Abstract
Despite the diversity of ant-myrmecophile associations, there are few examples of primary parasitism of ants and these are poorly documented, particularly in genera with only a few species such as the genus Ectatomma. We identified 18 associations that involve at least 16 taxa of primary parasitoids distributed in three families belonging to two invertebrate classes, and five of the 15 valid Ectatomma species. Among these, we report for the first time an endoparasitoid fly (probably a phorid) that attacks E. ruidum larvae and constitutes the second record of ant-larva endoparasitism by a dipteran. We provide a brief account of the interactions of these organisms with their hosts and their possible impact at the colonial or population level. Ectatomma ants, though being a small group, serve as a remarkable resource for the evolution of a wide variety of parasitoid organisms which, comparatively, are much more important than those associated with better-studied ant genera such as Myrmica or Formica. Considering the lack of studies dedicated to their parasites and parasitoids, the available information (almost limited to the three most studied Ectatomma species) suggests that, in spite of both their carnivorous diet and the aggressiveness typical of their workers, the diversity of these associations with Ectatomma might be much more important than previously expected. We stress the urgency of performing detailed inventories focused on these associations, not only for the genus Ectatomma, but for all the poorly studied ant communities (ectaheteromorphs, poneromorphs, arboreal ants) and endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The original definition of ‘parasitoid’ includes any organism where the juvenile stages parasitize a single host that is used as food source, whereas adult parasitoids are free-living (Reuter 1913). In general, parasitoid females lay their eggs on or inside the host body and one or more individuals can develop on the same host (Eggleton and Belshaw 1992; Godfray 1994). Any developmental stage of the host (egg, larva, nymph or adult) is liable to be attacked, though the majority of the parasitoids exhibit some preference for a specific stage. On no occasion does the female parasitoid attempt to transport the host to another location (prepared cache or nest). Generally, the parasitoid larva gradually kills its host while feeding on its tissues (Godfray 1994). Some authors as Eggleton and Belshaw (1992) restrict the use of the term parasitoid, excluding social parasites and castrators, but do apply it for some nematodes, while some others (Kathirithamby 2009) consider castrators such as the myrmecolacid strepsipterans as true parasitoids.
Although at the end of the 19th century and the beginning of the 20th century numerous studies have been published on the fauna associated with social insects, and particularly with ants (see reviews in Wheeler 1910; Donisthorpe 1927; Wilson 1971; Kistner 1982; Hölldobler and Wilson 1990; Schmid-Hempel 1998), few of them focused on the diversity of true parasitoids of ants, bees or wasps. Furthermore, in the few studies that did address this topic, the actual nature of the association with ants remains ambiguous in many cases and prevents determining whether the report is a true case of direct (primary) parasitism or rather is an indirect (interference) association through the primary parasitism of other guests that are present in ant colonies or are associated with them (myrmecophiles).
A recent survey of the literature between 1852 and 2011 on the diversity of the hymenopteran parasitoids of ants (Lachaud and Pérez-Lachaud 2012) showed that of a total of more than 500 species originally considered as ant parasitoids, only a fraction (138 species) actually belonged to this category. This figure may appear insignificant taking into consideration both the number of species for the whole family Formicidae, with 15,794 recognized species and valid subspecies distributed in 21 extant subfamilies of very variable size (Bolton 2003; AntWeb 2014), and the astounding number of potential parasitoids (Godfray 1994, 2007; Heraty 2009). In addition, the lack of reliable information becomes much more evident when focusing on the parasitoids associated with genera or subfamilies that include only a few species for which virtually nothing is known. Reviews on the macro and/or microfauna associated with some well-studied ant genera belonging to the formicines and myrmicines have recently been published (Formica: Parmentier et al. 2014; Myrmica: Witek et al. 2014), but such an endeavor has not been attempted for any poneromorph or ectaheteromorph ant (sensu Brady et al. 2006). Here, we provide a comprehensive review of the published information on parasitoids associated with species of the genus Ectatomma and add some original unpublished data.
The Neotropical ant genus Ectatomma F. Smith is composed of 15 valid species of which the most thoroughly studied are E. ruidum (Roger), E. tuberculatum (Olivier) and, to a lesser extent, E. brunneum F. Smith. These species have attracted a great deal of attention because they are conspicuous elements in different habitats, and have been used as model species for studies on a variety of topics (reviews in Brown 1958; Fernández 1991; Arias-Penna 2008; Breed et al. 2012; Poteaux et al. in press). With the single exception of E. parasiticum Feitosa and Fresneau, a social parasite of E. tuberculatum colonies (Fénéron et al. 2013), all Ectatomma species are generalist and opportunist predators (Fernández 1991) that actively hunt prey or collect corpses belonging to a large diversity of taxa (Ibarra-Núñez et al. 2001; Pie 2004; Lima and Antonialli-Junior 2013), but also forage on sugary food sources (Weber 1946; Jaffe et al. 1989; Passera et al. 1994). Due to their predatory impact on a diversity of insects, some species such as E. ruidum and E. tuberculatum have been considered as important, natural biological control agents (Cook 1905; Weber 1946; Ibarra-Núñez et al. 2001).
Mymecophiles are supposed to be more abundant and diverse in large colonies of ants (Wilson 1971; Kistner 1982; Hölldobler and Wilson 1990; Schmid-Hempel 1998). Consequently, as for the majority of the poneromorph and ectaheteromorph ants, both the small size of their colonies and the aggressiveness of their workers would lead intuitively to consider that the number of invertebrates associated with Ectatomma ants would be very small. However, accurate biological data are available only for a few Ectatomma species and most of the species of this genus have been very poorly documented (Fernández 1991; Arias-Penna 2008; Poteaux et al. in press).
Such a situation encouraged us to review the currently available information on the actual diversity of the primary parasitoids associated with ants in general and, specially, with the genus Ectatomma. We aimed to: (1) provide a comprehensive survey of the parasitoids known to attack this genus, and (2) suggest some directions where future studies are urgently needed.
Overview of the ant parasitoids
Entomophagous parasitoids are largely distributed among the invertebrates and currently represent about 10 % of all described insects (Eggleton and Belshaw 1992; Godfray 2007). Considering the most reliable estimations of the number of insect species on Earth, ranging between 2.5 and 5 million (Gaston 1991; Hamilton et al. 2010), this would give a conservative estimate of at least 250,000 to 500,000 parasitoid species, but some estimates reached up more than 680,000 species (Heraty 2009). Currently, there are about 88,300 species of known parasitoids most of which belong to three main orders: Hymenoptera (79.0 %), Diptera (17.7 %), and Coleoptera (1.8 %); the remaining 1.5 % belong to the order Strepsiptera and to a few species from other insect orders such as Neuroptera, Lepidoptera, and Trichoptera. Finally, some species belong to other invertebrate classes such as Mesostigmata mites and nematode worms (Eggleton and Belshaw 1992; González et al. 2004; Heraty 2009; Kathirithamby 2009; Goater et al. 2014). In this review, we only consider clearly established or at least plausible reports of primary parasitoid attack on ants. Furthermore, we restricted our survey to those cases that fit the original definition of parasitoid but also take into account those cases where the host is killed by the parasitoid just before its final molting at the moment of leaving the host as occurs for some post-parasitic nematode juveniles (Baker and Poinar 1995; Goater et al. 2014). In other examples, the parasitoid only sterilizes its host as in myrmecolacid strepsipterans (Kathirithamby 2009), or allows the incomplete development of the host (when the latter is sufficiently large in comparison with the parasitoid) and its survival at least for some time (Wheeler 1907). Taking into consideration these restrictions, the diversity of species that could actually be considered as primary ant parasitoids is extremely limited (Table 1), particularly when compared with both the size of the families of potential parasitoids and the size of the host family (Formicidae). As yet, only approximately 750 reliable cases of primary ant parasitoids have been reported (Table 1) involving five orders from three classes: insects (Insecta: Diptera, Hymenoptera, Strepsiptera), mites (Arachnida: Mesostigmata), and nematodes (Adenophorea: Mermithida).
Here, we provide a brief panorama of all the known cases of ant parasitoids for the whole formicid family, and contrast these data against those obtained for the genus Ectatomma for which we found a total of 18 associations involving parasitoids from two invertebrate classes and at least 16 taxa. We conclude by discussing the diversity of the parasitoids found in Ectatomma in view of the diversity of two recently reviewed, more species-rich genera, Formica and Myrmica.
Known parasitoids of Ectatomma
Hymenoptera (Eucharitidae: Eucharitinae)
Setting aside a questionable single case of primary ant parasitism by perilampid wasps (Table 1), parasitoids known to attack adult ants or their brood belong to eight families: Chalcididae, Encyrtidae, Eucharitidae, Eulophidae, and Eurytomidae (Chalcidoidea); Diapriidae (Diaprioidea); Braconidae and Ichneumonidae (Ichneumonoidea) (Kistner 1982; Hölldobler and Wilson 1990; Schmid-Hempel 1998; Lachaud and Pérez-Lachaud 2012; Pérez-Lachaud et al. 2012). Of these families, Eucharitidae is one of the smallest with less than 500 valid species; however, all eucharitids are exclusive, specific parasitoids of ant brood, and the family presents the highest number of known ant-parasitoid associations (Table 1) (Heraty 2002; Lachaud and Pérez-Lachaud 2012). Eucharitids have a highly modified life cycle (Clausen 1941; Heraty 2002; Lachaud and Pérez-Lachaud 2012). The females lay their eggs on or inside the tissues of certain plants and it is the very mobile first larval stage, termed “planidium”, which actively searches for its host, using foraging workers or prey of the ant-host which carry it by phoresis to the host nest (Carey et al. 2012). When reaching the nest, the planidium moves to an ant larva and waits for the host pupation. Then, the parasitoid feeds on the host and begins development (Clausen 1941; Heraty 2002; Pérez-Lachaud et al. 2006a). Generally, only one parasitoid develops per host but occasionally, between two and four individuals can complete development from a single host if it is large enough (Pérez-Lachaud et al. 2006a, 2010; Lachaud and Pérez-Lachaud 2009). However, the number of planidia observed on a single host larva can be greater and in some cases up to 11 planidia have been observed (Pérez-Lachaud et al. 2010). The cuticular hydrocarbon profile of eucharitids emerging within the host nest resembles that of their hosts and the parasitoids are not treated aggressively during the few hours after their emergence (Vander Meer et al. 1989; Howard et al. 2001). They are transported unharmed (Lachaud et al. 1998; Howard et al. 2001; Rocha et al. 2014) outside the host nest where mating takes place (Clausen 1941).
Eight associations involving eight species of eucharitine wasps and three Ectatomma species (E. brunneum, E. ruidum, E. tuberculatum) have been documented (Table 2). The eucharitine subfamily includes numerous species that attack ponerine and ectatommine ants (Lachaud and Pérez-Lachaud 2012), all of which pupate in a cocoon. The most frequently collected species are from the genus Kapala Cameron (Lachaud et al. 2012a). Though specificity for a particular host, at least at the genus level, is considered to be a relatively stable characteristic of eucharitids and parasitoids in general (Godfray 1994; Schmid-Hempel 1998; Heraty 2002), it is notable that some species of eucharitid wasps are able to use diverse hosts belonging to different ant genera, in some cases from phylogenetically unrelated taxa. This is the case for Kapala iridicolor Cameron that not only parasitizes E. ruidum but also three other ectatommine ants, Gnamptogenys regularis Mayr, G. striatula Mayr, G. sulcata (F. Smith), and even a ponerine ant, Pachycondyla stigma (Fabricius) (Pérez-Lachaud et al. 2006a), precisely ant groups belonging to two very different clades, the Formicoid and the Poneroid (see Brady et al. 2006; Moreau and Bell 2013).
Two examples of “co-occurrence” (the attack of the same host population by different parasitoid species), have been described. One for E. ruidum, parasitized by at least two species of the same genus (K. iridicolor and K. izapa Carmichael) (Pérez-Lachaud et al. 2006a; Lachaud and Pérez-Lachaud 2009), and the other for E. tuberculatum, parasitized by three species from three different genera (Dilocantha lachaudii Heraty, Isomerala coronata (Westwood) and Kapala sp.) (Pérez-Lachaud et al. 2006b, 2010). Ectatomma brunneum is also parasitized by three species from three different genera (Dicoelothorax platycerus Ashmead, Galearia latreillei (Guérin-Méneville) and Kapala sp.); however, in this case there is no co-occurrence as the three parasitoid species have been recorded in different populations (Lachaud et al. 2012a; Torréns and Heraty 2012; Torréns 2013). Finally, another phenomenon has been reported for E. tuberculatum: the only known example of multiparasitism (simultaneous development of two or more parasitoid species to the detriment of the same individual host, see Quicke 1997) in eucharitid wasps (Pérez-Lachaud et al. 2006b, 2010).
Due to their possible economic impact, the two most studied ant-eucharitid associations are euharitine wasps associated with E. ruidum and E. tuberculatum, and several orasemine species associated with the red imported fire ant (Solenopsis invicta Buren complex), the black imported fire ant (S. richteri Forel complex), and the little fire ant (Wasmannia auropunctata (Roger)). The observed parasitism is highly variable and very localized both in time and space (Lachaud and Pérez-Lachaud 2009; Pérez-Lachaud et al. 2010; Varone et al. 2010). Despite a local prevalence that is occasionally very high and may severely affect certain host-colonies, at a more global level the impact on the host population dynamics appears to be very limited (Lachaud and Pérez-Lachaud 2012).
Diptera (Phoridae: Phorinae)
There are numerous examples of dipteran-ant associations with more than 20 families of Diptera involved in a wide range of obligatory or facultative relationships with ants. Such relationships include: commensalism, detritivorous and saprophagous habits within the nest refuse (scavengers), thievery of stored food (cleptoparasitism), predation on the adults or the brood, or parasitism of the adults or the brood (Kistner 1982; Hölldobler and Wilson 1990; Feener and Brown 1997; Schmid-Hempel 1998; Pérez-Lachaud et al. 2014). Nevertheless, examples of primary parasitoid attack on ants are relatively scarce and are restricted, with a very uneven distribution, to only four families (Table 1): (1) a single tachinid species, Strongylogaster globula (Meigen), endoparasitoid of the foundress queens of various species of Lasius Fabricius (Gösswald 1950); (2) a single syrphid species of the Microdontinae subfamily, Hypselosyrphus trigonus Hull, ectoparasitoid of the prepupae of the arboricolous ponerine ant Neoponera villosa (Fabricius) in Mexico (Pérez-Lachaud et al. 2014); (3) a single chloropid species, Pseudogaurax sp., a novel ectoparasitoid fly attacking the larvae of the fungus-growing ant Apterostigma dentigerum Wheeler in Panama (González et al. 2014); and (4) numerous phorid species from 38 genera involved in more than 420 associations, all of them are endoparasitoids of adult workers (Disney 1994; Feener and Brown 1997; Brown and Feener 1998; Brown et al. 2012; Folgarait 2013) with the exception of a single unidentified species that is endoparasitic in ant larvae (Wheeler and Wheeler 1952, Fig. 2, p. 130).
To date, seven associations of dipteran parasitoids with the genus Ectatomma have been reported (Table 2), involving five species of phorid flies and four Ectatomma species (E. goninion Kugler and Brown, E. lugens Emery, E. ruidum, and E. tuberculatum). All of the phorid species attacking Ectatomma belong to the genus Apocephalus Coquillet and all are included within the miricaudata-group (see Brown 2000). In this group, the females are characterized by being attracted by the alarm pheromones of their host and certain chemical compounds released by injured and almost (or recently) dead workers, into which they lay their eggs (Feener et al. 1996). In this case, larval development is very rapid with Apocephalus larvae leaving their host after only 4–5 days to pupate in the soil (Feener and Brown 1997).
For almost all of the species reported in Table 2 (A. catholicus Brown, A. comosus Brown, A. glabriventris Brown, and A. lobicauda Brown), only attraction towards the injured potential host worker and some attempts at oviposition have been observed, making the parasitism hypothesis very plausible. However, there was no direct evidence of the presence of the eggs in the host or of the development of the parasitoid up to the adult state. The only fully reliable case is that of A. paraponerae Borgmeier attacking E. tuberculatum, for which the laying of several eggs (1.13 per host on average) and the development, at least up to the larval stage, could be ascertained after natural egg laying by the female parasitoid (Brown 2000). Through experimental egg transfers, Morehead and Feener (2000a) obtained 21.2 % of successful development up to the adult stage. Though the preferential host of A. paraponerae is the paraponerine ant Paraponera clavata (Fabricius) (Brown and Feener 1991), these flies can attack other ants such as Dolichoderus attelaboides (Fabricius), E. tuberculatum, E. ruidum, and different species of Pachycondyla and Neoponera (Brown 2000; Morehead and Feener 2000b). Brown (2000) reports that in the Peninsula of Osa in Costa Rica, A. paraponerae may survive exclusively on E. tuberculatum since P. clavata is not present in this region. Nevertheless, more recent results (Morehead et al. 2001), based on some behavioral characters and on body size differences, suggest that the populations of A. paraponerae that attack P. clavata and E. tuberculatum might in fact belong to different races or, maybe, to different cryptic species.
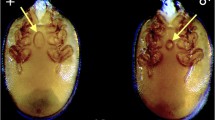
A novel, very special case of dipteran attack concerns two E. ruidum larvae from our collections in Chiapas (Izapa, Chiapas, Mexico; April 17/1997) that have been found to harbor a third-instar endoparasitoid fly larva, presumably of the phorid family (Fig. 1). In the absence of any adult parasitoid individual, successful parasitoid development cannot be confirmed; however, this appears likely because the dipteran larvae were fully grown (Fig. 1). It is noteworthy that the targets of both attacks were ant larvae since, until now, only a previous single case of endoparasitic attack of ant larvae by a phorid fly had been reported in another ectatommine ant, Gnamptogenys tortuolosa (F. Smith) (Wheeler and Wheeler 1952, p. 130 and 134). Unfortunately, the state of conservation of our material did not allow for its bar-coding and we had to maintain this record as an unidentified dipteran (presumably of the phorid family) in Table 2.
Finally, another association has been reported in Brazil (Lapola et al. 2003) between adults of an unidentified phorid species and E. brunneum, but as there was not any direct evidence of parasitoid attack and considering the fact that the adult flies were found seemingly free in a deep chamber inside the nest, it is more likely that they were scavengers or perhaps predators. As a consequence this report was not included in Table 2.
Nematoda (Mermithidae)
Poinar (2012) enumerates a list of 10 nematode families involved in associations with ants: Allantonematidae, Diplogastridae, Heterorhabditidae, Mermithidae, Panagrolaimidae, Physalopteridae, Rhabditidae, Seuratidae, Steinernematidae, and Tetradonematidae. However, only species from two of these families (Mermithidae and Tetradonematidae) meet our criteria for being considered true primary parasitoids of ants, by parasitizing only one host and killing it upon emergence (see Eggleton and Gaston 1990; Eggleton and Belshaw 1992; Wise de Valdez 2006; Goater et al. 2014). The best-known family is Mermithidae whose members have a relatively important specificity towards their hosts. The life cycle can follow two pathways: (1) direct, when development from the infectious stage (that occurs after emergence from the egg) up to emergence from the host is completed within the same host; or (2) indirect, when the infectious stage develops into a cyst and enters diapause inside an intermediary host (paratenic) before infecting the definitive host. When the growth of the nematode has been completed, the final phase entails the manipulation of the behavior of the host which is attracted towards an aquatic or semi-aquatic habitat. Then, the post-parasitic juvenile emerges from its host (generally after the rupture of the host abdomen and its death), performs its last molt and thus reaches the adult stage. Generally, ant parasitism by nematodes is easy to detect due to the notable increase of the host abdomen size (see Fig. 2). In many cases, it is accompanied by more or less important morphological modifications (head and thorax deformation, presence of ocelli, etc.), specific to each ant species, which can lead to the formation of intercastes (Wheeler 1907; Passera 1976).
Mermithized Ectatomma workers. a Parasitized E. ruidum (note its enlarged abdomen); b Close up of the abdomen of a mermithized E. ruidum worker, the second tergite of the gaster has been partially cut off to show a coiled up juvenile individual of Meximermis ectatommi; c Post-parasitic juvenile individual of Meximernis sp. emerging from a parasitized E. tuberculatum worker
We know only of two associations with the genus Ectatomma (Table 2), which involve two species of mermithid nematodes and two Ectatomma species (E. ruidum, E. tuberculatum). Emery (1890) first mentioned the presence of an E. tuberculatum worker with both a small head and a voluminous abdomen in his collection. However, the first detailed report of mermithized E. tuberculatum workers was by Wheeler (1930) who also indicated the deformation of the head, shorter and narrower, as well as the deformation of several other structures such as longer legs, shorter mandibles, a shorter and more compressed thorax, and thinner cuticular sculptures of the head, but without any indication of feminization or intercaste formation. An effect of reproductive castration exerted by the nematodes has also been observed since out of four mermithized, dealate females of E. tuberculatum found in a given nest, none was functionally reproductive (Pérez-Lachaud et al. 2011). In mermithized workers of E. ruidum, Weber (1946) also indicated some morphological deformations considered as the result of a marked feminization of certain characters (especially the presence of ocelli) that led to the development of intermediary individuals between workers and sexual females.
The nematode species associated with Ectatomma were initially reported, in both cases, as Mermis sp. (Wheeler 1930; Weber 1946). Nevertheless, the recent rearing of the post-parasitic juvenile stages up to the last molt, allowed determining that they belonged to a different new genus, Meximermis Poinar, Lachaud, Castillo and Infante (Poinar et al. 2006; Pérez-Lachaud et al. 2011), as well as identifying M. ectatommi Poinar, Lachaud, Castillo and Infante as the species associated with E. ruidum. In the case of E. tuberculatum, only adult nematode females were obtained and, in the absence of males, identification at species level was not possible.
Conclusions and perspectives
With the exception of the nomadic species (dorylimorphs), the nest of most ant species constitutes a relatively stable habitat that provides both food source and protection against other predators for a large range of organisms (Kistner 1982; Hölldobler and Wilson 1990; Hughes et al. 2008; Lachaud et al. 2012b, 2013). However, until very recently (see Kistner 1982; Hölldobler and Wilson 1990), it was considered that aggressive species where workers are provided with a powerful sting and exhibit a carnivorous diet, such as species within the genus Ectatomma, were unlikely to provide such services, thus offering an explanation for the lack of reports concerning the presence of associated guests in their nests. Furthermore, it has been considered that the abundance and diversity of myrmecophilous organisms are related to the size and longevity of the colonies (Wilson 1971; Kistner 1982), probably because alien organisms such as myrmecophiles are more likely to be spotted and culled in small colonies than in large colonies. Although longevity data for Ectatomma colonies under natural conditions are not available, the species of this genus are known for having relatively small colonies: a few dozen workers for the majority of the species and up to 1,200 individuals in E. tuberculatum (Lachaud et al. 1996; Poteaux et al. in press). Therefore, the association of any Ectatomma species with a diverse myrmecophilous fauna was assumed to be unlikely.
Nevertheless, even if we are a long way from the more than 300 associates reported for the single army ant species Eciton burchellii (Westwood) (Rettenmeyer et al. 2011) or from the diversity of associates and the highly complex interaction network found in the nests of the arboreal formicine ant Camponotus sp. aff. textor (Pérez-Lachaud and Lachaud 2014), the diversity of ant-parasitoid associations reported here for the genus Ectatomma seems to invalidate these a priori conclusions. Furthermore, the fact that Ectatomma ants, though being a small group, “serve as remarkable resource for the evolution of a wide variety of associated organisms” (Witek et al. 2014) appears all the more convincing since only parasitoid associates were considered in this study.
Two recent reviews, focusing on the associated organisms found with the Holarctic species of the genus Myrmica (Witek et al. 2014) and the red wood ants of the Formica rufa group (Parmentier et al. 2014), pointed out the high diversity of these parasitic communities and their impact on individuals and host colony fitness. However, both reviews almost completely neglected the numerous, complex associations with the primary parasitoids of these ants despite their occasional drastic impact at colony level as shown by Czechowski et al. (2007b) in M. rubra (Linnaeus), where about 25 % of the adult workers of a single colony were infested by parasitoid mermithid nematodes. On the basis of the species richness of each of these genera, the number of ant-parasitoid associations found with the 15 valid species of the genus Ectatomma would be expected to be far beyond that reported for the 198 and 175 extant species in the genera Myrmica and Formica, respectively. However, the situation is clearly distinct and the number of reliable ant-parasitoid associations reported for Myrmica (16) and Formica (43) (Tables S1, S2), involving 13 and 25 parasitoid taxa, respectively, appears to be extremely reduced in comparison with the 18 associations and 16 involved taxa reported here for Ectatomma. This figure is even more remarkable when we consider that only five species of Ectatomma were involved in these associations (and, actually, only three if we set aside the almost anecdotal mentions of E. goninion and E. lugens) while 10 and 22 species were involved for Myrmica and Formica, respectively (see Tables S1, S2).
Several studies have shown that attacks by parasitoids such as phorid flies (Feener 2000; Philpott et al. 2009) and eucharitid wasps (Lachaud and Pérez-Lachaud 2009; Pérez-Lachaud et al. 2010), or by entomopathogens (Keller 1995; Schmid-Hempel 1998; Naug and Camazine 2002) may constitute important factors of disturbance and mortality capable of affecting both the composition and dynamics of ant communities and their colony phenotype. The biology and ecology of various Ectatomma species are poorly documented, and in some cases almost no data is available; therefore it is no surprise that of the 15 species of Ectatomma currently recognized, primary parasitoids have been reported almost exclusively for the three most studied Ectatomma species. However, as our record (in E. ruidum) of the first dipteran endoparasitoid of Ectatomma larvae suggests, even these three species have not been thoroughly researched. Essentially, these figures emphasize the lack of knowledge on the exact relations that exist inside numerous communities of ants and the need to carry out exhaustive studies on their associated fauna. Considering the drastic changes suffered by numerous habitats and the dramatic loss of biodiversity in different zones of the Neotropics cataloged as biodiversity ‘hot-spots’ (Guénard et al. 2012; Lachaud et al. 2012b), there is an urgent need to conduct thorough surveys on the diversity of different ant communities which are still poorly known. This is particularly the case for most ectaheteromorphs and poneromorphs, but also for all the arboreal species (Pérez-Lachaud et al. 2012, 2013, 2014) and some other species found in very restricted habitats (e.g. Brown et al. 2012; Pérez-Lachaud and Lachaud 2014) that are already in a critical situation. These research aims should be the number one priority for our scientific community during the coming years.
References
AntWeb. Available from http://www.antweb.org. Accessed 17 November 2014
Arias-Penna T.M. 2008. Subfamilia Ectatomminae. In: Sistemática, Biogeografía y Conservación de las Hormigas Cazadoras de Colombia (Jiménez E., Fernández F., Arias T.M. and Lozano-Zambrano F.H., Eds), Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá. pp 53–107
Baker G.L. and Poinar G.O. Jr. 1995. Agamermis catadecaudata n. sp. (Nematoda: Mermithidae), a parasitoid of Orthoptera in south-eastern Australia. Fundam. Appl. Nematol. 18: 139–148
Bolton B. 2003. Synopsis and classification of Formicidae. Mem. Am. Entomol. Inst. 71: 1–370
Brady S.G., Schultz T.R., Fisher B.L. and Ward P.S. 2006. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl Acad. Sci. USA 103: 18172–18177
Breed M.D., Cook C. and Krasnec M.O. 2012. Cleptobiosis in social insects. Psyche 2012: Article ID 484765, 7 pages
Brown B.V. 2000. Revision of the “Apocephalus miricauda-group” of ant-parasitizing flies (Diptera: Phoridae). Contrib. Sci. 482: 1–62
Brown B.V. and Feener D.H. Jr. 1991. Behavior and host location cues of Apocephalus paraponerae (Diptera: Phoridae), a parasitoid of the giant tropical ant, Paraponera clavata (Hymenoptera: Formicidae). Biotropica 23: 182–187
Brown B.V. and Feener D.H. Jr. 1998. Parasitic phorid flies (Diptera: Phoridae) associated with army ants (Hymenoptera: Formicidae: Ecitoninae, Dorylinae) and their conservation biology. Biotropica 30: 482–487
Brown B.V., Bragança M.A.L., Gomes D.S., Queiros J.M. and Teixeira M.C. 2012. Parasitoid phorid flies (Diptera: Phoridae) from the threatened leafcutter ant Atta robusta Borgmeier (Hymenoptera: Formicidae). Zootaxa 3385: 33–38
Brown W.L. Jr. 1958. Contributions toward a reclassification of the Formicidae. II. Tribe Ectatommini (Hymenoptera). Bull. Mus. Comp. Zool. 118: 175–362
Carey B., Visscher K. and Heraty J. 2012. Nectary use for gaining access to an ant host by the parasitoid Orasema simulatrix (Hymenoptera, Eucharitidae). J. Hym. Res. 27: 47–65
Clausen C.P. 1941. The habits of the Eucharidae. Psyche 48: 57–69
Cockerell T.D.A. 1909. A new braconid of the genus Elasmosoma. Proc. Entomol. Soc. Washington 10: 168–169
Coman D. 1953. Mermithide freatice în fauna Republicii Populare România, Studii cercet. ştiinţ. 4: 123–152
Cook O.F. 1904. Notes on the habits of the Kelep, or Guatemalan cotton-boll-weevil ant. USDA Bull. # 49, pp 15
Cook O.F. 1905. The social organization and breeding habits of the cotton-protecting Kelep of Guatemala. USDA Techn. Ser. 10: 1–55
Coovert G.A. 2005. The ants of Ohio (Hymenoptera: Formicidae). Ohio Biol. Survey Bull. N.S. 15: 1–196
Csősz S. 2012. Nematode infection as significant source of unjustified taxonomic descriptions in ants (Hymenoptera: Formicidae). Myrmecol. News 17: 27–31
Csősz S. and Majoros G. 2009. Ontogenic origin of mermithogenic Myrmica phenotypes (Hymenoptera, Formicidae). Insect. Soc. 56: 70–76
Czechowski W., Czechowska W. and Radchenko A. 2007a. Strikingly malformed host morphology: Myrmica rugulosa Nyl. and Myrmica sabuleti Mein. (Hymenoptera: Formicidae) parasitised by mermithid nematodes. Fragm. Faun. 50: 139–148
Czechowski W., Radchenko A. and Czechowska W. 2007b. Mermithid infestation strikingly alters the morphology of Myrmica rubra (L.) (Hymenoptera: Formicidae): possible taxonomical involvements. Ann. Zool. 57: 325–330
Darling D.C. 2009. A new species of Smicromorpha (Hymenoptera, Chalcididae) from Vietnam, with notes on the host association of the genus. ZooKeys 20: 155–163
Davidson D.W. and Fisher B.L. 1991. Symbiosis of ants with Cecropia as a function of light regime. In: Ant—Plant Interactions (Huxley C.R. and Cutler D.F., Eds), Oxford University Press, Oxford. pp 289-309
Disney R.H.L. 1979. Natural history notes on some British Phoridae (Diptera) with comments on a changing picture. Entomol. Gaz. 30: 140–150
Disney R.H.L. 1994. Scuttle Flies: The Phoridae. Chapman and Hall, London
Disney R.H.L. 2000. Revision of European Pseudacteon Coquillet (Diptera, Phoridae). Bonn. Zool. Beitr. 49: 79–91
Disney R.H.L. 2002. Revisionary notes and new key to Aenigmatias Meinert (Diptera, Phoridae). Fragm. Faun. 45: 67–72
Donisthorpe H.St.J.K. 1910. Myrmecophilous notes for 1909. Entomol. Rec. J. Var. 22: 15–17
Donisthorpe H.St.J.K. 1913a. Aenigmatias blattoides Meinert, captured in Scotland. Entomol. Rec. J. Var. 25: 277–278
Donisthorpe H.St.J.K. 1913b. Rare myrmecophilous Diptera. Proc. Entomol. Soc. Lond. 1913: lxxv-lxxvi.
Donisthorpe H.St.J.K. 1914. Some notes on the genera Platyphora and Aenigmatias and a species new to Britain. Entomol. Rec. J. Var. 26: 276–278
Donisthorpe H.St.J.K. 1915. British Ants, Their Life-History & Classification. William Brendon and Son Limited, Plymouth
Donisthorpe H.St.J.K. 1927. The Guests of British Ants: Their Habits and Life-Histories. G. Routledge and Sons, London
Dupont S. and Pape T. 2007. Fore tarsus attachment device of the male scuttle fly, Aenigmatias lubbockii. J. Insect Sci. 7:54.
Eggleton P. and Belshaw R. 1992. Insect parasitoids: an evolutionary overview. Phil. Trans. R. Soc. Lond. B 337: 1–20
Eggleton P. and Gaston K.J. 1990. “Parasitoid” species and assemblages: convenient definitions or misleading compromises? Oikos 59: 417–421
Emery C. 1890. Studii sulle formiche della fauna neotropica. I. Formiche di Costa Rica. Boll. Soc. Entomol. Ital. 22: 33–80
Fahringer J. 1936. Opuscula braconologica. Band 4. Palaearktischen Region. Lieferung 1-3. Opusc. Braconol. 1935: 1–276
Falk S.J. and Chandler P.J. 2005. A review of the scarce and threatened flies of Great Britain. Part 2: Nematocera and Aschiza not dealt with by Falk (1991). Species Status 2: 1–189
Feener D.H. Jr. 2000. Is the assembly of ant communities mediated by parasitoids? Oikos 90: 79–88
Feener D.H. Jr. and Brown B.V. 1997. Diptera as parasitoids. Annu. Rev. Entomol. 42: 73–97
Feener D.H. Jr., Jacobs L.F. and Schmidt J.O. 1996. Specialized parasitoids attracted to a pheromone of ants. Anim. Behav. 51: 61–66
Fénéron R., Poteaux C., Boilève M., Valenzuela J. and Savarit F. 2013. Discrimination of the social parasite Ectatomma parasiticum by its host sibling species (E. tuberculatum). Psyche 2013: Article ID 573541, 11 pages
Fernández F. 1991. Las hormigas cazadoras de género Ectatomma (Formicidae: Ponerinae) en Colombia. Caldasia 16: 551–564
Folgarait P.J. 2013. Leaf-cutter ant parasitoids: current knowledge. Psyche 2013: Article ID 539780, 10 pages
Forel A. 1874. Les fourmis de la Suisse. Systématique. Notices anatomiques et physiologiques. Architecture. Distribution géographique. Nouvelles expériences et observations de moeurs. Neue Denkschriften der Allgemeinen Schweizerischen Gesellschaft für di gesammten Naturwissenschaften 26: 1–452
Forel A. 1890. Un parasite de la Myrmecia forficata Fabr. Ann. Soc. Entomol. Belg. 34: 8–10
Gahan A.B. 1940. A contribution to the knowledge of the Eucharidae (Hymenoptera: Chalcidoidea). Proc. U.S. Natl Mus. 88: 425–458
Gaston K.J. 1991. The magnitude of global insect species richness. Conserv. Biol. 5: 283–296
Gaulle J. de 1907. Catalogue systématique & biologique des Hyménoptères de France, suite. Feuille Jeunes Nat. 37: 185–189
Giraud J. 1871. Note sur l’Elasmosoma berolinense et description d’une espèce nouvelle (viennense) du même genre. Ann. Soc. Entomol. Fr. 1871: 299–302
Goater T.M., Goater C.P. and Esch G.W. 2014. Parasitism: The Diversity and Ecology of Animal Parasites, 2nd edn. Cambridge University Press, Cambrige
Godfray H.C.J. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press, Princeton, New Jersey
Godfray H.C.J. 2007. Parasitoids. In: Encyclopedia of Biodiversity (Levin S. Ed.), Academic Press. pp 1–13
Gómez Durán J.-M. and van Achterberg C. 2011. Oviposition behaviour of four ant parasitoids (Hymenoptera, Braconidae, Euphorinae, Neoneurini and Ichneumonidae, Hybrizontinae), with the description of three new European species. ZooKeys 125: 59–106
González C.T., Wcislo W.T., Wheeler T., Cambra R. and Fernández-Marín H. 2014. Pseudogaurax sp. (Chloropidae) a novel ectoparasitoid fly to ants, attacking the fungus-growing ant, Apterostigma dentigerum (Hymenoptera: Formicidae). In: Memorias 41° Congreso de la Sociedad Colombiana de Entomología (Rodríguez J., Ed.), SOCOLEN 2014, Cali, Colombia. p. 40
González V.V.E., Gómez L.L.A., and Mesa C.N.C. 2004. Observaciones sobre la biología y comportamiento del ácaro Macrodinychus sellnicki (Mesostigmata: Uropidae) ectoparasitoide de la hormiga loca Paratrechina fulva (Hymenoptera: Formicidae). Rev. Colomb. Entomol. 30: 143–149
Gösswald K. 1930. Weitere Beiträge zur Verbreitung der Mermithiden bei Ameisen. Zool. Anz. 90: 13–21
Gösswald K. 1934. Über Ameisengäste und –schmarotzer des mittleren Maingebiets. Entomol. Z. 48: 125–127
Gösswald K. 1938. Über bisher unbekannte, durch den Parasitismus der Mermithiden (Nemat.) verursachte Formveränderungen bei Ameisen. Z. Parasit. 10: 138–151
Gösswald K. 1950. Pflege des Ameisenparasiten Tamiclea globula Meig. (Dipt.) durch den Wirt mit Bemerkungen über den Stoffwechsel in der parasitierten Ameise. Verhandl. Deutsch. Zool. 1949: 256–264
Guénard B., Weiser M.D. and Dunn R.R. 2012. Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. Proc. Natl Acad. Sci. USA 109: 7368–7373
Hagmeier A. 1912. Beitrage zur Kenntnis der Mermithiden. I. Biologische Notizen und systematische Bechreibung einiger alter und neuer Arten. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 32: 521–612
Hamilton A.J., Basset Y., Benke K.K., Grimbacher P.S., Miller S.E., Novotný V., Samuelson G.A., Stork N.E., Weible G.D. and Yen J.D.L. 2010. Quantifying uncertainty in estimation of tropical arthropod species richness. Am. Nat. 176: 90–95
Heraty J.M. 1998. The genus Dilocantha (Hymenoptera: Eucharitidae). Proc. Entomol. Soc. Washington 100: 72–87
Heraty J.M. 2002. A revision of the genera of Eucharitidae (Hymenoptera: Chalcidoidea) of the world. Mem. Am. Entomol. Inst. 68: 1–367
Heraty J.M. 2009. Parasitoid biodiversity and insect pest management. In: Insect Biodiversity: Science and Society (Foottit R.G. and Adler P.H. Eds), Wiley-Blackwell, Chichester. pp 445–462
Hölldobler B. and Wilson E.O. 1990. The Ants. Harvard University Press, Cambridge, Massachusetts
Howard R.W., Pérez-Lachaud G. and Lachaud J.-P. 2001. Cuticular hydrocarbons of Kapala sulcifacies (Hymenoptera: Eucharitidae) and its host, the ponerine ant Ectatomma ruidum (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 94: 707–716
Huddleston T. 1976. A revision of Elasmosoma Ruthe (Hymenoptera, Braconidae) with two new species from Mongolia. Ann. Hist.-Nat. Mus. Nation. Hung. 68: 215–225
Hughes D.P., Pierce N.E. and Boomsma J.J. 2008. Social insect symbionts: evolution in homeostatic fortresses. Trends Ecol. Evol. 23: 672–677
Ibarra-Núñez G., García J.A., López J.A. and Lachaud J.-P. 2001. Prey analysis in the diet of some ponerine ants (Hymenoptera: Formicidae) and web-building spiders (Araneae) in coffee plantations in Chiapas, Mexico. Sociobiology 37: 723–755
Jaffe K., Pavis C., Vansuyt G. and Kermarrec A. 1989. Ants visit extrafloral nectaries of the orchid Spathoglotis plicata Blume. Biotropica 21: 278–279
Kathirithamby J. 2009. Host-parasitoid associations in Strepsiptera. Annu. Rev. Entomol. 54: 227–249
Keller L. 1995. Parasites, worker polymorphism, and queen number in social insects. Am. Nat. 145: 842–847
Kistner D.H. 1982. The social insects’ bestiary. In: Social Insects, vol. 3 (Hermann H.R. Ed.), Academic Press, New York, New York. pp 1–244
Kloft W. 1949. Ü-ber den Einfluss von Mermisparasitismus auf den Stoffwechsel und die Organbildung bei Ameisen. Z. Parasit. 14: 390–422
Komatsu T. and Konishi K. 2010. Parasitic behaviors of two ant parasitoid wasps (Ichneumonidae: Hybrizontinae). Sociobiology 56: 575–584
Lachaud J.-P. and Pérez-Lachaud G. 2009. Impact of natural parasitism by two eucharitid wasps on a potential biocontrol agent ant in southeastern Mexico. Biol. Control 48: 92–99
Lachaud J.-P. and Pérez-Lachaud G. 2012. Diversity of species and behavior of hymenopteran parasitoids of ants: a review. Psyche 2012: Article ID 134746, 24 pages
Lachaud J.-P., Cerdan P. and Pérez-Lachaud G. 2012a. Poneromorph ants associated with parasitoid wasps of the genus Kapala Cameron (Hymenoptera: Eucharitidae) in French Guiana. Psyche 2012: Article ID 393486, 6 pages
Lachaud J.-P., Lenoir A. and Hughes D.P. (eds.). 2013. Ants and Their Parasites 2013. Psyche Special Issue. Hindawi Publishing Corporation, New York, New York
Lachaud J.-P., Lenoir A. and Witte V. (eds.). 2012b. Ants and Their Parasites. Psyche Special Issue. Hindawi Publishing Corporation, New York, New York
Lachaud J.-P., López Méndez J.A., Schatz B., De Carli P. and Beugnon G. 1996. Comparison de l’impact de prédation de deux ponérines du genre Ectatomma dans un agroécosystème néotropical. Actes Coll. Insect. Soc. 10: 67–74
Lachaud J.-P., Pérez-Lachaud G. and Heraty J.M. 1998. Parasites associated with the ponerine ant Ectatomma tuberculatum (Hymenoptera: Formicidae): First host record for the genus Dilocantha (Hymenoptera: Eucharitidae). Fla Entomol. 81: 570–574
Lapola D.M., Antonialli Júnior W.F. and Giannotti E. 2003. Arquitetura de ninhos da formiga neotropical Ectatomma brunneum F. Smith, 1858 (Formicidae, Ponerinae) em ambientes alterados. Rev. Bras. Zooci. 5: 177–188
Le Breton J., Takaku G. and Tsuji K. 2006. Brood parasitism by mites (Uropodidae) in an invasive population of the pest-ant Pheidole megacephala. Insect. Soc. 53: 168–171
Lima L.D. and Antonialli-Junior W.F. 2013. Foraging strategies of the ant Ectatomma vizottoi (Hymenoptera, Formicidae). Rev. Bras. Entomol. 57: 392–396
Longino J. 2014. AntWeb. Available from: http://www.antweb.org/specimen.do?name=jtl022350. Accessed 17 November 2014.
Marshall T.A. 1899. I. A monograph of British Braconidae. Part VIII. Trans. Entomol. Soc. Lond. 47: 1–79
Moreau C.S. and Bell C.D. 2013. Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution 67: 2240–2257
Morehead S.A. and Feener D.H. Jr. 2000a. An experimental test of potential host range in the ant parasitoid Apocephalus paraponerae. Ecol. Entomol. 25: 332–340
Morehead S.A. and Feener Jr. D.H. 2000b. Visual and chemical cues used in host location and acceptance by a dipteran parasitoid. J. Insect Behav. 13: 613–625
Morehead S.A., Seger J., Feener Jr. D.H. Jr. and Brown B.V. 2001. Evidence for a cryptic species complex in the ant parasitoid Apocephalus paraponerae (Diptera: Phoridae). Evol. Ecol. Res. 3: 273–284
Morley C. 1909. Notes on Braconidae, X.: On the Pachylommatinae, with descriptions of new species. Entomol. Month. Mag. 45: 209–214
Muesebeck C.F.W. 1941. A new ant parasite (Hymenoptera, Braconidae). Bull. Brooklyn Entomol. Soc. 36: 200–201
Naug D. and Camazine S. 2002. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 215: 427–439
Nickle W.R. and Jouvenaz D.P. 1987. Tetradonema solenopsis n. sp. (Nematoda: Tetradenomatidae) parasitic on the red imported fire ant Solenopsis invicta Buren from Brazil. J. Nematol. 19: 311–313
Olivier E. 1893. Notes entomologiques. Bull. Séanc. Bull. Bibliogr. Soc. Entomol. Fr. 1: lxx-lxxi
Parmentier T., Dekoninck W. and Wenseleers T. 2014. A highly diverse microcosm in a hostile world: a review on the associates of red wood ants (Formica rufa group). Insect. Soc. 61: 229–237
Passera L. 1976. Origine des intercastes dans les sociétés de Pheidole pallidula (Nyl.) (Hymenoptera Formicidae) parasitées par Mermis sp. (Nematoda Mermithidae). Insect. Soc. 23: 559–576
Passera L., Lachaud J.-P. and Gomel L. 1994. Individual food source fidelity in the neotropical ponerine ant Ectatomma ruidum Roger (Hymenoptera Formicidae). Ethol. Ecol. Evol. 6: 13–21
Pérez-Lachaud G. and Lachaud J.-P. 2014. Arboreal ant colonies as ‘hot-points’ of cryptic diversity for myrmecophiles: the weaver ant Camponotus sp. aff. textor and its interaction network with its associates. PLoS ONE 9:e100155.
Pérez-Lachaud G., Gates M.W. and Lachaud J.-P. 2013. New host record for Camponotophilus delvarei (Hymenoptera: Eurytomidae), a parasitoid of microdontine larvae (Diptera: Syrphidae), associated with the ant Camponotus sp. aff. textor. Psyche 2013: Article ID 230601, 6 pages
Pérez-Lachaud G., Heraty J.M., Carmichael A. and Lachaud J.-P. 2006a. Biology and behavior of Kapala (Hymenoptera: Eucharitidae) attacking Ectatomma, Gnamptogenys, and Pachycondyla (Formicidae: Ectatomminae and Ponerinae) in Chiapas, Mexico. Ann. Entomol. Soc. Am. 99: 567–576
Pérez-Lachaud G., Jervis M.A., Reemer M. and Lachaud J.-P. 2014. An unusual, but not unexpected, evolutionary step taken by syrphid flies: the first record of true primary parasitoidism of ants by Microdontinae. Biol. J. Linn. Soc. 111: 462–472
Pérez-Lachaud G., López-Méndez J.A. and Lachaud J.-P. 2006b. Eucharitid parasitism of the Neotropical ant Ectatomma tuberculatum: parasitoid co-occurrence, seasonal variation, and multiparasitism. Biotropica 38: 574–576
Pérez-Lachaud G., López-Méndez J.A., Beugnon G., Winterton P. and Lachaud J.-P. 2010. High prevalence but relatively low impact of two eucharitid parasitoids attacking the Neotropical ant Ectatomma tuberculatum (Olivier). Biol. Control 52: 131–139
Pérez-Lachaud G., Noyes J. and Lachaud J.-P. 2012. First record of an encyrtid wasp (Hymenoptera: Chalcidoidea) as a true primary parasitoid of ants (Hymenoptera: Formicidae). Fla Entomol. 95: 1066–1076
Pérez-Lachaud G., Valenzuela J.E. and Lachaud J.-P. 2011. Is increased resistance to parasitism at the origin of polygyny in a Mexican population of the ant Ectatomma tuberculatum (Hymenoptera: Formicidae)? Fla Entomol. 94: 677–684
Philpott S.M., Perfecto I., Vandermeer J. and Uno S. 2009. Spatial scale and density dependence in a host parasitoid system: an arboreal ant, Azteca instabilis, and its Pseudacteon phorid parasitoid. Environ. Entomol. 38: 790–796
Pie M.R. 2004. Foraging ecology and behaviour of the ponerine ant Ectatomma opaciventre Roger in a Brazilian savannah. J. Nat. Hist. 38: 717–729
Poinar G. Jr. 2004. Behaviour and development of Elasmosoma sp. (Neoneurinae: Braconidae: Hymenoptera), an endoparasite of Formica ants (Formicidae: Hymenoptera). Parasitol. 128: 521–531
Poinar G. Jr. 2012. Nematode parasites and associates of ants: past and present. Psyche 2012: Article ID 192017, 13 pages
Poinar G. Jr. and Yanoviak S.P. 2008. Myrmeconema neotropicum n. g., n. sp., a new tetradonematid nematode parasitising South American populations of Cephalotes atratus (Hymenoptera: Formicidae), with the discovery of an apparent parasite-induced host morph. Syst. Parasitol. 69: 145–153
Poinar Jr. G., Lachaud J.-P., Castillo A. and Infante F. 2006. Recent and fossil nematode parasites (Nematoda: Mermithidae) of Neotropical ants. J. Invertebr. Pathol. 91: 19–26
Poteaux C., Prada-Achiardi F.C., Fernández F. and Lachaud J.-P. (in press). Diversidade genética e fenotípica no gênero Ectatomma. In: Poneromorfas do Brasil (Delabie J.H.C., Feitosa R., Serrão J.E., Mariano C. and Majer J., Eds)
Quicke D.L.J. 1997. Parasitic Wasps. Chapman and Hall, London
Rettenmeyer C.W., Rettenmeyer M.E., Joseph J. and Berghoff S.M. 2011. The largest animal association centered on one species: the army ant Eciton burchellii and its more than 300 associates. Insect. Soc. 58: 281–292
Reuter O.M. 1913. Lebensgewohnheiten und Instinkte der Insekten bis zum Erwachen der Sozialen Instinkte. R. Friedländer & Sohn, Berlin
Rocha F.H., Lachaud J.-P. and Pérez-Lachaud G. 2014. Fine individual specialization and elitism among workers of the ant Ectatomma tuberculatum for a highly specific task: intruder removal. Ethology 120: 1185–1198
Ruschka F. 1924. Die europäisch-mediterranen Eucharidinae und Perilampinae. (Hym. Chalc.). [Der Chalcididenstudien IV. und V. Teil.]. Deutsche Entomol. Z. 41: 82–96
Ruthe J.F. 1858. Beiträge zur Kenntniss der Braconiden. Berl. Entomol. Z. 2: 1–10
Schmid-Hempel P. 1998. Parasites in Social Insects. Monographs in Behavior and Ecology. Princeton University Press, Princeton, New Jersey
Schmiedeknecht O. 1914. Die Schlupfwespen (Ichneumonidea). In: Die Insekten Mitteleuropas insbesondere Deutschlands. Zweiter Band. Hymenopteren Zweiter Teil (Schröder C., Ed.), Franckh’sche Verlagshanlung, Stuttgart, Germany. pp 113–256
Schmitz H. 1914. Die myrmecophilen Phoriden der Wasmann’schen Sammlung. Zool. Jahrb. 37: 509–566
Schmitz H. 1941. Zwei neue Aenigmatistes aus Abessinien, nebst einem Verzeichnis aller bisher beschriebenen Aenigmatiinae. Natuurhist. Maandbl. 30: 104–108
Shaw S.R. 1992. Seven new North American species of Neoneurus (Hymenoptera: Braconidae). Proc. Entomol. Soc. Washington 94: 26–47
Shaw S.R. 1993. Observations on the ovipositional behavior of Neoneurus mantis, an ant-associated parasitoid from Wyoming (Hymenoptera: Braconidae). J. Insect Behav. 6: 649–658
Shaw S.R. 2007. A new species of Elasmosoma Ruthe (Hymenoptera: Braconidae: Neoneurinae) from the northwestern United States associated with the western thatching ants, Formica obscuripes Forel and Formica obscuriventris clivia Creighton (Hymenoptera: Formicidae). Proc. Entomol. Soc. Washington 109: 1–8
Tang C., Gu J., Li Q. 1999. Observation on the mermithid larvae from Formica gagates and Amphimermis chinensis in ant colony soil in Korquing pasture of inner Mongolia. Sichuan J. Zool. 18: 152–156
Tobias V.I. 1971. Obzor naezdnikov-brakonid (Hymenoptera) fauny SSSR. Trud. Vsesoy. Entomol. Obshch. 54: 156–268
Torréns J. 2013. A review of the biology of Eucharitidae (Hymenoptera: Chalcidoidea) from Argentina. Psyche 2013: Article ID 926572, 14 pages
Torréns J. and Heraty J.M. 2012. Description of the species of Dicoelothorax Ashmead (Chalcidoidea, Eucharitidae) and biology of D. platycerus Ashmead. ZooKeys 165: 33–46
Vandel A. 1930. La production d’intercastes chez la fourmi Pheidole pallidula sous l’action des parasites du genre Mermis. 1. Étude morphologique des individus parasites. Bull. Biol. Fr. Belg. 64: 457-494
Vander Meer R.K., Jouvenaz D.P. and Wojcik D.P. 1989. Chemical mimicry in a parasitoid (Hymenoptera: Eucharitidae) of fire ants (Hymenoptera: Formicidae). J. Chem. Ecol. 15: 2247–2261
Varone L., Heraty J.M. and Calcaterra L.A. 2010. Distribution, abundance and persistence of species of Orasema (Hym: Eucharitidae) parasitic on fire ants in South America. Biol. Control 55: 72–78
Wagner H.C., Koschuh A., Schatz I. and Stalling T. 2012. Die Myrmecophilen einer Lawinenrinne im Nationalpark Gesäuse (Steiermark). Abhandl. Zool.-Bot. Gesellsch. Österreich 38: 147–161
Wasmann E. 1894. Kritisches Verzeichniss der myrmekophilen und termitophilen Arthropoden. Mit Angabe der Lebensweise und mit Beschreibung neuer Arten. Verlag von Felix L. Dames, Berlin, Germany
Wasmann E. 1899. Die psychischen Fähigkeiten der Ameisen. Zoologica 11: 1-133 (1909).
Wasmann E. 1908. Weitere Beiträge zum sozialen Parasitismus und der Sklaverei bei den Ameisen. Biol. Zentralbl. 28: 726–731
Watanabe C. 1935. On two hymenopterous guests of ants in Japan. Ins. Matsumurana 9: 90–94
Weber N.A. 1946. Two common ponerine ants of possible economic significance, Ectatomma tuberculatum (Olivier) and E. ruidum Roger. Proc. Entomol. Soc. Washington 48: 1–16
Wheeler G.C. and Wheeler J. 1952. The ant larvae of the subfamily Ponerinae—Part I. Am. Midl. Nat. 48: 111–144
Wheeler G.C. and Wheeler J.N. 1986. The Ants of Nevada. Natural History Museum of Los Angeles County, Los Angeles, California
Wheeler W.M. 1907. The polymorphism of ants, with an account of some singular abnormalities due to parasitism. Bull. Am. Mus. Nat. Hist. 23: 1–93
Wheeler W.M. 1910. Ants, Their Structure, Development and Behavior. The Columbia University Press, New York, New York
Wheeler W.M. 1930. Two mermithergates of Ectatomma. Psyche 37: 48–54
Wilson E.O. 1971. The Insect Societies, Harvard University Press, Cambridge, Massachusetts
Wise de Valdez M.R. 2006. Parasitoid-induced behavioral alterations of Aedes aegypti mosquito larvae infected with mermithid nematodes (Nematoda: Mermithidae). J. Vector Ecol. 31: 344–354
Witek M., Barbero F. and Markó B. 2014. Myrmica ants host highly diverse parasitic communities: from social parasites to microbes. Insect. Soc. 61: 307–323
Acknowledgments
We thank Patricia Chacón de Ulloa, Inge Armbrecht, James Montoya Lerma, Jonathan Rodríguez and all the staff of the SOCOLEN 2014 for their invitation and their initial suggestion for the topic of this paper. We are indebted to Luis Antonio Gómez Laverde for having attracted our attention to the few and very poorly known cases of ant parasitoids in the uropodid mites. We also thank two anonymous reviewers for helpful comments. Although we have attempted to make the review as complete as possible, we may have overlooked some references, and so we would be grateful if readers could send additional information not included in the tables presented here.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lachaud, JP., Pérez-Lachaud, G. Ectaheteromorph ants also host highly diverse parasitic communities: a review of parasitoids of the Neotropical genus Ectatomma . Insect. Soc. 62, 121–132 (2015). https://doi.org/10.1007/s00040-015-0390-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-015-0390-x