ELISA. A class of C2-symmetric biphenyl compounds incorporating five-membered heterocycles was reported by Syntron [100], and representative compound 90 showed an IC50 value of 30.9 nM in HTRF assays.
The cyclic structure of biphenyl derivatives
In 2019, Guangzhou Dankang Medical Biological Co. Ltd. published a class of biphenyl cyclic PD-1/PD-L1 inhibitors [101], among which compounds 91 and 92 displayed IC50 values of 1.1 and 1.2 nM, respectively, in HTRF binding assays, compared with 39 nM for the BMS-202 control. In vitro metabolic stability experiments compared compounds 1164, 1250 and 1305 in patent CN106536515A, and some compounds showed better metabolic stability. In pharmacokinetic studies on compound 91 and reference compound 1250, compound 91 showed better pharmacokinetic characteristics and higher oral bioavailability; the average bioavailability of compounds 91 and 1250 was 66.38% and 22.90%, respectively. In 2020, Chemocentryx released a series of macrocyclic immunomodulators consisting of combined aromatic macrocyclic compounds [102]. The IC50 value measured by ELISA was <2uM, compared with <100 nM for compounds 93 and 94 (Table 9).
Conclusion
PD-1/PD-L1 protein-protein interaction (PPI) is a target with a large interacting surface, which makes it difficult to design small-molecule inhibitors. Small-molecule inhibitors based on biphenyls can induce dimerisation of PD-L1, effectively prevent binding between PD-1 and PD-L1, and regulate the activity of immune cells. In this work, we summarised small-molecule inhibitors based on biphenyls and performed SAR analysis (Fig. 3). For both biphenyls and biaryls, the activity of compounds could be significantly improved by rational design of the extension, the ether group junction and the tail. The linker is not an essential pharmacophore, but is useful for designing novel structures. In conclusion, the structure of existing compounds can serve as starting points for designing new molecular frameworks. However, due to the limited number of existing PD-1/PD-L1 small-molecule inhibitors, there is ample opportunity to explore novel inhibitors with optimised therapeutic efficacy. Structure-based drug design strategies driven by crystal structure and pharmacophore models may lead to improved small-molecule drugs. In addition, we believe that with the rapid development of small-molecule inhibitors of the PD-1/PD-L1 pathway, more compounds with significant immunomodulatory activities and good drug-like properties will appear in the future.
References
Zhang YY, Zhang ZM. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21. https://doi.org/10.1038/s41423-020-0488-6.
Shekarian T, Valsesia-Wittmann S, Caux C, Marabelle A. Paradigm shift in oncology: targeting the immune system rather than cancer cells. Mutagenesis. 2015;30:205–11. https://doi.org/10.1093/mutage/geu073.
Atkins MB, Sznol M. Cancer immunotherapy: Past Progress and Future Directions. Semin Oncol. 2015;42:518–22. https://doi.org/10.1053/j.seminoncol.2015.05.001.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. https://doi.org/10.1038/nm1100.
Rosenberg SA. Raising the Bar: The Curative Potential of Human Cancer Immunotherapy. Sci Transl Med. 2012;4. https://doi.org/10.1126/scitranslmed.3003634
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. https://doi.org/10.1038/nature10673.
Taefehshokr N, Baradaran B, Baghbanzadeh A, Taefehshokr S. Promising approaches in cancer immunotherapy. Immunobiology. 2020;225. https://doi.org/10.1016/j.imbio.2019.11.010
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 Antibody Promotes T Cell IFN-gamma-Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011;71:3540–51. https://doi.org/10.1158/0008-5472.can-11-0096.
Li H, Wu K, Tao KX, Chen LB, Zheng QC, Lu XM. et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–51. https://doi.org/10.1002/hep.25777.
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ. et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012;72:917–27. https://doi.org/10.1158/0008-5472.can-11-1620.
Sondak VK, Smalley KSM, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10:411–2. https://doi.org/10.1038/nrd3463.
Tan SG, Zhang CWH, Gao GF. Seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Sig Transd Targeted Ther. 2016;1. https://doi.org/10.1038/sigtrans.2016.29.
Lin X, Lu X, Luo GS, Xiang H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur J Med Chem. 2020;186. https://doi.org/10.1016/j.ejmech.2019.111876.
Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–63. https://doi.org/10.1016/j.it.2013.07.003.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84. https://doi.org/10.1038/nrd4591.
Keir ME, Butte MJ, Freeman GJ, Sharpel AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331.
Chen LP. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. https://doi.org/10.1038/nri1349.
Intlekofer AM, Thompson CB. At the Bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. https://doi.org/10.1189/jlb.1212621.
Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci USA. 2008;105:10483–8. https://doi.org/10.1073/pnas.0804453105.
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. https://doi.org/10.4049/jimmunol.173.2.945.
Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective Effects of PD-1 on Akt and Ras Pathways Regulate Molecular Components of the Cell Cycle and Inhibit T Cell Proliferation. Sci Signal. 2012;5. https://doi.org/10.1126/scisignal.2002796.
Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428. https://doi.org/10.1126/science.aaf1292.
Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. https://doi.org/10.1038/nri.2017.108.
Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med. 2011;84:409–21.
Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6. https://doi.org/10.1186/1756-8722-6-74
Ledford H, Else H, Warren M. Cancer immunologists scoop medicine Nobel prize. Nature. 2018;562:20–1. https://doi.org/10.1038/d41586-018-06751-0.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350. https://doi.org/10.1126/science.aar4060.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. https://doi.org/10.1111/j.1476-5381.2009.00190.x.
Beck KM, Dong JN, Geskin LJ, Beltrani VP, Phelps RG, Carvajal RD, et al. Disease stabilization with pembrolizumab for metastatic acral melanoma in the setting of autoimmune bullous pemphigoid. J Immunother Cancer. 2016;4. https://doi.org/10.1186/s40425-016-0123-3
Weinmann H. Cancer Immunotherapy: Selected Targets and Small-Molecule Modulators. Chemmedchem. 2016;11:450–66. https://doi.org/10.1002/cmdc.201500566.
Musielak B, Kocik J, Skalniak L, Magiera-Mularz K, Sala D, Czub M, et al. CA-170 - A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules. 2019;24. https://doi.org/10.3390/molecules24152804
Sasikumar PGN, Ramachandra M, Naremaddepalli S, Naremaddepalli SS, Nair Sasikumar PG, Setty Sudarshan N, et al. AURIGENE DISCOVERY TECHNOLOGIES LTD (REDY-C) AURIGENE DISCOVERY TECHNOLOGIES LTD (REDY-C) SASIKUMAR P G N (SASI-Individual) RAMACHANDRA M (RAMA-Individual) NAREMADDEPALLI S S S (NARE-Individual) NAREMADDEPALLI S S (NARE-Individual), assignee. New 1,3,4-oxadiazole/thiadiazole derivatives are programmed cell death-1 signaling pathway modulators useful for the treatment of e.g. bone cancer, pancreatic cancer, bacterial disease and viral disease patent WO2015033301-A1. 2015.
Sasikumar PGNB, IN), Ramachandra, Muralidhara (Bangalore, IN), Naremaddepalli, Seetharamaiah Sudarshan (Bangalore, IN), inventor Sasikumar Pottayil Govindan N.,Ramachandra Muralidhara,Naremaddepalli Seetharamaiah Sudarshan, assignee. 1,2,4-OXADIAZOLE AND THIADIAZOLE COMPOUNDS AS IMMUNOMODULATORS patent US20180044303--A1. 2018.
Sharpe AH, Butte MJ, Oyama S. HARVARD COLLEGE (HARD-C) SHARPE A H (SHAR-Individual) BUTTE M J (BUTT-Individual) OYAMA S (OYAM-Individual), assignee. New bis((hetero)aryl) sulfenyl/sulfonyl/carbonyl compounds used in pharmaceutical composition for treating or preventing e.g. herpes simplex virus infection, cancer, rheumatoid arthritis, multiple sclerosis, and type I diabetes patent WO2011082400-A2. 2011.
Chupak LS, Zheng X, Chupak Louis S. Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C), assignee. New immunomodulating compound useful for preparing a pharmaceutical composition, and for treating a disease or disorder associated with the inhibition of the programmed cell death 1/programmed death-ligand 1 interaction, e.g. cancer patent WO2015034820-A1. 2015.
Connolly TP, Frennesson DB, Grant-Young KA, Hewawasam P, Langley DR, Meng Z, et al. Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C), assignee. New substituted aromatic compound used in pharmaceutical composition for e.g. enhancing, stimulating, modulating and/or increasing immune response, and inhibiting growth, proliferation, or metastasis of cancer cells patent WO2017066227-A1. 2017.
Zak KM, Grudnik P, Guzik K, Zieba BJ, Musielak B, Domling A, et al. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1. Oncotarget. 2016;7:30323–35. https://doi.org/10.18632/oncotarget.8730.
Skalniak L, Zak KM, Guzik K, Magiera K, Musielak B, Pachota M. et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget. 2017;8:72167–81. https://doi.org/10.18632/oncotarget.20050.
Degorce F, Card A, Soh S, Trinquet E, Knapik GP, Xie B. HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications. Curr Chem Genom. 2009;3:22–32. https://doi.org/10.2174/1875397300903010022.
Feng Z, Chen X, Zhang L, Yang Y, Lai F, Ji M, et al. Chinese Acad Medical Sci (Msum-C), assignee. New bromo-substituted phenylether derivative used in pharmaceutical composition for manufacturing medicament useful for preventing and/or treating cancer, infectious disease and autoimmune disease patent CN107417666-A. 2017.
Yan J, YAN J (YANJ-Individual) ANHUI SHITE PHARM TECHNOLOGY CO LTD (ANHU-Non-standard), assignee. New (5-methyl-1-phenyl-2,3-dihydro-1H-pyrazol-3-yl)methanol compound is cell death receptor 1/programmed cell death ligand 1 inhibitor useful in preparing antitumor medicine patent CN111320606-A. 2020.
Ma J, Wang Y, Chen R, Liu J, Wu S. Univ Huaqiao (Uyhq-C), assignee. New 2-((dimethylamino)methyl)-5-(4-quinolyloxy)phenol derivative used in preparing pharmaceutical composition for treating diseases related to programmed cell death-1/programmed death ligand-1 patent CN112979562-A. 2021.
Guo JL, Luo LL, Wang ZH, Hu NJ, Wang W, Xie F. et al. Design, Synthesis, and Biological Evaluation of Linear Aliphatic Amine-Linked Triaryl Derivatives as Potent Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction with Promising Antitumor Effects In Vivo. J Med Chem. 2020;63:13825–50. https://doi.org/10.1021/acs.jmedchem.0c01329.
Wang Y, Xu Z, Wu T, He M, Zhang N. GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard), assignee. New aromatic acetylene or aromatic vinyl compound used in pharmaceutical composition, and in preparation of medicament for prevention, alleviation and treatment of cancer, infections and autoimmune diseases patent WO2018006795-A1. 2018.
Wang Y, Zhang N, Wu T, He M, Wang F. GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU ZAIJI PHARM TECHNOLOGY CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard) SHANGHAI MAXINOVEL PHARM CO LTD (SHAN-Non-standard) GUANGZHOU MAXINOVEL PHARM CO LTD (GUAN-Non-standard), assignee. New 1-phenyl-3-(2-phenylethyl)benzene derivative useful in pharmaceutical composition for preparing medicine for preventing and treating e.g. cancer and autoimmune disease patent WO2019128918-A1. 2019.
Jiang S, Hao H, Wang T, Wang M, Zhang W, Ni Y, et al. Univ China Pharm (Uycp-C) Univ China Pharm (Uycp-C), assignee. New phenyl-substituted five-membered heterocyclic compound used in pharmaceutical composition for preparing anti-tumor medicine patent CN111718310-A. 2020.
Lajkiewicz N, Wu L, Yao W, Wen-Qing Y. Incyte Corp (Inyt-C) Incyte Corp (Inyt-C) Incyte Corp (Inyt-C), assignee. New substituted phenyl-quinolin-8-yl-amine compounds are programmed cell death protein (PD) 1-PD-ligand 1 interaction inhibitors useful for e.g. treating viral infection and cancer patent CN108699001-A. 2018.
Li J, Wu L, Yao W. Incyte Corp (Inyt-C) Incyte Corp (Inyt-C) Incyte Corp (Inyt-C), assignee. New substituted heterocyclic compounds are programmed cell death protein 1/programmed death-ligand 1 interaction inhibitors useful to enhance, stimulate and/or increase immune response and to treat e.g. viral infection and cancer patent CN109641885-A. 2019.
Xiao K, Zhang F, Wu L, Yao W, Xiao KJ, Zhang FL, et al. Incyte Corp (Inyt-C) Incyte Corp (Inyt-C) Incyte Corp (Inyt-C) Incyte Corp (Inyt-C), assignee. New amido-substituted heterocyclic compound used in pharmaceutical composition useful for treating disease or disorder associated with inhibition of PD-1/PD-L1 interaction, and enhancing, stimulating or increasing immune response in patient patent CN109890819-A. 2019.
Lange C, Malathong V, McMurtrie DJ, Punna S, Singh R, Yang J, et al. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New substituted phenoxyindane compounds are programmed cell death-1 protein inhibitors, used to treat e.g. infectious disease, bacterial infectious disease, viral infectious disease, fungal infectious disease, solid tumor and cancer patent WO2018005374-A1. 2018.
Lange C, Punna S, Singh R, Yang J, Zhang P. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New substituted indan-1-yl-phenyl-amine compounds are used to e.g. modulate immune response mediated by the programmed cell death protein (PD)-1 signaling pathway and enhance, stimulate, modulate and/or increase the immune response patent WO2019165043-A2. 2019.
Xu Y, Huang L, Lin D, Hu H. GUANGZHOU DANKANG MEDICAL BIOLOGY CO LTD (GUAN-Non-standard), assignee. New fused ring compound useful in preparing medicine for treating and preventing disease associated with programmed cell death protein-1/programmed death-ligand-1 interaction and preparing immunomodulator patent CN110092740-A. 2019.
Fu B, Zhang Y, Wang Y, Liu X, Wang J, Ding L. BETA PHARM CO LTD (BETA-Non-standard) ZHEJIANG BETA PHARMA INC (ZHEJ-Non-standard) BETA PHARM CO LTD (BETA-Non-standard), assignee. New substituted heterocylic compounds are programmed cell death protein-1/programmed cell death-ligand1 interaction inhibitors, used to treat e.g. colon, gastric, thyroid, lung, pancreatic, brain, renal, prostate, ovarian and breast cancers patent CN112384500-A. 2021.
Qin MZ, Cao Q, Wu X, Liu CY, Zheng SS, Xie HB, et al. Discovery of the programmed cell death-1/programmed cell death-ligand 1 interaction inhibitors bearing an indoline scaffold. Eur J Med Chem. 2020;186. https://doi.org/10.1016/j.ejmech.2019.111856
Qin MZ, Meng YY, Yang HS, Liu L, Zhang HT, Wang SM. et al. Discovery of 4-Arylindolines Containing a Thiazole Moiety as Potential Antitumor Agents Inhibiting the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction. J Med Chem. 2021;64:5519–34. https://doi.org/10.1021/acs.jmedchem.0c01958.
Chen H, Wang K, Yang Y, Huang X, Dai X, Feng Z. Design, synthesis, and structure-activity relationship of programmed cell death-1/programmed cell death-ligand 1 interaction inhibitors bearing a benzo[d]isothiazole scaffold. Eur J Med Chem. 2021;217:113377. https://doi.org/10.1016/j.ejmech.2021.113377.
Muszak D, Surmiak E, Plewka J, Magiera-Mularz K, Kocik-Krol J, Musielak B. et al. Terphenyl-Based Small-Molecule Inhibitors of Programmed Cell Death-1/Programmed Death-Ligand 1 Protein-Protein Interaction. J Med Chem. 2021;64:11614–36. https://doi.org/10.1021/acs.jmedchem.1c00957.
Wang TY, Cai S, Wang MM, Zhang WH, Zhang KJ, Chen D. et al. Novel Biphenyl Pyridines as Potent Small-Molecule Inhibitors Targeting the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction. J Med Chem. 2021;64:7390–403. https://doi.org/10.1021/acs.jmedchem.1c00010.
Zhang HB, Xia Y, Yu CQ, Du HJ, Liu JC, Li H, et al. Discovery of Novel Small-Molecule Inhibitors of PD-1/PD-L1 Interaction via Structural Simplification Strategy. Molecules. 2021;26. https://doi.org/10.3390/molecules26113347
Yeung K, St Laurent DR, Romine JL, Scola PM, Yang G, St LDR. Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C), assignee. New isoquionline derivatives are programmed death-1 inhibitors used to enhance, stimulate, modulate and/or increase immune response, inhibit growth, proliferation, or metastasis of cancer cells and treat infectious disease and septic shock patent WO2018183171-A1. 2018.
Ma J, Huang K, Ni X, Chen R, Yu C, Yan Q, et al. Univ Huaqiao (Uyhq-C), assignee. New coumarin derivatives useful in preparing medicine for treating and preventing cancer diseases and other proliferative diseases patent CN109232498-A. 2019.
Chen J, Cheng B. Univ Southern Medical (Unsm-C), assignee. New substituted 2-(1,1’-biphenyl-3-yl)-4H-chromen-4-one compound useful for preparing programmed cell death protein 1/programmed death-ligand 1 inhibitor patent CN109336857-A. 2019.
Chen J, Cheng B. Univ Southern Medical (Unsm-C), assignee. New 2-methyl-6-((2-methylbiphenyl-3-yl)methoxy)naphthalene compound useful in preparing programmed cell death protein 1/programmed death-ligand 1 (PD1/PD-L1) inhibitor patent CN109438263-A. 2019.
Chen J, Cheng B. Univ Southern Medical (Unsm-C), assignee. New substituted biphenyl-containing chalcone derivative used for preparing programmed cell death receptor 1/programmed cell death ligand 1 inhibitor patent CN109456284-A. 2019.
Sun H, Liu L, Yao Z. Univ China Pharm (Uycp-C), assignee. New 7-(biphenyl-3-ylmethoxy)-6,7-dihydro-benzo(1,2,5)oxadiazol-5-ol compounds are programmed cell death protein 1 and programmed death ligand 1 inhibitors used for tumor immunotherapy patent CN109776445-A. 2019.
Yang P, Xiao Y, Sun C, Deng H, Cheng Y, Xie S, et al. Univ China Pharm (Uycp-C), assignee. New substituted phthalimide compound used in pharmaceutical composition for treating disease including autoimmune disease, cancer and infectious disease patent CN112979532-A. 2021.
Wang Z, Fan G, Zeng Z, Wang X, Jiang R. SHANGHAI LONGWOOD BIOPHARMACEUTICALS CO (SHAN-Non-standard) SHANGHAI CHENGSEN PHARM CO LTD (SHAN-Non-standard), assignee. New heteroaryl compound used in pharmaceutical composition for preparing PD-1/PD-L1 inhibitor to treat cancer treating cancer e.g. melanoma, lung cancer and bladder cancer, is formed by performing ring fusion of diheteroaryl compounds patent WO2019076343-A1. 2019.
Wang Z, Zhang J, Zeng Z. SHANGHAI CHENGSEN PHARM CO LTD (SHAN-Non-standard), assignee. New substituted aromatic compound used in pharmaceutical composition for preventing and/or treating disease associated with programmed cell death protein 1/programmed death-ligand 1 patent CN111039942-A. 2020.
Wang Z, Bai H, Li D, Qian A, Win BH, Lan Q. SHANGHAI CHENGSEN PHARM CO LTD (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARM CO (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARMACEUTICALS CO (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARM CO (SHAN-Non-standard), assignee. New 1,2,3,4-tetrahydronaphthalene compounds useful for preventing/treating diseases related to activity or expression of programmed cell death 1 receptor/ligand programmed cell death ligand 1 patent CN112457305-A. 2021.
Wang Z, Bai H, Li D, Qian A, Win BH, Lan Q. SHANGHAI CHENGSEN PHARM CO LTD (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARMACEUTICALS CO (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARM CO (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARM CO LTD (SHAN-Non-standard) SHANGHAI LONGWOOD BIOPHARM CO (SHAN-Non-standard), assignee. New tricyclic aromatic heterocyclic compound used e.g. for pharmaceutical composition for preventing and treating diseases related to activity or expression amount of programmed cell death 1 receptor/programmed cell death ligand 1 patent CN112457308-A. 2021.
Venkateshappa C, Duraiswamy AJ, Putta RKVP, Rajagopal S, Putta RKV. JUBILANT BIOSYS LTD (JUBI-Non-standard) JUBILANT BIOSYS LTD (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard), assignee. New 2-(benzyloxy)pyrimidine derivatives are programmed cell death protein-1 inhibitors used to treat and/or prevent e.g. proliferative disorder or cancer, HIV, influenza, herpes virus, hepatitis A, hepatitis B, hepatitis C and hepatitis D patent WO2019087214-A1. 2019.
Venkateshappa C, D AJ, Pendyala M, Sivanandhan D, Rajagopal S, A JD, et al. JUBILANT BIOSYS LTD (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT PRODEL LLC (JUBI-Non-standard) JUBILANT BIOSYS LTD (JUBI-Non-standard), assignee. New bicyclic compounds are PD-1 interaction inhibitors useful for treating and/or preventing various diseases, including cancer and infectious diseases patent WO2019175897-A1. 2019.
Fan P, Lange CW, Lui RM, McMurtrie DJ, Scamp RJ, Yang J, et al. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New heteroaryl-biphenyl amines are programmed cell death protein-1 inhibitors used to treat e.g. infectious disease, bacterial infectious disease, viral infectious disease, fungal infectious disease, inflammatory disease and cancer patent WO2021076688-A1. 2021.
Fan P, Lange CW, Lui RM, McMurtrie DJ, Scamp RJ, Yang J, et al. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New heteroaryl-biphenyl amides derivatives are programmed death-1 inhibitors to treat e.g. infectious disease, bacterial infectious disease, solid tumor, immune disorder, inflammatory and viral infectious disease fungal infectious disease patent WO2021076691-A1. 2021.
Yang Q, Lu X, Li Z, Xin L, Song Y, Fu C. SHENZHEN CHIPSCREEN BIOSCIENCES LTD (SHEN-Non-standard) SHENZHEN CHIPSCREEN BIOSCIENCES LTD (SHEN-Non-standard) SHENZHEN CHIPSCREEN BIOSCIENCES LTD (SHEN-Non-standard), assignee. New substituted amine based urea compounds useful in medicinal composition and medicament for preventing disease associated with PD-1/PD-L1 signaling pathway including cancer, autoimmune diseases and chronic infectious diseases patent CN108250159-A. 2018.
Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–14. https://doi.org/10.1038/nrd.2016.211.
Lim S-O, Li C-W, Xia W, Cha J-H, Chan L-C, Wu Y. et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–39. https://doi.org/10.1016/j.ccell.2016.10.010.
Cheng BB, Ren YC, Cao H, Chen JJ. Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1. Eur J Med Chem. 2020;199. https://doi.org/10.1016/j.ejmech.2020.112377.
Chamberlain PP, Hamann LG. Development of targeted protein degradation therapeutics. Nat Chem Biol. 2019;15:937–44. https://doi.org/10.1038/s41589-019-0362-y.
Zeidner JF, Knaus HA, Zeidan AM, Blackford AL, Montiel-Esparza R, Hackl H. et al. Immunomodulation with pomalidomide at early lymphocyte recovery after induction chemotherapy in newly diagnosed AML and high-risk MDS. Leukemia. 2020;34:1563–76. https://doi.org/10.1038/s41375-019-0693-4.
Wang YB, Zhou YY, Cao S, Sun Y, Dong ZQ, Li C, et al. In vitro and in vivo degradation of programmed cell death ligand 1 (PD-L1) by a proteolysis targeting chimera (PROTAC). Bioorg Chem. 2021;111. https://doi.org/10.1016/j.bioorg.2021.104833
Frennesson DB, Grant-Young KA, Hewawasam P, Langley DR, Meng Z, Mull E, et al. Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol Myers Squibb Co (Brim-C), assignee. New immunomodulatory derivative used for treating cancer and infectious diseases, where additional agent is antimicrobial agent, antiviral agent, cytotoxic agent, gene expression modulatory agent or immune response modifier patent CN109863146-A. 2019.
Feng Z, Chen X, Yang Y, Zhou C, Lai F, Ji M, et al. Chinese Acad Medical Sci (Msum-C), assignee. New bromo-substituted phenylether derivative used in pharmaceutical composition for manufacturing medicament useful for preventing and/or treating cancer, infectious disease and autoimmune disease patent CN107417564-A. 2017.
Xu Y, Huang L, Lin D, Hu H. GUANGZHOU DANKANG MEDICAL BIOLOGY CO LTD (GUAN-Non-standard), assignee. New chlorobenzene compound useful in preparing medicine for preventing/treating diseases related to interaction solid cancer and advanced solid cancer (e.g. non-small cell lung cancer) and for preparing medicine and immunomodulators patent CN111747927-A. 2020.
Sun H, Liu L, Wang S, Yao Z, Xie T, Wu G, et al. Univ China Pharm (Uycp-C) Univ China Pharm (Uycp-C), assignee. New substituted 5-((3-(((1,1’-biphenyl)-3-yloxy)methyl)phenoxy)methyl)-2H-1,3-benzodiazole compound used in pharmaceutical composition for preparing immunomodulator drugs used for preventing or treating tumors or autoimmune diseases patent CN111909108-A. 2020.
Guzik K, Zak KM, Grudnik P, Magiera K, Musielak B, Torner R. et al. Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimerization of PD-L1. J Med Chem. 2017;60:5857–67. https://doi.org/10.1021/acs.jmedchem.7b00293.
Yeung K, Grant-Young KA, Sun L, Langley DR, St Laurent DR, Scola PM, et al. Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C) Bristol-Myers Squibb Co (Brim-C), assignee. New substituted phenyl compounds are programmed death-ligand 1-programmed death 1 interaction inhibitors used to e.g. enhance, stimulate, and/or increase immune response, and inhibit growth, proliferation or metastasis of cancer cells patent WO2018118848-A1. 2018.
Yu J, Xin L, Shan S, Wang X, Fu C, Li Z, et al. SHENZHEN CHIPSCREEN BIOSCIENCES LTD (SHEN-Non-standard) SHENZHEN CHIPSCREEN BIOTECHNOLOGY CO LTD (SHEN-Non-standard), assignee. New biphenyl compounds are programmed cell death receptor-1 and PROGRAMMED cell death protein ligand-1 inhibitors used for e.g. improving, stimulating, regulating and increasing immune response, and treat infectious disease caused by virus patent WO2020043154-A1. 2020.
Liu B, Yang T, Yu X, Sun D, Zhang Y. Sunshine Lake Pharma Co Ltd (Susn-C) Guangdong Dongyangguang Pharm Co Ltd (Gddy-C), assignee. New substituted phenol compound useful in medicinal composition for treating, preventing and improving cancer in which immortalized cell exists in organ/body tissue including e.g. bone cancer, infectious diseases and autoimmune diseases patent WO2019174533-A1. 2019.
Liu B, Zhang Y, Yang T, Yu X, Sun D. DONGGUAN HEC NEW DRUG RES & DEV CO LTD (DONG-Non-standard) GUANGDONG DONGYANGGUANG PHARM CO LTD (GDDY-C), assignee. New programmed cell death protein 1/programmed cell death protein 1 and its ligand small molecule inhibitor i.e. substituted aromatic compound in preparing medicine for treating e.g. cancer, infectious disease and autoimmune disease patent CN111793077-A. 2020.
Du Z, Graupe M, Machicao Tello PA, Medley JW, Metobo SE, Parkhill EQ, et al. Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C), assignee. New substituted heterocyclic compounds are programmed death-1 inhibitors, used to treat e.g. pancreatic cancer, bladder cancer, colorectal cancer, breast cancer, prostate cancer, renal cancer, hepatocellular cancer, lung and ovarian cancers patent WO2020086556-A1. 2020.
Xu Y, Zhang H, Zhu Q, Du H, Xia Y, Yu C, et al. Univ China Pharm (Uycp-C), assignee. New biphenyl compound useful in preparing programmed cell death protein 1 and programmed death-ligand 1 inhibitor medicine and in preparing medicine for treating tumor patent CN113135895-A. 2021.
Zhang H, Zhou J, Zhang J, Zhu P, Wang T. Univ China Pharm (Uycp-C), assignee. New heterocyclic substituted biphenyl compound is programmed cell death protein 1/programmed death-ligand 1 inhibitor, useful in preparing medicine for preventing and treating e.g. solid tumor, late solid tumor and melanoma patent CN113307779-A. 2021.
Basu S, Yang J, Xu B, Magiera-Mularz K, Skalniak L, Musielak B. et al. Design, Synthesis, Evaluation, and Structural Studies of C-2-Symmetric Small Molecule Inhibitors of Programmed Cell Death1/Programmed Death-Ligand 1 Protein-Protein Interaction. J Med Chem. 2019;62:7250–63. https://doi.org/10.1021/acs.jmedchem.9b00795.
Wu L, Qian D, Lu L, Lajkiewicz N, Konkol LC, Li Z, et al. INCYTE CORP (INYT-C) INCYTE CORP (INYT-C) LU L (LULL-Individual) ZHANG F (ZHAN-Individual) LI J (LIJJ-Individual) WANG H (WANG-Individual) XIAO K (XIAO-Individual), assignee. New heterocyclic compound used in pharmaceutical composition treating disease or disorder associated with inhibition of PD-1/PD-L1 interaction patent CN110267953-A. 2019.
Aktoudianakis E, Appleby T, Cho A, Du Z, Graupe M, Guerrero JA, et al. Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C) Gilead Sci Inc (Gile-C), assignee. New substituted heterocyclic compounds are programmed cell death-1 inhibitors, used to treat cancer e.g. cancer of pancreatic, bladder, colorectal, breast, prostate, renal, hepatocellular, lung, ovarian, cervical, gastric and esophageal patent WO2018195321-A1. 2018.
Wang Y, Fu B, Zhang Y, Liu X, Wang J, Ding L. BETA PHARM CO LTD (BETA-Non-standard) ZHEJIANG BETA PHARMA INC (ZHEJ-Non-standard) BETA PHARM CO LTD (BETA-Non-standard) BETA PHARM CO LTD (BETA-Non-standard) BETA PHARM CO LTD (BETA-Non-standard), assignee. New substituted 1,1’-biphenyl-3,3’-diamine compounds are PD-1-PD-L1 interaction inhibitors used for treating cancer of e.g. gastric, thyroid, lung, renal, and prostate and enhancing, stimulating and/or increasing immune response patent WO2019192506-A1. 2019.
Wang Y, Zhang Y, Fu B, Wang J, Ding L, Chen K, et al. BETA PHARM CO LTD (BETA-Non-standard) ZHEJIANG BETA PHARMA INC (ZHEJ-Non-standard) BETA PHARM CO LTD (BETA-Non-standard) BETA PHARM CO LTD (BETA-Non-standard) BETA PHARM CO LTD (BETA-Non-standard), assignee. New substituted fused cyclic compounds are programmed cell death-1/programmed death-ligand 1 interaction inhibitor, used to treat e.g. colon cancer, brain cancer and melanoma, and enhancing, stimulating and/or increasing immune response patent WO2020156323-A1. 2020.
Malathong V, Mali VR, McMurtrie DJ, Punna S, Roth HS, Singh R, et al. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New substituted fused cyclic compounds are programmed cell death protein-1 (PD-1) inhibitors, used to e.g. modulating immune response mediated by PD-1 signaling pathway, increasing immune response and inhibiting metastasis of cancer cells patent WO2021007386-A1. 2021.
Jiang S, Xiao Y, Hao H, Wang T, Zhang K, Wang M, et al. Univ China Pharm (Uycp-C), assignee. New five-membered heterocyclic substituted biphenyl compounds are programmed cell death ligand inhibitor and immune checkpoint inhibitors, useful in preparing medicine for treating cancer patent CN113121464-A. 2021.
Xu Y, Huang L, Lin D, Hu H. GUANGZHOU DANKANG MEDICAL BIOLOGY CO LTD (GUAN-Non-standard), assignee. New (phenoxymethyl)benzene-containing derivative used for treating and/or preventing programmed death-1/programmed death-ligand 1 interaction-related diseases patent CN110092799-A. 2019.
Malathong V, McMahon J, McMurtrie DJ, Punna S, Roth HS, Singh R, et al. Chemocentryx Inc (Chcx-C) Chemocentryx Inc (Chcx-C), assignee. New macrocyclic immunomodulator used for inhibiting growth, proliferation, or metastasis of cancer cells, and treating disease or disorder mediated by programmed cell death protein 1 signaling pathway, e.g. immune disorder or cancer patent WO2019032547-A1. 2019.
Price C, Zhou X, Li W, Wang L. Real-Time Measurement of Solute Transport Within the Lacunar-Canalicular System of Mechanically Loaded Bone: Direct Evidence for Load-Induced Fluid Flow. J Bone Min Res. 2011;26:277–85. https://doi.org/10.1002/jbmr.211.
Acknowledgements
This work was supported by the Postdoctoral Science Foundation of Beijing [2022-ZZ-025].
Author contributions
YLW and SRW designed the research; SRW performed the collection and analysis of data; SRW and HY drafted the article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Wang, Y. & Yan, H. Progress on biphenyl derivatives as PD-1/PD-L1 inhibitors. Med Chem Res 32, 2089–2115 (2023). https://doi.org/10.1007/s00044-023-03127-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03127-6