Abstract
Cancer, which is considered to be the world’s most serious illness cause 8.2 million deaths and this rate may double by 2030. We herein report a new series of 3-(2-(p-substituted)-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)-2-(p-substituted)ethylidene)indolin-2-one (15–19) and 5-substituted-5′-substituted phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one derivatives (20–24) as potent anticancer agents. These compounds were evaluated for in vitro antitumor activity against the National Cancer Institute panel of 60 cancer cell lines. Among all the synthesized compounds, two compounds 15 and 16 showed remarkable antitumor activity with GI50 (MG-MID) values of 0.65 & 0.72 µM, respectively against Non-small cell lung cancer. To gain insight for mode of binding with Epidermal Growth Factor Receptor kinase enzyme, these compounds were further subjected to docking studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer, a life-threatening disease is the second common cause of death after cardiovascular diseases (Jemal et al. 2011). The American Chemical Society defines cancer as a group of diseases characterized by uncontrolled growth, and the spread of abnormal cells that left untreated may lead to death (Garcia et al. 2007). According to WHO global cancer report 2014, it is expected to increase 57% worldwide in next 20 years (Vineis and Wild 2014). By 2030, it is projected that there will be ∼26 million new cancer cases and 17 million cancer deaths per year (Thun et al. 2010; Boyle and Levin 2008). The projected increase will be driven largely by growth and aging of populations and will be largest in low-resource and medium-resource countries (Abraham 2014).
Isatins are endogenous molecules present in human and mammals which exhibits diverse pharmacological profiles especially antimicrobial (Thadhaney et al. 2010; Bari et al. 2015), antitumor (Havrylyuk et al. 2012; Liang et al. 2014; Havrylyuk et al. 2015; Thi Lan Huong et al. 2015; Lesyk et al. 2015), antiviral (Varma and Khan 2014), anti-inflammatory (Socca et al. 2014), and antioxidant activities (Dandia et al. 2014). The isatin moiety present in wide range of compounds can act as inhibitors of apoptosis (Medvedev et al. 2007) targeting proteases (Zhou et al. 2006), caspases (Chapman et al. 2002) kinases (Cao et al. 2009) extracellular signal regulated protein kinase (ERK) (Cane et al. 2000). Isatin hybrids possessing heterocyclic analogues have been identified as potential antitumor agents and the most promising hybrid molecules are shown in Fig. 1 (Ibrahim et al. 2015; Eldehna et al. 2015; Ribeiro et al. 2016; Monteiro et al. 2014; Fares et al. 2015).
In recent days, chemists have gained considerable attention on five membered aromatic systems having three heteroatoms at the symmetrical positions (i.e., pyrazolines, and thiadiazolines) due to their interesting biological activities (Yusuf and Jain 2014; Shih et al. 2015; Altıntop et al. 2015). On the other hand, various thiadiazole (Bursavich et al. 2007; Nikalje et al. 2015) and pyrazoline derivatives (Havrylyuk et al. 2012; Karthikeyan et al. 2015) as a potential chemotherapeutic agents. Spirooxindole were reported as an important class of heterocyclic scaffolds with promising anticancer activity (Reddy et al. 2015; Ziarani et al. 2016). Pyrazoline derivatives, are nitrogen heterocyclic compounds with electron rich property (Schmidt and Dreger 2011), widely occur in nature in the form of alkaloids (Shaaban et al. 2012), vitamins, pigments, and as constituents of plant and animal cell (Singh et al. 2009; Fall et al. 2002). Thiadiazole acts as “hydrogen binding domain” and “two electron donor system” with a constrained pharmacophore, has structural frameworks similar to several naturally occurring alkaloids that show a wide range of pharmaceutical and industrial importance (Chen et al. 2012).
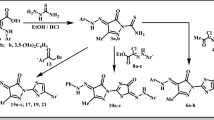
In the present study, we herein report the synthesis of 3-(2-(p-substituted)-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)-2-(p-substituted)ethylidene)indolin-2-one (15–19) and 5-substituted-5′-substituted phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one derivatives (20–24) (Fig. 2). The new compounds were screened for their in vitro antitumor activity using the NCI’s disease-oriented human cell lines assay. Docking simulations were performed using the X-ray crystallographic structure of the EGFR in complex with an inhibitor to explore the binding modes of these compounds at the active site.
Experimental
General methods
Melting points (°C) were determined on Toshniwal melting point apparatus and are uncorrected. Elemental analyses were performed on a Perkin-Elmer 240 elemental analyzer. Reactions were monitored using thin layer chromatography (TLC) on aluminum-backed precoated silica gel 60 F254 plates (E Merck). The FT-IR spectra of the compounds were recorded on thermo Nicolet Nexus 670S series.1H NMR and 13C NMR were recorded on a Avance-300 MHz instrument using TMS as an internal standard (chemical shifts in δ, ppm). Mass spectra were recorded on LC-MSD-Trap-SL using ESI(+) method. Column chromatography was performed by using Qualigen’s silica gel for column chromatography (60–120 mesh). All the fine chemicals and reagents used were purchased from Sigma-Aldrich (St. Louis, USA).
Synthesis of 2-amino-5-aryl-1,3,4-thiadiazole (1–3)
Compounds 1–3 were synthesized from substituted benzaldehyde and thiosemicarbazide and subsequently cyclized with bromine in glacial acetic acid. The solids were obtained and the residue was recrystallized from suitable solvent.
General procedure for the synthesis of isatin chalcones (11–14)
To a solid homogenous mixture of substituted isatin (4–6) (1 mmol) and acetophenones (7–10) (1 mmol) with catalytic amount of dimethylamine was taken in 250 mL conical flask and stirred for 15–30 min. The reaction mixture was cooled overnight, a colorless solid was formed. Twenty milliliter of glacial acetic acid and five drops of concentrated HCl was added to this precipitate and the mixture was warmed at 80 °C for 30 min and after dehydration, gave isatin chalcones (11–14).
General procedure for synthesis of various imines derivatives (15–19)
A mixture of 2-Amino-5-aryl-1, 3, 4-thiadiazole (1–3) (2 mmol) and substituted isatin chalcones (11–14) (2 mmol) was taken in 20 mL of absolute ethanol in 250 mL conical flask. The resulting mixture was refluxed for 5–8 h and the reaction mixture was cooled on overnight and solvent was evaporated under reduced conditions, the residue thus obtained was recrystallized from methanol gave imine derivatives (15–19).
3-(2-Phenyl-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)ethylidene)indolin-2-one (15)
Yield 83%; m.p. 190–191 °C; IR (KBr) ṽ max cm−1: 3426 (NH str), 3161 (C=CH, str), 1681 (C=O), 1650(C=N), 1458 (Ar–C=C), 1385(Ar–C=N), 1015 (Ar–C–S),.1H NMR (300 MHz, DMSO-d 6 ) δ 9.96 (s, 1H), 8.93 (s, J = 6.8, 1H), 8.32–8.25 (m, 4H), 7.93–7.81 (m, 6H), 7.41 (t, J = 7.2, 1H), 7.23 (t, J = 7.2, 1H), 7.06 (d, J = 8.4, 1H), 6.21(s, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 191.0, 179.2 (2C), 169.3, 164.8, 143.2, 137.5, 136.6, 133.8 (2C), 132.7 (2C), 128.9 (4C), 128.0 (2C), 126.4 (2C), 122.9 (2C), 120.6, 110.2. ESI-MS m/z: 409.1 [M+H]+; Anal. Calcd. for C24H16N4OS: C, 70.57; H, 3.95; N, 13.72; found: C, 70.59; H, 3.98; N, 13.71.
3-(2-((5-(4-Chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)-2-phenylethylidene)indolin-2-one (16)
Yield 80%; m.p. 189–190 °C; IR (KBr) ṽ max cm−1: 3477 (NH str), 3106 (C=CH, str), 1681 (C=O), 1655 (C=N), 1385 (C=C), 1320 (Ar–C=N), 1015 (Ar–C–S), 735 (para-Cl). 1H NMR (300 MHz, DMSO-d 6 ) δ 9.13 (s, 1H), 8.89 (s, J = 7.2, 1H), 8.34–8.26 (m, 4H), 7.89–7.83 (M, 5H), 7.43 (t, J = 6.4, 1H), 7.22 (t, J = 7.2, 1H), 7.09 (d, J = 7.6, 1H), 6.18 (s, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 190.6, 178.8 (2C), 168.3, 163.9, 144.1, 139.3, 138.6, 136.3 (2C), 135.8, 133.3 (2C), 133.1 (2C), 132.4 (3C), 131.6, 126.5, 125.4, 122.8, 120.9, 110.8. ESI-MS m/z: 443.3 [M+H]+; Anal. Calcd. for C24H15ClN4OS: C, 65.08; H, 3.41; N, 12.65; found: C, 65.11; H, 3.48; N, 12.60.
3-(2-Phenyl-2-((5-(p-tolyl)-1,3,4-thiadiazol-2-yl)imino)ethylidene) indolin-2-one (17)
Yield 78%; m.p. 186–187 °C; IR (KBr) ṽ max cm−1: 3458 (NH str), 3106 (C=CH, str),2996 (C–H, str), 1681 (C=O), 1660 (C=N), 1446 (C=C), 1355 (Ar–C=N), 972 (Ar–C–S). 1H NMR (300 MHz, DMSO-d 6 ) δ 10.09 (s, 1H), 8.84 (d, J = 7.5 Hz, 1H), 8.29–7.83 (m, 7H), 7.53–7.49 (m, 3H), 7.39 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 7.2 Hz, 1H), 5.99 (s, 1H), 2.48 (s, 3H). 13C NMR (75 MHz, DMSO-d 6 ) δ 188.3, 177.4, 168.9 (2C), 162.3, 138.8 (2C), 137.8, 137.1, 136.2, 133.6 (2C), 133.3 (2C), 131.6 (2C), 130.3, 129.5 (2C), 126.8, 125.3, 122.1, 120.3, 110.5, 21.6. ESI-MS m/z: 423.3 [M+H]+; Anal. Calcd. for C25H18N4OS:C, 71.07; H, 4.29; N, 13.26; found: C, 71.15; H, 4.32; N, 13.29.
3-(2-(4-Hydroxyphenyl)-2-(5-phenyl-1,3,4-thiadiazol-2-ylimino)ethylidene)indolin-2-one (18)
Yield 80%; m.p. 182–183 °C; IR (KBr) ṽ max cm−1: 3456 (NH str), 3300 (OH, str), 3100 (C=CH, str), 1670 (C=N), 1385 (C=C), 1320 (Ar–C=N), 1015 (Ar–C–S), 1H NMR (300 MHz, DMSO-d 6 ) δ 9.98 (s, 1H), 8.89–8.86 (m, 3H), 8.39 (d, J = 6.8 Hz, 2H), 8.10–7.89 (m, 4H), 7.43 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 7.6 Hz, 1H), 6.91 (d, J = 6.8 Hz, 2H), 6.01 (s, 1H), 5.39 (s, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 191.5, 178.4, 169.1, 165.3, 163.9, 144.2, 138.9, 136.8 (2C), 136.2 (3C), 134.1 (2C), 132.5, 131.3, 127.2, 126.2, 125.1, 124.2, 120.6, 116.7 (2C), 110.8. ESI-MS m/z: 425[M+H]. Anal. Calcd. for C24H16N4O2S.C, 67.91; H, 3.80; N, 13.20; found: C, 67.98; H, 3.86; N, 13.18.
3-(2-((5-(4-Chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)-2-(4-hydroxyphenyl) ethylidene)indolin-2-one (19)
Yield 80%; m.p. 218–219 °C; IR (KBr) ṽ max cm−1: 3495 (NH str), 3350 (OH, str), 3110 (C=CH, str), 1685 (C=N), 1385 (C=C), 1365 (Ar–C=N), 926 (Ar–C–S), 735 (para-Cl). 1H NMR (300 MHz, DMSO-d 6 ) δ: 10.02 (s, 1H), 8.78–8.74 (m, 3H), 8.34(d, J = 7.2 Hz, 2H), 8.13–7.93 (m, 3H), 7.36 (t, J = 7.6 Hz, 1H), 7.09 (d, J = 7.2 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 6.03 (s, 1H), 5.36 (s, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 189.3, 179.3 (2C), 170.8, 165.5, 154.3, 149.8, 138.9 (2C), 136.3, 134.6 (2C), 133.8 (2C), 132.6 (2C), 131.3, 128.4, 127.1, 126.2, 123.6, 121.4, 116.9, 111.2. ESI-MS m/z: 459.7 [M+H]+; Anal. Calcd. for C24H15ClN4O2S: C, 62.81; H, 3.29; N, 12.21; found: C, 62.87; H, 3.31; N, 12.15.
General procedure for the synthesis of 5-substituted-5′-substituted phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one derivatives (20–24)
A mixture of substituted isatin chalcones 11–14 (1 mmol) and hydrazine hydrate (1 mmol) was taken in 30 mL of absolute ethanol in 25 mL conical flask. Then the reaction mixture was refluxed for about 1 h. Then the reaction mixture was cooled on overnight and the precipitate was collected by filtration. Thus, obtained spiro derivatives (20–24) were purified by column chromatography and subsequently by recrystallization with absolute ethanol.
5′-Phenyl-2′,4′-dihydrospiro[indol-3,3′-pyrazol]-2-one (20)
Yield 84%; m.p. 200–201 °C; IR (KBr) ṽ max cm−1: 3481 (NH str), 3422 (NH str), 1709 (C=O), 1600 (C=N). 1H NMR (300 MHz, DMSO-d 6 ) δ 9.16 (s, 1H), 8.09 (s, J = 7.2 Hz, 1H), 7.63 (d, J = 7.2 Hz, 2H), 7.56–7.51 (m, 3H), 7.22–7.16 (m, 2H), 6.92 (t, J = 7.6 Hz, 1H), 6.20 (s, 1H), 3.43 (d, J = 15.0 Hz, 1H), 3.72 (d, J = 15.0 Hz, 1H). 13C NMR (75 MHz, DMSO- d 6 ) δ 180.3, 151.3, 140.1, 132.5, 130.9, 130.1, 129.3, 129.0, 128.3, 128.4, 127.6, 126.3, 122.4, 113.8, 70.3, 45.9. ESI-MS m/z: 264.1 [M+H]+; Anal. Calcd. for C16H13N3O: C, 72.99; H, 4.98; N, 15.96; found: C, 72.87; H, 5.02; N, 15.93.
5′-(4-Chlorophenyl)-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (21)
Yield 71%; m.p. 222–223 °C; IR (KBr) ṽ max cm−1: 3461 (NH, str), 3279 (NH str), 1712 (C=O), 1609 (C=N). 1H NMR (300 MHz, DMSO-d 6 ) δ9.32 (s, 1H), 8.02 (s, J = 7.6 Hz, 1H), 7.64 (t, J = 6.8 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.26–7.21 (m, 2H), 6.97 (t, J = 7.6, 1H), 6.33 (s, 1H), 3.58 (d, J = 15.0 Hz, 1H), 3.43 (d, J = 15.0 Hz, 1H). 13C NMR (75 MHz, DMSO- d 6 ) δ 178.5, 147.5, 141.7, 133.9, 132.3 (2C), 129.7 (2C), 128.9, 127.8 (2C), 124.0, 122.8, 110.1, 70.1, 44.1.ESI-MS m/z: 298.5 [M+H]+; Anal. Calcd. for C16H12ClN3O: C, 64.54; H, 4.06; N, 14.11; found: C, 64.47; H, 4.02; N, 14.02.
1-Benzyl-5′-phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (22)
Yield 71%; m.p. 232–235 °C; IR (KBr)ṽ max cm−1: 3476 (NHstr), 3276 (NH str), 1706 (C=O), 1603 (C=N). 1H NMR (300 MHz, DMSO-d6) δ9.19 (s, 1H),7.71 (d, J = 7.2 Hz, 2H), 7.61–7.55 (m, 3H), 7.41–7.25 (m, 8H), 6.98 (d, J = 6.8, 1H), 4.66 (s, 2H), 3.58 (d, J = 15.0 Hz, 1H), 3.46 (d, J = 15.0 Hz, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 177.3, 147.8, 142.9, 141.8, 131.8, 131.6, 130.2, 129.9, 125.6 (2C), 125.3 (2C), 125.0 (2C), 124.7, 123.7(3C), 123.2, 116.4, 67.1, 54.6, 44.8.ESI-MS m/z: 354.3 [M+H]+; Anal. Calcd. for C23H19N3O: C, 78.16; H, 5.42; N, 11.89; found: C, 78.12; H, 5.48; N, 11.87.
5-Bromo-5′-phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (23)
Yield 83%; m.p. 232–234 °C; FTIR (KBr) ṽ max cm−1: 3462 (NH, str), 3278 (NH, str), 1707 (C=O), 1618 (C=N). 1H NMR (300 MHz, DMSO-d 6 ), 9.12 (s, 1H), 8.11 (s, J = 7.5 Hz, 1H), 7.61 (d, J = 8.8 Hz, 2H), 7.53–7.47 (m, 3H), 7.21 (d, J = 7.6 Hz, 1H), 7.02 (d, J = 7.2 Hz, 1H), 6.89 (s, 1H), 3.43 (d, J = 15.0 Hz, 1H), 3.34 (d, J = 15.0 Hz, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 178.2, 147.6, 147.2, 131.8, 130.1, 128.9, 128.6, 127.1, 123.8 (2C), 123.1 (2C), 122.6, 120.8, 68.4, 43.4.ESI-MS m/z: 343.1 [M+H]+; Anal. Calcd. for C16H12BrN3O.C, 56.16; H, 3.53; N, 12.28; found: C, 56.20; H, 3.58; N, 12.20.
5′-(4-Methoxyphenyl)-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (24)
Yield 83%; m.p. 232–234 °C; IR (KBr) ṽ max cm−1: 3438 (NH, str), 3391 (NH str), 1710 (C=O), 1617 (C=N) , 1301 (OCH3). 1H NMR (300 MHz, DMSO-d 6 ) δ 8.02 (s, 1H), 7.64 (d, J = 8.8 Hz, 2H), 7.33 (d, J = 7.6 Hz, 1H), 7.28 (d, J = 6.8 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.91 (t, J = 10.0 Hz, 3H), 6.02 (s, 1H), 3.84 (s, 3H), 3.72 (d, J = 15.0 Hz, 1H), 3.42 (d, J = 15.0 Hz, 1H). 13C NMR (75 MHz, DMSO-d 6 ) δ 178.7, 159.5, 147.4, 141.4, 132.3, 129.0, 127.1 (2C), 125.3, 123.5, 122.1, 114.0 (2C), 109.6, 69.5, 56.1, 43.9.ESI-MS m/z: 294.2 [M+H]+; Anal. Calcd. for C17H15N3O2: C, 69.61; H, 5.15; N, 14.33; found: C, 69.68; H, 5.12; N, 14.39.
Molecular docking studies
To gain more insight about the binding modes of synthesized derivatives (15–24), we herein performed docking studies (Rekulapally et al. 2015) on Epidermal Growth Factor Receptor (EGFR). In this study, X-ray crystal structure of human EGFR at resolution 1.9 Å (PDB ID: 4ICZ) was used further to identify binding modes involved in the inhibition activity. The protein monomer was optimized for geometry correction followed by energy optimization using Merck Molecular Force Field (MMFF). This optimized receptor was used for docking simulation. Molecular docking studies for synthesized analogues were carried out using GRIP batch docking method available in BioPredicta tools of VLife Molecular Design Suite 4.3 software. GRIP docking employees the PLP scoring function for ligand receptor interactions (i.e., hydrogen bonding, steric interactions, vanderwaal’s interactions, hydrophobic interactions and electrostatic interactions) with the active site of EGFR protein. The PLP scores were compared with gefitinib (Pao et al. 2004), an EGFR inhibitor used for breast, lung, and other cancers. The best conformers and the dock score for each ligand was shown in Table 1.
Antitumor activity
The cytotoxic activity of synthesized compounds was evaluated at National Cancer Institute (NCI), Bethesda, Maryland, USA in an in vitro 60 human tumor cell lines panel. The human tumor cell line derived from nine neoplastic cancer types i.e., leukemia, non-small cell lung, prostate, melanoma, breast, colon, CNS, ovarian, and renal cancers. In vitro cytotoxic assays were performed according to the USA NCI protocol. The compounds were first evaluated at single dose primary anticancer assay towards 60 cancer lines (concentration 10−5 M). Compounds which exhibit significant growth inhibition are evaluated against the 60 cell panel at five concentration levels (Andreani et al. 2008; Grever et al. 1992; Shoemaker 2006; Alley et al. 1988).
Results and discussion
Chemistry
As a starting point for the study, new derivatives were synthesized from the intermediate isatin chalcones (11–14), which were prepared by the reaction of acetophenones (7–10) with isatin (4–6) in a solvent free condition using dimethylamine by refluxing in glacial acetic acid and Conc HCl. The compounds isatin-thiadiazole hybrids (15–19) were synthesized from isatin chalcones (11–14) and 2-amino-5-aryl-thiadiazoles (1–3). The 2-amino-5-aryl-thiadiazole was synthesized from benzyladehyde and thiosemicarbazide under reflux to give thiosemicarbazone and subsequently cyclized with bromine in acetic acid. The synthesis of spiro isatin-pyrazolines (20–24) was carried as reported earlier in the literature (Mohammadizadeh 2006) (Scheme 1)
Derivatives obtained were fully characterized by FT-IR, 1H and 13C NMR and Mass (ESI) spectral data. All compounds have shown an excellent agreement between calculated and experimentally obtained data for CHN analysis. The IR spectrum of compounds isatin-thiadiazole hybrids (15–19) exhibited absorption bands in the range of 3200–3100 cm−1 due to -ene C–H stretching bonds, 1650–1500 cm−1 due to imine C=N stretching and 800–700 cm−1 due to aromatic deformation. The 1H NMR peaks in the range of δ 8.93–6.88 ppm were due to different aromatic protons. δ5.0–6.0 ppm was characteristic to =CH protons; and around δ 9.0–11.0 ppm was assigned to –NH proton of isatin. The 1H NMR spectrum of compounds 20–24 exhibited two doublets (δ 3.42–3.84 ppm) due to –CH2 protons of pyrazoline ring and multiplets (δ 6.5–8.00 ppm) for the aromatic protons. The singlet protons at around δ 9.16 and 6.20 are assigned for –NH proton of isatin and pyrazoline, respectively.
Molecular docking
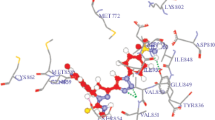
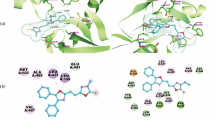
The synthesized compounds 15–24 were docked with 4ICZ binding site of EGFR protein wherein some of the compounds showed better docking score than the standard drug gefitinib. Docking results showed that compound 3-(2-phenyl-2-((5-phenyl-1,3,4-thiadiazol-2-yl) imino)ethylidene) indolin-2-one (15) has highest dock score of −79.11 with hydrogen bond and vanderwaals interactions between protein and ligand. VLife Sciences 4.3 was employed for the docking studies to explore the binding mode of ligands. To validate the docking simulations, gefitinib was used as the reference ligand. The original ligand score obtained for gefitinib was −71.05, confirming the ability of the method to accurately predict the binding confirmation. All ligands exhibited negative docking scores and were comparable with the reference gefitinib. From the dock score, compounds 15, 16, 17, 18, 19, 21, and 22 were found to have highest negative dock score ranging from −79.11 to −59.45 (Table 1). All the docked compounds were analyzed for various types of interactions such as hydrophobic bonding, charge, hydrogen, and vanderwaal’s interactions. Figure 3 shows the docking of the ligands in the receptor cavity. All the ligands and reference exhibited same interactions such as hydrogen, charge, hydrophobic, and vanderwaal’s interactions as shown in Table 1.
Antitumor activity
Seven newly synthesized compounds (15, 16, 20, 21, 22, 23, and 24) were selected by National Cancer Institute (NCI) Developmental Therapeutic Program (http://www.dtp.nci.nih.gov), Bethesda, MD, U.S.A. All the derivatives were subjected to the NCI’s disease oriented human cell lines screening assay for the evaluation of their in vitro antitumor activity. The compounds were tested at a single-dose concentration of 10 µM, and the percentage of growth over the 60 tested cell lines were determined and illustrated in (Table 2).
Among all, 3-(2-(p-substituted)-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)-2-(p-substituted)ethylidene)indolin-2-one derivatives (15 and 16) and 5-substituted-5′-substituted phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (20–24) analogs showed a distinctive pattern of selectivity. Compound 3-(2-phenyl-2-((5-phenyl-1,3,4-thiadiazol-2-yl) imino)ethylidene) indolin-2-one (15) and 3-(2-((5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)-2-phenyl ethylidene) indolin-2-one (16) showed remarkably lowest cell growth promotion against Non-small lung NCI-H522 cancer cell line with cell growth promotion of −59.34 and −95.65 respectively and also showed lethality against Melanoma LOX IMVI cancer cell line with −86.29 and −79.30 respectively. Lowest cell growth promotion are also observed by compound 15 against Leukemia CCRF-CEM (−45.05), HL-60 (−32.88), MOLT-4 (−24.19), RPMI-8226 (−17.47), and SR (−22.97); Colon cancer HCT-116 (−51.94). The compound 16 also showed significant cytotoxic activity against Leukemia CCRF-CEM (−42.66), HL-60 (−31.81), MOLT-4 (−26.44), RPMI-8226 (−14.37), and SR (−27.60) Colon cancer HCT-116 (−58.54). Compounds 15, 16, 20 and 23 showed growth promotion values of 2.53, 0.63, 31.37, and 30.54, against Colon COLO 205 respectively; while compound 20 and 23 showed values of 8.14 and 12.15 against Colon HT29. Through evaluation of the data revealed in (Table 2) showed compounds 15, 16, 20, and 23 are the most potent of this study, displaying usefulness towards various cell types belong to distinct tumor subpanels. Compounds 15, 16, 20, and 23 showed higher percent mean inhibition than 21, 22, and 24. Compound with chloro substitution on phenyl ring was more active than compound 15 with unsubstituted phenyl ring. Also compound 20 and 23 were more active than 21, 22, and 24 (Fig. 4).
Compounds 15, 16, 20, and 23 passed the prime anticancer assay at strength of 10 µM. Subsequently, these active compounds tested towards a panel of sixty distinct tumor cell types at a 5-log dose range. Two of these four analogues were selected for a repeat screen (15 and 16) and the results obtained are shown in Table 3, as the average of these two runs. Compounds 15, 16, 20, and 23 showed remarkable broad-spectrum antitumor activity.
The entire cell lines (about 60), representing nine tumor subpanels were incubated at five different concentrations (0.01, 0.1, 1, 10, and 100 µM). Three response parameters GI50, TGI, and LC50 were calculated for each cell line. Tested compounds 15, 16, 20, and 23 displayed effective growth inhibition GI50 (MG-MID) values of 3.01, 2.81, 6.02, and 4.07 μM, respectively, beside cytostatic activity TGI (MG-MID) values of 8.70, 8.51, 69.18, and 30.9 μM, respectively (Fig. 5). In addition, they exhibited some cytotoxic activity with LC50 (MG-MID) values of 29.5, 30.9, 100, and 95.4 μM, respectively as shown in Fig. 5.
Compound 3-(2-phenyl-2-((5-phenyl-1,3,4-thiadiazol-2-yl) imino)ethylidene) indolin-2-one (15) demonstrated remarkable anticancer activity towards almost all of the tested cell lines on behalf of nine distinct subpanels with GI50 values between “0.65–9.85 µM”, expect two cell lines SF-295 and SNB-19 of CNS cancer subpanel showing GI50 at a concentration of 17.09 and 12.29 µM respectively. The results are shown in Table 3, with regard to sensitivity against some individual cell lines the compound showed high activity against Non-Small Cell Lung Cancer NCI-H522 and HOP-92 with GI50 0.65 and 0.99 µM respectively. GI50, TGI, and LC50 MG-MID values of 3.01, 8.70, and 29.5 μM respectively, proved to be the most active member in this study. On the other hand, 3-(2-((5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)-2-phenyl ethylidene) indolin-2-one (16) showed nearly the same pattern of activity as 15 but to a lesser extent. This compound is active towards non-small cell lung cancer (NCI-H522 and HOP-92) and colon (HCT-116) with GI50 values 0.72, 0.97, and 1.07 µM respectively. The results are presented in Table 3.
5-Bromo-5′-phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (23) is active against CNS cancer (SNB-75), colon cancer (HCC-2998), and non-small lung cell cancer (HOP-92) showing GI50 values 1.35, 1.59, and 1.66 µM respectively. The results of compound 23 at five dose level in μM are shown in Table 3. The compounds displayed relatively weaker growth inhibition, cytostatic, and cytotoxic patterns when compared with compound 15 and compound 16 (GI50, TGI, and LC50MG-MID values 4.07, 30.9, and 95.49 μM, respectively). Finally, 5′-phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one (20) active against Melanoma (MDA-MB-435), non-small cell lung cancer (HOP-92) and renal cancer (A498) with GI50 values 1.66, 1.65, and 1.85 µM respectively. The compound 20 shows significant activity against all cancer cell lines except on ovarian (OVCAR-4 and OVCAR-5) and non-small cell lung cancer (NCI-H226). The results are shown in Table 3. The compound has shown weaker growth inhibition, cytostatic, and cytotoxic patterns with GI50, TGI, and LC50 MG-MID values 6.02, 69.18, and 100 μM (Fig. 5).
Conclusion
In conclusion, we have synthesized a new series of isatin based thiadiazoline and pyrazoline derivatives as a novel class of antitumor agents. Among these compounds 3-(2-Phenyl-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)ethylidene)indolin-2-one (15) and 3-(2-((5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)-2-phenylethylidene)indolin-2-one (16) displayed significant selective cytotoxic activity against non-small cell lung cancer (NCI-H522) with GI50 0.65 and 0.72 µM respectively. Thus, structure-activity investigation revealed that isatin-thiadiazole conjugates displayed high significant activity compared to that of spiroisatin–pyrazoline derivatives. Docking studies performed on the synthesized compounds by using VLife Molecular Design Suite 4.3 software, has confirmed that inhibitors fit into the binding pocket of the EGFR, 4ICZ protein. From the results, we found that for successful docking, hydrogen bonding and hydrophobic interactions between the ligand and the receptor are very important. Further studies will be undertaken to elucidate the antitumor mechanism of action involved.
References
Abraham J (2014) Paving the way for biosimilars in oncology, Part 2: Focus on safety and clinical trial considerations. Semin Oncol, 41:S1–S2
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48(3):589–601
Altıntop MD, Özdemir A, Turan-Zitouni G, Ilgın S, Atlı Ö, Demirel R, Kaplancıklı ZA (2015) A novel series of thiazolyl–pyrazoline derivatives: synthesis and evaluation of antifungal activity, cytotoxicity and genotoxicity. Eur J Med Chem 92:342–352
Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Calonghi N, Cappadone C (2008) Antitumor activity of new substituted 3-(5-Imidazo [2, 1-b] thiazolylmethylene)-2-indolinones and 3-(5-imidazo [2, 1-b] thiadiazolylmethylene)-2-indolinones: selectivity against colon tumor cells and effect on cell cycle-related events (1). J Med Chem 51(23):7508–7513
Bari S, Mandab S, Ugalec V, Jupallyb VR, Akenab V (2015) Rational design and synthesis of benzothiazolo-isatins for antimicrobial and cytotoxic activities. Indian J Chem B 54(3):418–429
Boyle P, Levin B (2008) World cancer report 2008. IARC Press, International Agency for Research on Cancer, Lyon, France
Bursavich MG, Gilbert AM, Lombardi S, Georgiadis KE, Reifenberg E, Flannery CR, Morris EA (2007) 5′-Phenyl-3′H-spiro[indoline-3,2′-[1,3,4]thiadiazol]-2-one inhibitors of ADAMTS-5 (Aggrecanase-2). Bioorg Med Chem Lett 17(20):5630–5633. doi:10.1016/j.bmcl.2007.07.048
Cane A, Tournaire M-C, Barritault D, Crumeyrolle-Arias M (2000) The endogenous oxindoles 5-hydroxyoxindole and isatin are antiproliferative and proapoptotic. Biochem Biophys Res Commun 276(1):379–384
Cao J, Gao H, Bemis G, Salituro F, Ledeboer M, Harrington E, Wilke S, Taslimi P, Pazhanisamy S, Xie X (2009) Structure-based design and parallel synthesis of N-benzyl isatin oximes as JNK3 MAP kinase inhibitors. Bioorg Med Chem Lett 19(10):2891–2895
Chapman JG, Magee WP, Stukenbrok HA, Beckius GE, Milici AJ, Tracey WR (2002) A novel nonpeptidic caspase-3/7 inhibitor,(S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl) sulfonyl] isatin reduces myocardial ischemic injury. Eur J Pharmacol 456(1):59–68
Chen M, Lin S, Li L, Zhu C, Wang X, Wang Y, Jiang B, Wang S, Li Y, Jiang J (2012) Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1, 2, 4-thiadiazole rings from the root of Isatis indigotica. Org Lett 14(22):5668–5671
Dandia A, Saini D, Bhaskaran S, Saini DK (2014) Ultrasound promoted green synthesis of spiro [pyrano [2, 3-c] pyrazoles] as antioxidant agents. Med Chem Res 23(2):725–734
Eldehna WM, Altoukhy A, Mahrous H, Abdel-Aziz HA (2015) Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur J Med Chem 90:684–694
Fall Y, Barreiro C, Fernández C, Mouriño A (2002) Vitamin D heterocyclic analogues. Part 1: A stereoselective route to CD systems with pyrazole rings in their side chains. Tetrahedron Lett 43(8):1433–1436. doi:10.1016/S0040-4039(02)00031-X
Fares M, Eldehna WM, Abou‐Seri SM, Abdel‐Aziz HA, Aly MH, Tolba MF (2015) Design, synthesis and in vitro antiproliferative activity of novel isatin‐quinazoline hybrids. Arch Pharm (Weinheim) 348(2):144–154
Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, Thun M (2007) Global cancer facts & figures 2007. American cancer society, Atlanta, GA, p 52. 1 (3)
Grever MR, Schepartz SA, Chabner BA (1992) The National Cancer Institute: cancer drug discovery and development program. In: Seminars in oncology, 1992. vol 6. WB SAUNDERS CO INDEPENDENCE SQUARE WEST CURTIS CENTER, STE 300, PHILADELPHIA, PA 19106-3399, pp 622-638
Havrylyuk D, Zimenkovsky B, Lesyk R (2015) Synthesis, biological activity of thiazolidinones bearing indoline moiety and isatin based hybrids. Mini Rev Org Chem 12(1):66–87
Havrylyuk D, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R (2012) Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J Med Chem 55(20):8630–8641
Ibrahim HS, Abou-Seri SM, Tanc M, Elaasser MM, Abdel-Aziz HA, Supuran CT (2015) Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumor-associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 103:583–593
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Karthikeyan C, SH Narayana Moorthy N, Ramasamy S, Vanam U, Manivannan E, Karunagaran D, Trivedi P (2015) Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov 10(1):97–115
Lesyk R, Havrylyuk D, Lelyukh M (2015) Synthesis and anticancer activity of isatin, oxadiazole and 4-thiazolidinone based conjugates, Chem & Chem Technol 9(1):29–36
Liang C, Xia J, Lei D, Li X, Yao Q, Gao J (2014) Synthesis, in vitro and in vivo antitumor activity of symmetrical bis-Schiff base derivatives of isatin. Eur J Med Chem 74:742–750
Medvedev A, Buneeva O, Glover V (2007) Biological targets for isatin and its analogues: implications for therapy. Biol Targets Ther 1(2):151
Mohammadizadeh MR (2006) One-pot rapid and efficient synthesis of new spiro derivatives of 11H-indeno [1, 2-b] quinoxalin-11-one, 6H-indeno [1, 2-b] pyrido [3, 2-e] pyrazin-6-one and isatin-based 2-pyrazolines. Arkivoc 11:47–58
Monteiro Â, Gonçalves LM, Santos MM (2014) Synthesis of novel spiropyrazoline oxindoles and evaluation of cytotoxicity in cancer cell lines. Eur J Med Chem 79:266–272
Nikalje APG, Shaikh SI, Khan FAK, Shaikh S, Sangshetti JN (2015) Molecular sieves promoted, ultrasound-mediated synthesis, biological evaluation and docking study of 3-(5-substituted-1, 3, 4-thiadiazol-2-ylimino) indolin-2-ones as a potential anticonvulsant agents. Med Chem Res 24(12):4058–4069
Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America 101(36):13306–13311
Reddy CN, Nayak VL, Mani GS, Kapure JS, Adiyala PR, Maurya RA, Kamal A (2015) Synthesis and biological evaluation of spiro [cyclopropane-1, 3′-indolin]-2′-ones as potential anticancer agents. Bioorg Med Chem Lett 25(20):4580–4586
Rekulapally S, Jarapula R, Gangarapu K, Manda S, Vaidya JR (2015) In silico and in vitro studies of novel 7-azaindole and 7-azaisatin derivatives as potent anticancer agents. Med Chem Res 24(9):3412–3422
Ribeiro CJ, Amaral JD, Rodrigues CM, Moreira R, Santos MM (2016) Spirooxadiazoline oxindoles with promising in vitro antitumor activities. Med Chem Comm 7(3):420–425
Schmidt A, Dreger A (2011) Recent advances in the chemistry of pyrazoles. Properties, biological activities, and syntheses. Curr Org Chem 15(9):1423–1463
Shaaban MR, Mayhoub AS, Farag AM (2012) Recent advances in the therapeutic applications of pyrazolines. Expert Opin Ther Pat 22(3):253–291
Shih M-H, Xu Y-Y, Yang Y-S, Lin G-L (2015) A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives. Molecules 20(4):6520–6532
Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6(10):813–823
Singh S, Bharti N, Mohapatra PP (2009) Chemistry and biology of synthetic and naturally occurring antiamoebic agents†. Chem Rev 109(5):1900–1947
Socca EAR, Luiz-Ferreira A, de Faria FM, de Almeida AC, Dunder RJ, Manzo LP, Brito ARMS (2014) Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by isatin: a molecular mechanism of protection against TNBS-induced colitis in rats. Chem Biol Interact 209:48–55
Thadhaney B, Sain D, Pemawat G, Talesara G (2010) Synthesis and antimicrobial evaluation of ethoxyphthalimide derivatized spiro [indole-3, 5’-(1, 3) thiazolo (4, 5-c) isoxazol]-2 (1H)-ones via ring closure metathesis. Indian J Chem B 49(3):368
Thi Lan Huong T, Thi Mai Dung D, Thi Kim Oanh D, Thi Bich Lan T, Thi Phuong Dung P, Loi VD, Woo Han B, Yun J, Soon Kang J, Kim Y (2015) 5-Aryl-1, 3, 4-thiadiazole-based hydroxamic acids as histone deacetylase inhibitors and antitumor agents: synthesis, bioevaluation and docking study. Med Chem 11(3):296–304
Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM (2010) The global burden of cancer: priorities for prevention. Carcinogenesis 31(1):100–110
Varma RS, Khan IA (2014) Isatins as potential biologically active agents. Def Sci J 28(4):191–202
Vineis P, Wild CP (2014) Global cancer patterns: causes and prevention. Lancet 383(9916):549–557
Yusuf M, Jain P (2014) Synthesis and biological significances of 1, 3, 4-thiadiazolines and related heterocyclic compounds. Arab J Chem 7(5):525–552
Zhou L, Liu Y, Zhang W, Wei P, Huang C, Pei J, Yuan Y, Lai L (2006) Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors. J Med Chem 49(12):3440–3443
Ziarani GM, Moradi R, Lashgarib N (2016) Synthesis of spiro-fused heterocyclic scaffolds through multicomponent reactions involving isatin. ARKIVOC 1:1–81
Acknowledgements
The authors would like to thank the National Cancer Institute (NCI), Bethesda, MD, USA for performing the antitumor testing of the synthesized compounds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gangarapu, K., Thumma, G., Manda, S. et al. Design, synthesis and molecular docking of novel structural hybrids of substituted isatin based pyrazoline and thiadiazoline as antitumor agents. Med Chem Res 26, 819–829 (2017). https://doi.org/10.1007/s00044-017-1781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1781-5