Abstract
A set of novel Schiff bases of isatin were synthesized and characterized by reaction of isatin with various aromatic or heterocyclic primary amines. Cytotoxic activities for some of the synthesized compounds were evaluated by MTT assay in three human cancer cell lines (HeLa, LS180 and Raji). Half of the tested compounds showed good cytotoxicity in HeLa cells. 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one was found to be the most potent molecule among the studied isatin derivatives. Docking studies of 3-substituted indolin-2-one scaffolds on vascular endothelial growth factor receptor 2 (VEGFR-2) involved in cell proliferation and angiogenesis was performed. 3-(naphthalen-1-ylimino) indolin-2-one and 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one exhibited higher docking binding energies with receptor. For 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one, H-bond interaction with Cys917 residue of target active site was in common with reported crystallographic benzoimidazole derivative (PDB code: 2OH4). New key H-bonds involving Glu915, Asn921, and Arg1049 residues in VEGFR-2 active site could be detected for 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one. Extended lipophilic rings containing H-bond acceptors on the 3 position of indoline scaffold seemed to be important factors in developing potent VEGFR-2 inhibitors virtually. Based on the ligand efficiency indices, some isoxazole or thiazole substituted isatin derivatives may be regarded as efficient candidates for further molecular developments of anticancer agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isatin is an endogenous compound isolated in 1988 (Glover et al., 1988) and reported to possess a wide range of central nervous system activities (d’Ischia et al., 1988; Varma and Nobles, 1975). It has also been found as a metabolic derivative of adrenaline in humans (d’Ischia et al., 1988). Isatin is a natural product found in a number of plants including those of the genus isatis and also has been found as a metabolic derivative of humans (d’Ischia et al., 1988). Various derivatives of isatin are known to possess a wide range of pharmacological properties (Varma and Nobles, 1975; Varma and Khank, 1977). Among the important pharmacological effects, antibacterial (Pandeya et al., 2000, 1999a; Sarangapani and Reddy, 1994; Varma and Nobles, 1975; Sridhar et al., 2001), antifungal (Pandeya et al., 2000; Pandeya et al., 1999), antiviral (Varma and Nobles, 1967; Singh et al., 1983; Logan et al., 1975), and anti-HIV (Pandeya et al., 1999b; Pandeya et al., 2000) activities are worth noting. Within the context of enzyme inhibitors, isatins (also known as 2,3-dioxindoles) have seen recent applications in the inhibition of cysteine and serine proteases (Iyer and Hanna, 1995; Webber et al., 1996). Thus, isatin is a biologically validated starting point for the design and synthesis of chemical libraries directed at these targets (Shuttleworth et al., 2000).
Various isatin derivatives have been reported to possess cytotoxic activity (Matesic et al., 2008; Hossain et al., 2008; Vine et al., 2007; Pervez et al., 2011). Increasing knowledge of the biological activities of some simple isatin derivatives guide many researchers to the development of antitumor agents, capable of causing apoptosis, a programmed cell death involved in many physiological and pathological processes.
Due to the privileged nature of isatin, libraries designed and synthesized around the basic structure of this scaffold should yield medicinally active compounds with high hit rates at significantly reduced library size compared to large classical libraries obtained from combinatorial chemistry efforts based on non-privileged templates. Schiff bases and Mannich bases of isatin were reported to possess as an extension of this study and we have now focused our attention on Schiff bases of isatin especially heterocyclic imines.
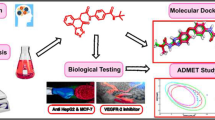
In continuation to our study on biologically active isatin derivatives (Azizian et al., 2002; Azizian et al., 2001), here, we report the synthesis and spectroscopic characterization of some hydrazones and Schiff bases of isatins in fairly good yields along with their cytotoxicity assay. Furthermore, synthesized compounds were subjected to molecular docking simulations to preliminary find out the potential molecular target and at the same moment further support the experimental cytotoxic tests. The efficiency of Autodock 4.2 program has been well demonstrated in several studies (Sousa et al., 2006; Sellers et al., 2010). We performed our docking study with Autodock 4.2 program. The target was chosen as VEGFR-2 on the basis of its involvement in cell proliferation/angiogenesis (Phosrithong and Ungwitayatorn, 2010) and previous reports on the inhibitory activity of 3-substituted-indolin-2-ones against VEGFR-2 (Sun et al., 1999). VEGFR-2 is a cell surface receptor for vascular endothelial growth factors. VEGFR-2 is a key pharmacological target in angiogenesis of tumor cells (Strawn et al., 1996). Moreover, it has been established that VEGFR-2 provokes proliferation through activation of the extracellular signal-regulated kinases pathway (Holmes et al., 2007). Anti-tumor drug development targeting the VEGFR-2 signaling pathway is now regarded as a prominent choice in the clinical trials (Holmes et al., 2007).
Experimental
Instruments
Melting points were determined on the Electro-thermal Melting Point apparatus and were uncorrected. Infrared spectra were recorded on the Shimadzu-420 infrared spectrophotometer. 1H-NMR and 13C NMR spectra were recorded in DMSO-d 6 on Brucker 300 MHz spectrometers (chemical shifts are given in parts per million (PPM)). Elemental analyses (C, H, N) were performed by the Microanalytical Unit.
General procedure for the synthesis of isatin schiff base derivatives
A mixture of isatin (5 mmol) and aromatic or heterocyclic primary amines (5 mmol) are refluxed in ethanol (50 ml) in the presence of acetic acid as the catalyst for 0.5–2 h. After completion of the reaction (monitored by TLC), the solvent was removed under reduced pressure and the crude product was washed with water and recrystallized from ethanol to give pure products (Table 1).

Some synthesized compounds are compared with those reported in earlier literature (3–10) (Silva et al., 2001).
Characteristic data for new isatin Schiff base are as follows:
(Z)-3-(6-chloro-2-methylpyrimidin-4-ylimino) indolin-2-one (11)
Yellow powder. Yield 83%, m.p. 193–194°C. IR (KBr, cm−1): 3412, 3110, 2923, 1702, 1667, 1450, 1130 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.11 (s, 1H, OH), 7.82 (s, 1H, pyr), 6.95–7.58 (m, 4H, Ar), 2.44 (s, 3H, Me). 13CNMR (DMSO-d 6) δ:160–187 (pyr), 173.1(C=O), 115–152 (Ar). Anal. Calcd for C13H9ClN4O: C, 57.26; H, 3.33; N, 20.55. Found: C, 57.21; H, 3.42; N, 20.46.
(dihydrothiazol-2-ylimino) indolin-2-one (12)
Red powder. Yield 83%, m.p. 194–195°C. IR (KBr, cm−1): 3385, 3225, 2915, 1710, 1667, 1450 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.34 (s, 1H, OH), 7.11–7.72 (m, 4H, Ar), 5.23 (d, 1H, heterocycl), 5.35 (s, 1H), 5.95 (d, 1H, heterocycl). 13CNMR (DMSO-d 6, 300 MHz) δ: 168 (C=O), 120- 155 (Ar), 112.5, 127.1 (heterocycl), 48.4 (C–S). Anal. Calcd for C11H9N3OS: C, 57.13; H, 3.92; N, 18.17; S, 13.86. Found: C, 57.23; H, 3.88; N, 18.25; S, 13.81.
(Z)-3-(5-chloropyridin-2-ylimino) indolin-2-one (13)
Yield 83%, m.p. 197–199°C. IR (KBr, cm−1): 3385, 3225, 2915, 1710, 1667, 1450 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.34 (s, 1H, OH), 7.11–7.72 (m, 4H, Ar), 5.23 (d, 1H, heterocycl), 5.35 (s, 1H), 5.95 (d, 1H, heterocycl). 13CNMR (DMSO-d 6, 300 MHz) δ: 168 (C=O), 120–155 (Ar), 112.2, 127.7 (heterocycl), 48.2 (C–S). Anal. Calcd for C11H9N3OS: C, 57.13; H, 3.92; N, 18.17; S, 13.86. Found: C, 57.23; H, 3.88; N, 18.25; S, 13.81.
(Z)-3-(1H-benzo[d]imidazol-2-ylimino) indolin-2-one (14)
Yield 75%, m.p. 154–155°C. IR (KBr, cm−1): 3305, 3365, 2915, 1705, 1607, 1450 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.57 (s, 1H, OH), 7.11–7.72 (m, 8H, Ar), 5.15 (sbr, 1H, N–H). 13CNMR (DMSO-d 6, 300 MHz) δ: 167 (C=O), 117–148 (Ar), 136–157 (imidazole). Anal. Calcd for C15H10N4O: C, 68.69; H, 3.84; N, 21.36. Found: C, 68.62; H, 3.88; N, 21.28.
(Z)-3-(benzo[d]thiazol-2-ylimino) indolin-2-one (15)
Red powder. Yield 73%, m.p. 168–171°C. IR (KBr, cm−1): 3296, 3325, 2903, 1715, 1613, 1446 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.39 (s, 1H, OH), 7.15–7.66 (m, 8H, Ar). 13CNMR (DMSO-d 6, 300 MHz) δ: 173 (C=O), 110–152 (Ar), 130–163 (thiazole). Anal. Calcd for C15H9N3OS: C, 64.50; H, 3.25; N, 15.04; S, 11.48. Found: C, 64.56; H, 3.29; N, 15.11; S, 11.42.
(3Z)-3-(5-methylisoxazol-2(5H)-ylimino) indolin-2-one (16)
Yield 85%, m.p. 153–154°C. IR (KBr cm−1): 3318, 3185, 2900, 1721, 1600, 1442 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.45 (s, 1H, OH), 7.12–7.73 (m, 4H, Ar), 6.15 (d, 1H, isoxazole), 4.45 (d, 1H, isoxazole), 2.27 (Me). 13CNMR (DMSO-d 6, 300 MHz) δ:170.3 (C=O), 112-147 (Ar),142.2 (isoxazole), 105.8 (isoxazole), 17.1 (Me). Anal. Calcd for C12H11N3O2: C, 62.87; H, 4.84; N, 18.33. Found: C, 62.82; H, 4.79; N, 18.37.
(Z)-3-(4-chlorothiazol-2-ylimino) indolin-2-one (17)
Brown powder. Yield 81%, m.p. 172–174°C. IR (KBr, cm−1): 3300, 3137, 2929, 1708, 1642, 1435 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.64 (s, 1H, OH), 7.34–7.70 (m, 4H, Ar), 7.31 (s, 1H, chlorothiazole). 13CNMR (DMSO-d 6, 300 MHz) δ: 169.7 (C=O), 120-14752 (Ar),140.6 (C–Cl), 111.8 (chlorothiazole). Anal. Calcd for C11H6ClN3OS: C, 50.10; H, 2.29; N, 15.93; S, 12.16. Found: C, 50.16; H, 2.35; N, 15.88; S, 12.11.
(Z)-3-(4-methylisoxazol-3-ylimino) indolin-2-one (18)
Yellow powder. Yield 83%, m.p. 179–181°C. IR (KBr, cm−1): 3327, 3124, 2910, 1695, 1639, 1413 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.62 (s, 1H, OH), 7.30–7.68 (m, 4H, Ar), 7.27 (s, 1H, isoxazole). 13CNMR (DMSO-d 6, 300 MHz) δ: 174.2 (C=O), 118–145 (Ar), 150.5 (C–O), 148.9 (N–C–N), 11.5 (Me). Anal. Calcd for C12H9N3O2: C, 63.43; H, 3.99; N, 18.49. Found: C, 63.48; H, 3.92; N, 18.53.
(Z)-3-(5-methylisoxazol-3-ylimino) indolin-2-one (19)
Yellow powder. Yield 83%, m.p. 193–195°C. IR (KBr, cm−1): 3320, 3122, 2915, 1690, 1651, 1417 cm−1. 1HNMR (300 MHz, DMSO-d 6) δ: 10.60 (s, 1H, OH), 7.32–7.65 (m, 4H, Ar), 6.12 (s, 1H, isoxazole). 13CNMR (DMSO-d 6, 300 MHz) δ: 170.9 (C=O), 113–138 (Ar), 152.3 (C–O), 148.1 (N–C–N), 12.9 (Me). Anal. Calcd for C12H9N3O2: C, 63.43; H, 3.99; N, 18.49. Found: C, 63.48; H, 3.92; N, 18.53.
Cytotoxicity assay
RPMI 1640, fetal bovine serum (FBS), trypsin and phosphate buffered saline (PBS) were purchased from Biosera (Ringmer, UK). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (Saint Louis, MO, USA) and penicillin/streptomycin was purchased from Invitrogen (San Diego, CA, USA). Doxorubicin and dimethyl sulphoxide were obtained from EBEWE Pharma (Unterach, Austria) and Merck (Darmstadt, Germany), respectively.
HeLa (human cervical adenocarcinoma), LS-180 (human colon adenocarcinoma), and Raji (human B lymphoma) cells were obtained from the National Cell Bank of Iran, Pasteur Institute, Tehran, Iran. All cell lines were maintained in RPMI 1640 supplemented with 10% FBS, and 100 units/ml penicillin-G and 100 μg/ml streptomycin. Cells were grown in monolayer cultures, except for Raji cells, which were grown in suspension, at 37°C in humidified air containing 5% CO2.
Cell viability following exposure to synthetic compounds was estimated by using the MTT reduction assay (Mosmann, 1983; Miri et al., 2011). HeLa, LS-180, and Raji cells were plated in 96-well flat-bottomed microplates at densities of 25,000, 100,000, and 50,000 cells/ml, respectively (100 μl per well). Control wells contained no drugs and blank wells contained only growth medium for background correction. After overnight incubation at 37°C, half of the growth medium was removed and 50 μl of medium supplemented with different concentrations of synthetic compounds dissolved in DMSO were added in quadruplicate. Plates with Raji cells were centrifuged before this procedure. Maximum concentration of DMSO in the wells was 0.5%. Cells were further incubated for 72 h, except for HeLa cells, which were incubated for 96 h. At the end of the incubation time, the medium was removed and MTT was added to each well at a final concentration of 0.5 mg/ml and plates were incubated for another 4 h at 37°C. Then, formazan crystals were solubilized in 200 μl DMSO. The optical density was measured at 570 nm with background correction at 655 nm using a Bio-Rad microplate reader (Model 680). The percentage of viability compared to control wells was calculated for each concentration of the compound and IC16 and IC50 values were calculated with the software CurveExpert version 1.34 for Windows. Each experiment was repeated 3–4 times. Data are presented as mean ± SD.
Docking study
AutoDock is a free to use program (The Scripps Research Institute, La Jolla, CA, http://autodock.scripps.edu/). Lamarckian Genetic Algorithm of the AutoDock 4.2 program was used to perform the flexible-ligand docking studies (Morris et al., 2009). Receptor X-ray crystal structure obtained from the Brookhaven protein data bank was applied in docking studies (2OH4; http://www.pdb.org/). Optimization and pre-processing of molecules under study was done using AM1 method and AutoDock Tools 1.5.4 program (ADT) (Morris et al., 2008). The AM1 optimization method was performed using Polak-Ribiere (conjugate gradient) algorithm with termination condition as RMS gradient of 0.1 (Kcal/Å mol) and 405 up to 495 maximum cycles based on the ligand structures.
Treatment of all molecular structures was done carefully in a uniform and consistent manner to avoid introduction of bias. Ligands were submitted to 100 independent genetic algorithms (GA) runs for search. For Lamarckian GA method, a maximum number of 2,500,000 energy evaluations; 27,000 maximum generations; a gene mutation rate of 0.02; and a crossover rate of 0.8 were used. A grid of 60, 60, and 60 points in x-, y-, and z-direction, respectively, with grid spacing of 0.375 Å was built centered on the center of mass of the catalytic site of considered receptor. Cluster analysis was performed on the docked results using a RMS tolerance of 2 Å. The best docking result in each case was considered to be the conformation with the lowest binding energy. Hydrogen bindings between docked potent agents and related macromolecule were analyzed using Autodock tools program (ADT, Version 1.5.4).
Results and discussion
Some new isatin-Schiff base compounds were obtained by condensation of different aromatic and heterocyclic amines and isatin, under mild conditions in ethanolic solution (see Fig. 1). The isolated compounds were then characterized by elemental analyses, IR and NMR spectroscopy. Spectroscopic properties were compared to those of related compounds that previously isolated and described to correlate their structural features. Electronic spectra of all the complexes were carried out in aqueous, DMSO or CH3CN solution, depending on their solubility.
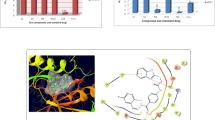
The in vitro cytotoxic activities for ten synthesized isatin Schiff base derivatives are shown in Table 2.
All the compounds under study showed superior cytotoxic activity on the HeLa cells. Compound 2 showed higher cytotoxicity in all of the tested cells. This indicated that arylhydrazone indolin-2-one scaffold bearing electron withdrawing/hydrogen acceptor groups is a favorable isatin structure for designing cytotoxic agents. This rational may also be established for arylimino indoline-2-one compounds 6, 13, and 17 in HeLa cell line. These compounds possess electron withdrawing chlorine atom on their aryl rings. However, the effect is more pronounced for compound 17 which contains five-member heterocyclic chlorothiazol ring bearing an electronegative chlorine atom at its fourth position.
It seemed that five-member heterocyclic rings may also promote cytotoxic activity on HeLa cells in arylimino indoline-2-ones since compounds 5 and 10 having electron donating groups show higher cytotoxic activity than compounds 6 and 13 which possess electron withdrawing groups but not five-member rings bearing two hetero atoms. However, extended cytotoxicity assays on diverse sets of isatin molecules need to be performed to establish more rational structure activity relationships.
Docking studies
Docking validation step was performed by re-docking of the co-crystallized conformation of cognate ligands into 3D structure of VEGFR-2 (Hevener et al., 2009). In this way, validation of the method for prediction of the known binding poses would be supported (Cosconati et al., 2010). If the RMSD is below 2 Å, it is generally considered a successful prediction (Vyas et al., 2008). The outputs of validation studies for different targets under study are shown in Table 3.
3-(arylimino) indolin-2-one compounds (Table 1) were all docked into the active site of selected receptor. Docking results are shown in Table 4. Top ranked binding energies (kcal/mol) in AutoDock dlg output file were considered as response in each run.
Docking results were supported almost by high cluster populations. This could be expected since literature evidence implied that docking studies with compounds bearing less active torsions can significantly promote the docking success rates due to the limited conformational degrees of freedom (Erickson et al., 2004).
Possible key hydrogen bonds between synthesized docked compounds and VEGFR-2 active site were estimated and gathered in Table 5. A key hydrogen bonding has been reported between carbamate NH and Cys917 in a crystallographic benzoimidazole-urea derivative (Hasegawa et al., 2007). Compound 2 exhibited a similar electrostatic interaction via its indolinone carbonyl oxygen with Cys917 NH (Table 5). However, the crystallographic data revealed a shorter distance of H-bond in comparison to our results. Asp1044 has been reported to make H-bond with urea oxygen in a cognate benzoimidazole-urea ligand and this interaction could not be observed in our studies. However, the spatial direction of compound 2 with regard to Asp1044 revealed a requirement of para-hydrogen acceptor substituents in the phenyl ring of indolinone scaffold. Possible interaction profiles for other docked compounds are also provided in Table 6.
Regarding the data obtained for binding energies with docked target (Table 4), docking outputs supported that higher binding energy of 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one (compound 2) can be interpreted by additional para-nitro substituent on phenyl ring which may provide additional H-bonds with the active sites of the receptor. This additional H-bond acceptor site would support two key H-bond interactions with Arg1049 and Asn921 residues through oxygen atoms of a nitro substituent (Fig. 2a). Further, H-bond may also be considered between indoline NH and Glu915 (Fig. 2a). Our experimental cytotoxicity data were in good agreement with docking binding energies since compound 2 was the most potent compound in all the tested cell lines (Table 2).
Docking data revealed that 3-(naphthalen-1-ylimino) indolin-2-one (compound 10) was the top-ranked docking result in terms of its binding free energy to the receptor (Table 4). The most characteristic feature of this compound may be attributed to its naphthalene scaffold. This may emphasize on the importance of hydrophobic interactions in the active site of VEGFR-2 (Fig. 2b). In our study, the geometric orientation of this ligand in the target active site provided a key H-bond interaction with Glu915 while no key H-bonds with other residues responsible for interactions of compound 2 could be detected. As was mentioned above, possible key hydrophobic interactions with active site residues may play an important role in interaction profile of compound 10 (Ala864, Val914, Phe1045, Cys1043, and Leu1033; Fig. 2b).
It is worth noting that compounds such as 14 (Table 4) or 15 possessing additional fused phenyl ring to their heterocyclic moieties may also be noticeable examples in this regard. Generally speaking, our results showed that designing indoline-2-ones including extended hydrophobic rings and appropriate H-bond acceptors on the third position of indoline scaffold may provide potent VEGFR-2 inhibitors for further drug development strategies.
Ligand efficiency indices
Another criterion which has recently absorbed much attention in binding studies is the ligand efficiency (LE) parameter. The concept of analyzing ligand binding in terms of the free energy per heavy atom was first proposed by Andrews (Andrews et al., 1984). Concept of the binding energy per atom or binding efficiency of a ligand could be a useful parameter in the selection of a lead compound, considering the real potency of a compound and hence optimizing fragments (Hopkins et al., 2004). Molecules that achieve a given potency with fewer heavy atoms are by definition more efficient. All of the synthesized compounds could pass the generally accepted filter for ligand efficiency as being efficient binding molecules themselves or as fragments to develop efficient biological molecules (Abad-Zapatero and Metz, 2005).
Putting LE concept in mind, one may realize that compounds 18 and 10 (Table 4) which contain 5-methylisoxazolyl and naphthyl moieties, respectively, are the most efficient molecules among our evaluated scaffolds. Compound 18 contains appropriate hydrogen bond donor and acceptor sites and compound 10 possess extendable hydrophobic skeleton to provide desirable hydrophobic interactions with VEGFR-2 active residues (Fig. 2b). From the least point of view; heterocycles bearing 3-(arylimino) indolin-2-one or 3-(arylhydrazono) indolin-2-one moieties may be regarded as promising candidates for further molecular extensions to develop potent cytotoxic agents in future.
Conclusion
Versatile new isatin derivatives (3-(arylimino) indolin-2-ones) bearing heterocyclic substituents were synthesized and identified. All the tested compounds showed higher cytotoxic effect on HeLa cancer cell lines compared to LS180 and Raji cancer cells. Electron withdrawing groups along with heterocyclic rings bearing more hetero atoms seemed to be necessary factors in providing higher cytotoxic activities in HeLa cell lines. Docking simulation technique with VEGFR-2 revealed that similar binding patterns could be observed for all studied isatin compounds and VEGFR-2 may be a potential molecular target for further drug development strategies based on 3-substituted-indoline-2-ones, confirming previous results. Biological and computational results revealed that arylhydrazono indolin-2-ones would be more potent cytotoxic agents than arylimino indolin-2-ones. Based on the obtained data, extended lipophilic rings bearing H-bond acceptors on the third position of indoline scaffold provide potent VEGFR-2 inhibitors for further drug development strategies. Isoxazole/thiazole-based compounds demonstrated to be appropriate scaffolds extendable to highly efficient cytotoxic agents due to their higher ligand efficiency indices in binding to the receptor.
References
Abad-Zapatero C, Metz JT (2005) Ligand efficiency indices as guideposts for drug discovery. Drug Discov Today 10(7):464–469
Andrews PR, Craik DJ, Martin JL (1984) Functional group contributions to drug-receptor interactions. J Med Chem 27(12):1648–1657
Azizian J, Soozangarzadeh S, Jadidi K (2001) Microwave-induced one-pot synthesis of some new spiro [3H-indole-3,5″(4″ H)-[1,2,4]-triazoline]-2-ones. Synth Commun 31(7):1069–1073
Azizian J, Morady AV, Soozangarzadeh S, Asadi A (2002) Synthesis of novel spiro-[3H-indole-3,3′-[1,2,4] triazolidine]-2-ones via azomethine imines. Tetrahedron Lett 43(52):9721–9723
Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ (2010) Virtual screening with AutoDock: theory and practice. Expert Opin Drug Discov 5(6):597–607
d’Ischia M, Palumbo A, Prota G (1988) Adrenalin oxidation revisited. New products beyond the adrenochrome stage. Tetrahedron 44(20):6441–6446
Erickson JA, Jalaie M, Robertson DH, Lewis RA, Vieth M (2004) Lessons in molecular recognition: the effects of ligand and protein flexibility on molecular docking accuracy. J Med Chem 47(1):45–55
Glover V, Halket JM, Watkins PJ, Clow A, Goodwin BL, Sandier M (1988) Isatin: identity with the purified endogenous monoamine oxidase inhibitor tribulin. J Neurochem 51(2):656–659
Hasegawa M, Nishigaki N, Washio Y, Kano K, Harris PA, Sato H, Mori I, West RI, Shibahara M, Toyoda H (2007) Discovery of novel benzimidazoles as potent inhibitors of TIE-2 and VEGFR-2 tyrosine kinase receptors. J Med Chem 50(18):4453–4470
Hevener KE, Zhao W, Ball DM, Babaoglu K, Qi J, White SW, Lee RE (2009) Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J Chem Inf Model 49(2):444–460
Holmes K, Roberts OL, Thomas AM, Cross MJ (2007) Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 19(10):2003–2012
Hopkins AL, Groom CR, Alex A (2004) Ligand efficiency: a useful metric for lead selection. Drug Discov Today 9(10):430–431
Hossain MM, Islam N, Khan R, Islam M (2008) Cytotoxicity study of dimethylisatin and its heterocyclic derivatives. Bangladesh J Pharmacol 2(2):66–70
Iyer RA, Hanna PE (1995) N-(carbobenzyloxy) isatin: a slow binding [alpha]-keto lactam inhibitor of [alpha]-chymotrypsin. Bioorganic Med Chem Lett 5(1):89–92
Logan JC, Fox MP, Morgan JH, Makohon AM, Pfau CJ (1975) Arenavirus inactivation on contact with N-substituted isatin beta-thiosemicarbazones and certain cations. J Gen Virol 28(3):271
Matesic L, Locke JM, Bremner JB, Pyne SG, Skropeta D, Ranson M, Vine KL (2008) N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorganic Med Chem 16(6):3118–3124
Miri R, Motamedi R, Rezaei MR, Firuzi O, Javidnia A, Shafiee A (2011) Design, synthesis and evaluation of cytotoxicity of novel chromeno [4, 3-b] quinoline derivatives. Archiv der Pharmazie (Epub ahead of print)
Morris GM, Huey R, Olson AJ (2008) Using AutoDock for ligand-receptor docking. Curr Protoc Bioinformatics 11(3):34–37
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Pandeya SN, Sriram D, Nath G, De Clercq E (1999a) Synthesis antibacterial, antifungal and anti HIV activity of Schiff’s and Mannich bases of isatin with N-[6-chlorobenz thiazole-2-yl]thiosemicarbazide. Indian J Pharm Sci 61:358–361
Pandeya SN, Sriram D, Nath G, De Clercq E (1999b) Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm Acta Helv 74(1):11–17
Pandeya SN, Yogeeswari P, Sriram D, De Clercq E, Pannecouque C, Witvrouw M (1999c) Synthesis and screening for anti-HIV activity of some N-Mannich bases of isatin derivatives. Chemotherapy 45(3):192–196
Pandeya SN, Sriram D, Nath G, De Clercq E (2000) Synthesis, antibacterial, antifungal and anti-HIV activities of norfloxacin Mannich bases. Eur J Med Chem 35(2):249–255
Pervez H, Ramzan M, Yaqub M, Mohammed Khan K (2011) Synthesis, cytotoxic and phytotoxic effects of some new N4-aryl substituted isatin-3-thiosemicarbazones. Lett Drug Des Discov 8(5):452–458
Phosrithong N, Ungwitayatorn J (2010) Molecular docking study on anticancer activity of plant-derived natural products. Med Chem Res 19:817–835
Sarangapani MR, Reddy VM (1994) Pharmacological evaluation of 1-(N,N-disubstituted aminomethyl)-3-imino-(2-phenyl-3,4-dihydro-4-oxo-quinazolin-3-yl) indolin-2-ones. Indian J Pharm Sci 56:174–177
Sellers RP, Alexander LD, Johnson VA, Lin CC, Savage J, Corral R, Moss J, Slugocki TS, Singh EK, Davis MR (2010) Design and synthesis of Hsp90 inhibitors: exploring the SAR of Sansalvamide A derivatives. Bioorganic Med Chem 18(18):6822–6856
Shuttleworth SJ, Nasturica D, Gervais C, Siddiqui MA, Rando RF, Lee N (2000) Parallel synthesis of isatin-based serine protease inhibitors. Bioorganic Med Chem Lett 10(22):2501–2504
Silva JFM, Garden SJ, Pinto AC (2001) The chemistry of isatins: a review from 1975 to 1999. J Brazilian Chem Soc 12(3):273–324
Singh SP, Shukla SK, Awasthi LP (1983) Synthesis of some 3-(4′-nitrobenzoylhydrazone)-2-indolinones as potential antiviral agents. Curr Sci 52(16):766–769
Sousa SF, Fernandes PA, Ramos MJ (2006) Protein-ligand docking: current status and future challenges. Proteins 65:15–26
Sridhar SK, Saravanan M, Ramesh A (2001) Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Med Chem 36(7–8):615–625
Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A (1996) Flk-1 as a target for tumor growth inhibition. Cancer Res 56(15):3540
Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver LK, McMahon G, Tang C (1999) Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl) methylidenyl] indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J Med Chem 42(25):5120–5130
Varma RS, Khan IA (1977) Potential biologically active agents. X. Synthesis of 3-arylimino-2-indolinones, and their 1-methyl-and 1-morpholino/piperidinomethyl derivatives as excystment and cysticidal agents against Schizopyrenus russelli. Polish J Pharmacol Pharm 29(5):549
Varma RS, Nobles WL (1967) Synthesis and antiviral and anti-bacterial activity of certain N-dialkylaminomethylisatin beta-thiosemicarbazones. J Med Chem 10:972–974
Varma RS, Nobles WL (1975) Antiviral, antibacterial, and antifungal activities of isatin N Mannich bases. J Pharm Sci 64(5):881–882
Vine KL, Locke JM, Ranson M, Pyne SG, Bremner JB (2007) In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorganic Med Chem 15(2):931–938
Vyas V, Jain A, Gupta A (2008) Virtual screening: a fast tool for drug design. Sci Pharm 76:333–360
Webber SE, Tikhe J, Worland ST, Fuhrman SA, Hendrickson TF, Matthews DA, Love RA, Patick AK, Meador JW, Ferre RA (1996) Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J Med Chem 39(26):5072–5082
Acknowledgments
Financial supports of this project by research council of Shiraz University of Medical Sciences are acknowledged. We gratefully acknowledge the financial support from the Medicinal and Natural Products Chemistry Research Centre, Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azizian, J., Mohammadi, M.K., Firuzi, O. et al. Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med Chem Res 21, 3730–3740 (2012). https://doi.org/10.1007/s00044-011-9896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9896-6