Abstract
Despite the extraordinary diversity of organisms associated with ants, few species or genera have been inventoried for the myrmecophilous communities they host. Here, we review the known information on Lepidoptera associated with the ant genus Ectatomma, based on: (a) extensive colony sampling and observations on six focal species (E. tuberculatum, E. brunneum, and four cryptic species of the E. ruidum species complex) over a period of 43 years in Mexico, French Guiana, and Colombia, (b) a follow-up of the available literature, and (c) an analysis of Ectatomma images from various photographic databases available online and direct observations in Brazil and Suriname. No lepidopteran species were found inside the nests, but a wide variety of facultative mutualisms were observed outside on plants bearing extrafloral nectaries and/or honeydew-producing hemipterans; however, around 15% involved a form of commensalism, with no direct physical butterfly–ant interaction. Various new associations, previously unnoticed, are reported, and we illustrate a new symbiotic association between Rekoa palegon and E. ruidum sp. 2 in Mexico. At least 29 lepidopteran species from 19 genera, belonging to four tribes in three subfamilies and three families, participate in 41 associations involving only 5 of the 18 known Ectatomma species, all 5 characterized by visiting liquid food sources on foliage. Specialized interactions with Ectatomma ants were only found in three Riodinidae species, while in Lycaenidae interactions were all facultative. A greater sampling effort is needed, including nocturnal sampling and studies on little-studied species of this genus, to obtain a comprehensive picture of the extent of Ectatomma–Lepidoptera interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ants interact in many ways with a multitude of other arthropods, establishing relationships ranging from facultative to highly specialized (Kistner 1982; Schmid-Hempel 1998; Lachaud et al. 2012, 2013; Hölldobler and Kwapich 2022). However, despite the extraordinary diversity of organisms associated with their societies, few ant species or genera have been the focus of a detailed inventory of the myrmecophilous communities they host. To date, the best-known and most striking example is certainly that reported by Rettenmeyer et al. (2011) for the army ant Eciton burchellii, a keystone species, with its 557 associates, ranging from birds to insects and mites, recorded over the course of 55 years of field studies. With the exception of red wood ants of the genus Formica, which are of interest in forest and heathland ecosystems (Parmentier et al. 2014), or red ants of the genus Myrmica, which are important model species for many biological, physiological, chemical, and ecological studies (Witek et al. 2014), almost no comprehensive review has been carried out on the more than 330 other existing ant genera. In many cases, even regarding ant genera that have been the subject of detailed studies due to their importance as tramp species, agricultural pests, or potential biological control agents (e.g., Anoplolepis, Linepithema, Atta, Oecophylla), information is widely scattered in the literature. For less charismatic or less well-known genera, or those perceived as less important from an ecological or economic point of view, information is even scarcer. Nevertheless, numerous reports on various associations are available, even within species-poor ant genera, albeit widely dispersed as adventitious information or part of the gray literature. In addition, a growing body of information has recently emerged from citizen science records available on the Internet, such as public photo repositories (Flickr, GBIF, iNaturalist, and similar databases), with photographic records increasingly becoming recognized resources in the study of biodiversity (Rousselet et al. 2013; Leighton et al. 2016; Suzuki-Ohno et al. 2017; Hochmair et al. 2020; Roberts et al. 2022; Szentivanyi and Vincze 2022).
Species of the genus Ectatomma are mainly predatory, hunting a variety of prey, but they also scavenge on dead arthropods or animals, and some species exploit sugary resources (fruit pulp, floral and extrafloral nectary, honeydew, or similar excretions) (Lachaud 2021). The genus, exclusively neotropical but widely distributed, contains few species: 14 extant and 1 fossil species currently recognized, and at least 4 cryptic species in the E. ruidum species complex, which, due to the presence of extensive mitochondrial heteroplasmy and the current impossibility of accurately distinguishing them on the basis of morphological characters, are still referred to as E. ruidum sp. 1, 2, 3, 4 (Kugler and Brown 1982; Feitosa et al. 2008; Nettel-Hernanz et al. 2015; Aguilar-Velasco et al. 2016; Meza-Lázaro et al. 2018, 2022; Peña-Carrillo et al. 2021, 2022). Several of them, such as E. tuberculatum and E. ruidum sp. 1 and sp. 2, are considered dominant and important natural control agents in various agroecosystems (Perfecto 1990; Lachaud 1990, 2021; Majer et al. 1994; Ibarra-Núñez et al. 2001; Schatz and Lachaud 2008). Because Ectatomma ants commonly display aggressive behavior, providing efficient protection to plants against herbivores and nectar thieves (Leal et al. 2006; Sanz-Veiga et al. 2017; Melati and Leal 2018; Ibarra-Isassi and Oliveira 2018), species of this genus have long been considered poor candidates to harbor an abundant community of myrmecophiles or to establish stable mutualistic associations. However, over the last few decades, several cases of invertebrate associates have occasionally been reported when excavating nests of various Ectatomma species (e.g., Antonialli and Giannotti 1997, 2001; Vieira et al. 2007; Lachaud and Pérez-Lachaud 2012, 2015; Torréns 2013; Pérez-Lachaud et al. 2019) or by studying interactions between foragers and other organisms outside the nests (e.g., Wood 1984; DeVries et al. 1992; Kronauer 2004; Espadaler et al. 2012; Lapèze 2021). Until now, no systematic and comprehensive review of any myrmecophile taxa associated with Ectatomma has been attempted, except for cases that involve ant primary parasitoids (Lachaud and Pérez-Lachaud 2015). Here, we focus on associations between ants of this genus and both lepidopteran caterpillars and adults. Our aim was to gather data from different sources and supplement them with our own observations.
Lepidoptera is one of the most widespread and recognizable insect orders in the world, with about 180,000 described species, present on Earth for at least 190 million years. Adults play an important role as pollinators (Willmer 2011; Walton et al. 2020) and are at the base of the food chain for many predatory species (Hooks et al. 2003; Nyffeler et al. 2018; Siddiqui et al. 2023); conversely, their larvae are essentially phytophagous and often considered problematic in agriculture (Zhang 1994; Hill 2008). The larvae of a minority of species are predators, social parasites or parasitoids of other insects, especially ant-associated species (Cottrell 1984; Pierce 1995). Some lepidopteran species are toxic to predators by sequestering chemicals from the plants they eat or by making their own toxins (Nishida 2002); some other species use multimodal strategies as camouflage or mimicry to protect themselves from predators (Quicke 2017; Casacci et al. 2019).
The caterpillars of many species belonging predominantly to the sister butterfly families Riodinidae and Lycaenidae can form symbiosis with ants, with relationships ranging from parasitic to mutualistic, and from facultative to obligate (Fiedler 1991, 2021; Pierce et al. 2002; Pierce and Dankowicz 2022). Caterpillar traits such as a protective thick cuticle, the production of appeasing substances or rewards in the form of semiochemicals rich in amino acids and sugary secretions, as well as the production of ant-mimicking vibratory calls or the exhibition of several strategies of chemical imitation of their host ants contribute to minimize predation risk by ants (Schönrogge et al. 2017; Cassacci et al. 2019); in return, they can obtain a degree of protection from their natural enemies. Several very specialized species are even able to overcome the host colony defenses and succeed in entering the ant nests and integrate the host colony social life (Malicky 1970; DeVries 1988, 1990, 1991a; Fiedler 1991, 2012, 2021; Pierce et al. 2002; Kaminski et al. 2021; Pierce and Dankovicz 2022). Social parasitism has emerged in Lycaenidae several times through different eco-evolutionary routes (Fiedler 1998). In the most emblematic case, species of Phengaris (= Maculinea) have phyto-predacious caterpillars, that is, they have an initial herbivorous phase followed by the adoption by ants and invasion of the ant nest, where they can be predators or kleptoparasites (Henning 1983; Als et al. 2004; Schönrogge et al. 2017; Casacci et al. 2019).
Many ant species have developed mutualistic interactions with plants that provide nectar from floral and extrafloral nectaries (EFNs) that are also attractive to many other insect taxa, including both caterpillars and adult butterflies (DeVries 1991a). In both facultative and obligate myrmecophilous butterflies, the oviposition pattern of females is ant mediated, as they tend to lay eggs in plants visited by ant species that engage in stable associations with their larvae (Atsatt 1981a; Pierce and Elgar 1985; Kaminski et al. 2010). For example, the occurrence of facultative myrmecophilous caterpillars of Synargis calyce (Riodinidae) in the EFN-bearing shrub Banisteriopsis malifolia (Malpighiaceae) is based on the abundance of the ant species Camponotus blandus, as the females of this butterfly oviposit predominantly in plants where C. blandus are more abundant (Alves-Silva et al. 2018). In many cases, the myrmecophilous butterflies exploit a pre-existing ant–plant mutualism to their advantage, monopolizing aggressive ants by offering more attractive resources than the plant’s EFNs through secretions from specialized ant organs (e.g., perforated cupola organs (PCOs), tentacle organs (TOs) and dorsal nectary organ (DNO) in Lycaenidae; anterior tentacle organs (ATOs), tentacle nectary organs (TNOs) and vibratory papillae (VP) in Riodinidae) (DeVries and Baker 1989; Blüthgen and Fiedler 2004; Katayama et al. 2013; Alves-Silva et al. 2018). For other myrmecophilous lepidopteran species, interaction with ants is weaker, with no direct protection of eggs and larvae, or without any food reward for ants, which, by their mere presence, reduce the action of potential parasitoids and predators that might disrupt egg laying, and thus act as oviposition cues of enemy-free space (Atsatt 1981b).
Materials and methods
Compilation of known associations
We evaluated the Lepidoptera present in nests or attended by foragers through extensive colony sampling spanning 43 years and observations on six focal species of Ectatomma (E. brunneum, E. tuberculatum, and four species (Ectatomma sp. 1, sp. 2, sp. 3, and sp. 4) of the E. ruidum species complex) in Mexico, French Guiana, and Colombia, and unpublished natural history records from Brazil and Suriname by LAK also available in a citizen science project in iNaturalist (Kaminski 2021a). The first four species are the most common and most studied Ectatomma species and those with the widest distribution within the genus (Nettel-Hernanz et al. 2015). In addition, we reviewed all available literature on all Ectatomma species since the description of the genus by F. Smith in 1858. A literature search was performed on several platforms, including Web of Science, Google Scholar, antcat.org, and antwiki.org using the keywords “Ectatomma”, “butterfly”, “Lepidoptera”, “associate”, “interaction”, and “caterpillar”. We also reviewed all the information available on the web, especially images involving any Ectatomma ant found on public photo repositories such as Flickr, Facebook, GBIF, iNaturalist (Kaminski 2021a), and similar databases, and we directly contacted the authors of the photographs when supplementary information was needed. In this review, only images where the associated lepidopteran species could be reliably identified, at least at genus level, were considered.
New association record
As part of a project on ant parasitoids, where colonies of E. tuberculatum and E. ruidum sp. 2 were collected in a coffee plantation at the INIFAP experimental station of Rosario Izapa, Tuxtla Chico Municipality, Chiapas, Mexico (14°57′42″N–92°09′21″W, 403 m asl), casual observations were made on Melanthera nivea (Asteraceae) flowers located at the edge of a dirt road where E. ruidum workers were foraging. A caterpillar was incidentally recorded interacting with ants (see “Results”), around 10:00–11:00 am, on two successive cloudy days, on February 28 and March 1, 2010.
Melanthera nivea, commonly known as “snow squarestem”, “pineland squarestem”, or “yerba de cabra”, is native to North America and the Caribbean region, and ranges from Illinois and southeastern USA to northern South America (Colombia, Ecuador, Peru, and Venezuela), and the Greater and Lesser Antilles (Wagner and Robinson 2001). In Mexico, it is present in Campeche, Chiapas, Colima, Guerrero, Jalisco, Michoacan, Nayarit, Oaxaca, Queretaro, Quintana Roo, San Luis Potosi, Tabasco, Tamaulipas, Veracruz, and Yucatan (Villaseñor Ríos and Espinosa García 1998). Very variable ecologically and morphologically, it is a perennial, herbaceous wildflower or shrub forming dense clusters along roadsides and reproducing by seeds and by stolons which allow vegetative propagation. Both floral and EFNs (at the junction of the veins on the abaxial side of the leaf) attract a wide range of arthropods. A study conducted in Costa Rica (Mexzón and Chinchilla 2000) ranked it as one of the plant species that attracts the largest number of insect families. Apart from various species of ants, the main groups of visitors are flies of the families Otitidae, Richardiidae, Sepsidae, Syrphidae, wasps of the families Braconidae, Chalcididae, Evaniidae, Ichneumonidae, and Pteromalidae (Mexzón and Chinchilla 2000), and both adults and caterpillars of numerous lepidopterans.
Results
Since 1981, we collected and revised at least 2422 Ectatomma nests (56 of E. brunneum, 227 of E. tuberculatum, 609 of E. ruidum sp. 1, 1454 of E. ruidum sp. 2, 49 of E. ruidum sp. 3, and 27 of E. ruidum sp. 4). Several invertebrate associates have been recorded, but no lepidopteran specimen has ever been found inside the nests, and no mention of such presence in Ectatomma nests has been reported in the literature. However, our literature review provided a wide variety of facultative comensalisms and mutualisms observed outside the nests. On numerous occasions, workers of several Ectatomma species have been observed tending caterpillars for excretions produced by specialized organs. In most cases, the ants can solicit droplets of honeydew through rapid antennation. In other cases, Ectatomma workers were observed resting near lepidopteran adults without direct physical interaction. The most conservative assessment of the number of associations recorded between butterflies and Ectatomma species is 41, involving 29 lepidopteran species of 19 genera from four tribes in three subfamilies and three families (all true butterflies, Papilionoidea), but only 5 of the 18 extant Ectatomma species (Table 1). These associations are listed below in alphabetical order for species within each tribe, and taxonomic arrangements follow Seraphim et al. (2018), Robbins et al. (2022), and Warren et al. (2024).
Lycaenidae (Theclinae, Eumaeini)
Allosmaitia strophius (Jantheclina): The Strophius Hairstreak is widely distributed from southern USA (Texas) to southern Brazil and the province of Misiones in Argentina (GBIF.org 2023). While adults are usually observed feeding on the nectar of small flowers of various families, larvae are specialized in feeding exclusively on reproductive tissues (buds and flowers) of Malpighiaceae (Fiedler 1991; Kaminski and Freitas 2010; Silva et al. 2014) and occasional cannibalism by larvae on individuals in prepupal and pupal phases has been observed (Silva et al. 2014). Females lay several eggs per inflorescence, but only two eggs per bud (Kaminski and Freitas 2010). The oviposition is mediated by the ant presence (Bächtold et al. 2014, 2017). Various associations with E. tuberculatum have been observed on two EFN-bearing shrubs (Malpighiaceae) that occur in the Brazilian savanna: Banisteriopsis malifolia (Bächtold et al. 2016) and Peixotoa tomentosa (Bächtold et al. 2016, 2017). Two associations with E. edentatum have also been found on P. tomentosa (Bächtold et al. 2017). Although A. strophius larvae exhibit several behavioral and morphological adaptations related to myrmecophily (absence of a “beat reflex” (rearing up the body, curling and wriggling vigorously), dendritic setae, thick cuticle, perforated cupola organs, dorsal nectar organ (but apparently non-functional)), assumed to prevent ant attacks (Kaminski and Freitas 2010), no direct symbiotic interactions were observed in the field between individuals of both Ectatomma species (or any other ant) and A. strophius eggs or larvae that co-occurred with them on the same host plant (Bächtold et al. 2017). This species appears to be a commensal which does not offer rewards to ants, although the caterpillars are occasionally touched by ants close to the PCOs; moreover, the caterpillars are chemically camouflaged (Lima et al. 2021). However, ant presence seems to facilitate oviposition as more eggs were found in branches patrolled by Camponotus blandus or E. tuberculatum foragers and all direct observations of female oviposition occurred only on plants with E. tuberculatum present (Bächtold et al. 2017).
Arawacus lincoides (Strymonina): The Lincoides Hairstreak or Side-striped Hairstreak or Stripe-streak is distributed from Honduras to western Ecuador, Colombia, and French Guiana and has been reported in Trinidad (Robbins 1991a; GBIF.org 2023). In Panama, larvae commonly feed on several species of Solanum (Solanaceae), and females oviposit on the leaves and twigs of Solanum lancaeifolium and S. ochraceo-ferrugineum (Robbins and Aiello 1982). Ants ignore the first two instars, but larvae of the two last instars secrete fluid through their dorsal nectar organ that is actively removed by several Pheidole sp. foragers simultaneously or by one worker at a time for both E. ruidum and E. tuberculatum (Robbins and Aiello 1982; Robbins 1991a).
Arawacus separata (Strymonina): The Separated Stripestreak or Zebra Teaser is distributed from Ecuador to the provinces of Misiones and Santa Fe in Argentina and the province of Rivera northern Uruguay; it has been captured as far north as Panama and Guatemala (only one report in each case, but the identification needs to be confirmed) (GBIF.org 2023). In Ecuador, larvae feed on species of Solanaceae such as Solanum stramoniifolium (referred to as S. coconilla) (Robbins and Aiello 1982). It is commonly found along forest edge habitats. In a study carried out in Brazil, eggs were laid isolated or in small clusters (2–5) near the tip of the twig and immatures were found on young leaves of Solanaceae often accompanied by aggregations of mealybugs and membracids tended by ants. Larvae are solitary and have a functional DNO, at least from the second instar onward, attracting Crematogaster sp., Solenopsis invicta, and various species of Camponotus and Pheidole (Dresch 2021). In addition, all larval stages can be found in association with ants, which feed on the liquids exuding from the damaged tissues of leaves cut by caterpillars. A pair of photographs taken on April 9, 2022 (Fig. 1), in Bolivia (Sara province, Santa Cruz state) (Kawakami 2022) illustrate a fourth-instar larva of A. separata observed on Solanum sp. and attended by two E. tuberculatum workers.
Calycopis sp. (Calycopidina): The genus is widely distributed from northern USA (Michigan) to Argentina (Buenos Aires province), but most species are found in the Neotropics. Larvae of some species are detritivorous (Duarte et al. 2005; Duarte and Robbins 2009), whereas in other species such as C. mimas (Silva et al. 2011, 2014) they feed on inflorescences of Lythraceae and Melastomataceae. Larvae of Calycopis have not been reported being tended by ants; however, larvae present PCOs and specialized dendritic setae, but mature larvae apparently lack a DNO (Duarte et al. 2005; Duarte and Robbins 2009). A non-agonistic interaction between an E. tuberculatum worker and an adult of Calycopis sp. visiting simultaneously a pericarpial nectary of Tocoyena formosa (Rubiaceae) has been reported in southeastern Brazil (Palmeira da Serra farm, Pratânia, São Paulo) (Sanz-Veiga et al. 2017).
Parrhasius polibetes (Parrhasiina): The Blackspot Haistreak is widespread from Nuevo León in Mexico to the province of Misiones in Argentina and the central part of Rio Grande do Sul in southern Brazil (Nicolay 1979; GBIF.org 2023). Eggs are laid on a wide range of plant hosts from at least 19 families, mainly Euphorbiaceae and Fabaceae (Fiedler 1991), and larvae can be found close to the flower buds, underneath the leaves during molting (Kaminski et al. 2012). Several ant species are facultatively associated with the larvae in the “cerrado” savanna of Brazil, namely E. edentatum attending aggregations of the treehopper Guayaquila xiphias on shrubs of the Araliaceae Didymopanax vinosum (Oliveira and Del-Claro 2005); infestations by P. polibetes appear to be higher when tending ants are associated with ant-tended treehoppers (Kaminski et al. 2010; Mota and Oliveira 2016). Larvae of P. polibetes have also been recorded in association with E. tuberculatum on Banisteriopsis malifolia in Brazilian savanna open area (Bächtold et al. 2016), and a series of 11 photographs (Kaminski 2024a) taken on April 14, 2008, in Brazil (Bosque das Palmeiras, Campinas, São Paulo) shows two workers of E. brunneum tending a P. polibetes last-instar larva (Fig. 2) on Eugenia bimarginata (Myrtaceae). Although caterpillars have a functional DNO since the third-larval instar (Kaminski et al. 2012), individuals of E. tuberculatum were not observed tending any larvae that co-occurred with them on the host plant (Bächtold et al. 2016); conversely, E. brunneum workers feed on the DNO of last-instar larvae (Kaminski 2024a).
Rekoa marius (Strymonina): The Marius Hairstreak is found in wet and dry disturbed areas from lowlands to mountains up to 3000 m. It is distributed from northwestern Sonora in Mexico and southern Texas in USA, south to La Paz province in Bolivia and the northeastern region of Buenos Aires province in Argentina (Robbins 1991b; GBIF.org 2023). Adults take nectar from a variety of flowers (Euphorbiaceae, Trigoniaceae, and Sapindaceae, among others), while larvae feed on plants from several families, mainly Fabaceae, Bignoniaceae, Malpighiaceae, and Ochnaceae (Fiedler 1991; Monteiro 1991; Robbins 1991b), consuming buds and flowers or, occasionally, petals and young fruits (Silva et al. 2014) and even leaves (Santos-Murgas et al. 2020); food deprivation triggers larval cannibalism (Monteiro 1991). Myrmecophilous interactions between third- and fourth-instar larvae of R. marius and several ant species have been reported from Brazil (Camponotus crassus, C. rufipes, C. cingulatus, Solenopsis sp., Dorymyrmex sp., Monteiro 1991) and from Panama (Camponotus lindigi on Acalypha wilkesiana (Euphorbiaceae) that does not have EFNs, Santos-Murgas et al. 2020). Antennation by the ants on the DNO and PCOs was observed, but excretions were only provided by the DNO (Monteiro 1991). Association with E. tuberculatum has been reported on Banisteriopsis malifolia (Bächtold et al. 2016) in Brazilian savanna open area; however, individuals of E. tuberculatum were not observed tending any larvae that co-occurred with them on the host plant. A series of five photographs taken on July 20, 2008, in Brazil (São João Batista do Glória, Minas Gerais) illustrate an association between a larva of Rekoa cf. marius and two workers of E. tuberculatum on Camptosema sp. (Fabaceae) (Fig. 3), but, in this case also, without any contact (Kaminski 2023a).
Rekoa palegon (Strymonina): New association. The Gold-bordered Hairstreak is found in wet and dry areas from sea level to above 2000 m. It is distributed from southern Texas and northern Mexico (Tamaulipas) to Santiago del Estero and Santa Fe Provinces in Argentina and southern Rio Grande do Sul province in Brazil, and has been reported in the Lesser Antilles (St. Vincent) and in Trinidad and Tobago (Robbins 1991b; GBIF.org 2023). Adults feed on the nectar from flowers of different species of Asteraceae and Boraginaceae and larvae feed on floral buds or flowers of a wide range of plants, including Verbenaceae, Solanaceae, Asteraceae, and Polygonaceae (Robbins 1991b; Monteiro 1991, 2000; Silva et al. 2014; Santos-Murgas et al. 2020). Although larvae of R. palegon possess myrmecophilous organs (PCOs which are spread throughout the body of the larva and DNO located in the seventh abdominal segment) and exhibit facultative associations with various ant species (Malicky 1969, 1970; DeVries 1990; Monteiro 1991; Robbins 1991a), in 1991 Robbins noted that no R. palegon larvae had yet been recorded being tended by ants in the field (Robbins 1991b). Since this publication, a few ant species have been reported interacting with R. palegon, mainly on Asteraceae, such as Camponotus crassus, Crematogaster sp. and Azteca sp. (Monteiro 1991, 2000; DeVries pers. comm. in Fiedler 2021), antennating the larvae on the DNO and PCOs but obtaining rewards only from the DNO (Monteiro 1991). However, to date, no species of Ectatomma has been reported to interact with this butterfly.
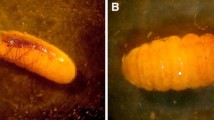
At the Rosario Izapa experimental station study site, several insects were associated with M. nivea flowers and EFNs (ants, bees, hemipterans, butterflies, Fig. 4a), including E. ruidum sp. 2 workers and R. palegon adults (Fig. 4b). On the morning of February 28, 2010, we observed a green, spiny caterpillar of R. palegon feeding on a Melanthera inflorescence (Fig. 5a). A few minutes later, a worker of E. ruidum sp. 2 was observed foraging on the same inflorescence (Fig. 5b), but without attacking the caterpillar. After approaching and contacting the caterpillar with rapid strokes of its antennae all over the larva's body (Fig. 5c), the ant licked different parts of the body and the spines covering the larva's body (Figs. 5c, 6a, b), but with no apparent preference for the seventh abdominal segment where the opening of the DNO is located. No liquid secretion by the caterpillar could be observed directly, but on prolonged contact, some liquid (probably originating from the PCOs) clearly accumulated at the base of the ant's mandibles (Fig. 6a). No more than two E. ruidum sp. 2 individuals were seen simultaneously tending a single R. palegon larva, walking on the caterpillar's body and palpating it (Fig. 6c).
Terenthina terentia (Strephonotina): The Terentia Hairstreak or Orange-Patched Hairstreak is found in rainforests and cloud forests, at altitudes between 200 and 1800 m. It is distributed from Puntarenas province in Costa Rica to southwestern Peru, northern Bolivia, and northern Mato Grosso in Brazil (GBIF.org 2023). Adults feed on nectar from various flowering plants including Asteraceae and Verbenaceae. Observations made in Tambopata, Madre de Dios province in Peru (Pomerantz 2015), show that larvae feed on the flower buds of Apodanthes caesariae (Apodanthaceae), a parasitic plant on Casearia trees. Caterpillars exhibit myrmecophilous organs and form symbiotic associations with E. tuberculatum workers which antennate the caterpillar until it excretes a droplet of nectar through the DNO that is readily collected (Pomerantz 2015).
Thereus lausus (Strymonina): The Lausus Hairstreak or Café-au-lait Haistreak occurs widely in lowland forests from southern Mexico (Chiapas) and Central America to Ecuador, Suriname, and Brazil (Amazon and Atlantic Forest region). The larvae feed on mistletoe (Loranthaceae) and exhibit facultative myrmecophily (Glassberg and Robbins 2005; Robbins et al. 2022; K. Nishida in Warren et al. 2024). A pair of photographs taken on March 23, 2023, in Trinidad and Tobago (Cocoa Palace, Brasso Seco Village, Tunapuna-Piarco), show a worker of one of the cryptic species (sp. 1 or sp. 2) of the E. ruidum complex exploring a last-instar caterpillar of T. lausus (Tran 2023).
Tmolus echion (Strephonotina): The Red-spotted Hairstreak or Lantana Hairstreak is found from southern Texas in USA and northern Mexico (Sinaloa and Tamaulipas) to southern Rio Grande do Sul province in Brazil (GBIF.org 2023). Larvae feed on leaves of Stigmaphyllon lindenianum (Malpighiaceae) and flowers of Mangifera indica (Anacardiaceae) in Brazil, Lantana camara (Verbenaceae) in Brazil, Costa Rica, and Hawaii, and Cordia sebestena (Boraginaceae), Datura arborea, Solanum nodiflorum, and S. sanitwongsei (Solanaceae) in Hawaii (Robbins and Aiello 1982). They have been reported only from dicots. In Panama, a female of T. echion was observed ovipositing on a flower of Aphelandra deppeana (Acanthaceae) among numerous workers of an unidentified Ectatomma sp. that were feeding on secretions from EFNs on the flower bracts (Robbins and Aiello 1982).
Unidentified caterpillar (Atlidina): Observations of a last instar of an unidentified species, found near an Ectatomma tuberculatum worker collecting nectar on an EFN of Inga sp. (Fabaceae) and apparently showing no aggressive behavior toward the caterpillar, were made in Ecuador (Cube, Quinindé, Esmeralda Province) on May 28, 2022 (Fierro-Minda 2022). The sub-tribe Atlidina includes non-myrmecophilous species with non-functional DNO (Ballmer and Pratt 1991; Kaminski, unpubl.). Larvae of some genera of Atlidina are specialists on mistletoes (Loranthaceae and Viscaceae) and others are generalists (Robbins et al. 2022).
Riodinidae (Riodininae, Eurybiini)
Eurybia elvina (Eurybiina): The Blind Eurybia or Blind Sheenmark is distributed from the states of Nayarit, Jalisco, and Veracruz in Mexico to Peru (Cusco province) and Brazil (São Paolo state) (GBIF.org 2023). In Mexico, adults are commonly found on the undersurface of leaves of various Calathea (Marantaceae), Piper (Piperaceae), and ferns, while larvae feed on buds and flowers or even on developing fruits and their seeds (for the late instars) of various plants, and cannibalism is frequent under laboratory conditions (Horvitz et al. 1987). Eggs are laid singly or in small, spaced clusters (2–5). All larval instars have trophobiotic associations with several ant species attracted by the secretion produced by the TNOs on the eighth abdominal segment and some of them, such as Solenopsis geminata and Paratrechina sp., even provide protection in the form of soil shelters constructed over late instars of E. elvina on the inflorescences (Horvitz et al. 1987). Caterpillars position themselves in the inflorescence such that the TNOs are presented to the ants at roughly the same level as the plant EFNs. The pupal stages were also attended by ants even though they produce no exudates. Larvae have been found in association with E. ruidum and E. tuberculatum on flowers of Calathea spp. in Panama (DeVries et al. 1992).
Eurybia lycisca (Eurybiina): The Blue-winged Eurybia or Blue-winged Sheenmark is found from Mexico (Sinaloa) to Ecuador (Santo Domingo de los Tsáchilas and Pichincha provinces) and Peru (Madre de Dios) (GBIF.org 2023). Adults collect nectar on Calathea flowers and larvae feed on the same host plants. Larvae have been found associated with E. ruidum on flowers of Calathea spp. in Costa Rica and Ecuador and flowers of Calathea latifolia in Panama, and with E. tuberculatum on flowers of Calathea spp. in Costa Rica and Ecuador and flowers of Ischnosiphon pruinosus (Marantaceae) in Costa Rica (De Vries et al. 1992).
Eurybia sp. nr. molochina hyacinthina (Eurybiina): Commonly known as the Hyacinth Sheenmark, it is distributed from Peru (Arequipa) to Brazil (São Paulo) (GBIF.org 2023). Larvae were found associated with E. tuberculatum on flowers of Renealmia sp. (Zingiberaceae) in Ecuador (DeVries et al. 1992).
Riodinidae (Riodininae, Nymphidiini)
Annulata annulifera (Lemoniadina): The Annulifera Metalmark, found in primary rainforests between 400 and 700 m, is distributed from Venezuela (Bolivar state) to Peru (Madre de Dios) and Brazil (Rondônia state) (Hall 2018; GBIF.org 2023). In the Tambopata National Reserve, in Peru, adults and caterpillars of A. annulifera feed on the EFN sugary secretions of the tip of shoots of two bamboo species of the genus Guadua (Poaceae), in association with several species of ants (E. tuberculatum, Paraponera clavata, Megalomyrmex balzani, and Pheidole sp.), with only one species of ant being present on each bamboo plant. The ants chase away other insects, including other ant species, but apparently ignore the butterflies, and E. tuberculatum foragers have been observed feeding at the same EFNs as both larval and adult A. annulifera (Torres and Pomerantz 2016). Eggs of A. annulifera are laid in small clusters (4–5) at the tips of the new shoots of bamboo and larvae feed at the EFNs of the bamboo. Ant organs (TNOs and one pair of VP) are present from the third instar onward and the larvae are actively tended by the ant species present on the plant. Adult A. annulifera also display an unusual kleptoparasitic behavior, stealing liquids collected by E. tuberculatum from EFNs of the bamboos and held between their mandibles, without reciprocal reward to the ants (Torres and Pomerantz 2016).
Aricoris domina (Lemoniadina): Commonly known as Domina Metalmark, it is distributed from Costa Rica to Panama. A female A. domina (referred to as Audre domina) has been observed in Costa Rica laying eggs on a twig of Vismia baccifera (Guttiferae) near several workers of an unidentified Ectatomma species (different from E. tuberculatum) that were tending membracids; however, the lepidopteran flew away when approached by one of the ants (Robbins and Aiello 1982). In Panama, another female of A. domina was observed by the same authors hovering over Turnera panamensis (Turneraceae) plants before landing when encountering and being approached by a group of E. tuberculatum workers. The female butterfly then turned, presented them with her opened wings that were repeatedly attacked by the ants with their mandibles, and laid several eggs before flying off to another group of ants (Robbins and Aiello 1982). According to these authors, eggs collected in the field hatched, but all caterpillars died in the first or second instar without feeding, suggesting that the caterpillars have an obligate interaction with Ectatomma and possibly diapause in ant nests. Based on the presence of greasy wings in Aricoris adults, it has been suggested that the caterpillars of this genus may be aphytophagous (Hall and Harvey 2002).
Nymphidium caricae (Nymphydiina): The Linnean Hemmark or Common Nymphidium found in gallery forest and forest edges is distributed from Colombia (Meta) and Suriname to the states of Madre de Dios in Peru and Goiás in Brazil (Callaghan 2001; GBIF.org 2023). Adults commonly collect nectar from plants of various families, while caterpillars have been recorded on Inga vera (Fabaceae) (Sepp 1848). Larvae lack setae, but exhibit myrmecophilous organs and are found in association with several ant species. An association with E. tuberculatum has been reported in Inga sp. in French Guyana (Brévignon and Gallard 1999), a second one was observed by one of us in Brazil (L.A. Kaminski pers. obs. in Pérez-Lachaud et al. 2021 Suppl. Mat.), and two series of photographs taken on October 1 (9 photographs) and October 6 (20 photographs), 2023, in Suriname (Margaretha, Commewijne district) (Kaminski 2023b) show an association between a third instar (Fig. 7a) and a fourth instar (Fig. 7b), respectively, of N. caricae with a worker of one of the species of the E. ruidum complex.
Nymphidium sp. (Nymphydiina): Observations made on Barro Colorado Island in Panama (Kursar et al. 2006) point to another unidentified species of Nymphidium, in association with an unidentified species of Ectatomma, two lepidopteran larvae being attended by three ant workers on Inga umbellifera. However, the poor quality of the photograph (plate 1, p. 3065, in Kursar et al. 2006) does not allow confirming the identity of the ant, even at the genus level.
Parvospila cilissa (Pandemina): The Cilissa Metalmark or Silver-streaked Mimic-skipper is distributed from the province of Atlántida in Honduras to the province of Córdoba in Colombia (Hall 2018; GBIF.org 2023). Larvae feed on Malpighiaceae. Larvae have been found associated with E. ruidum on leaves of Stygmaphyllon spp. in Costa Rica (DeVries et al. 1992).
Parvospila emylius (Pandemina): The Orange-speckled Grayler or Emylius Metalmark is distributed from Venezuela (Bolivar), Trinidad and Tobago, and Suriname to Peru (Cusco, Madre de Dios), Bolivia (Cochabamba), and Brazil (Mato Grosso) (Hall 2018; GBIF.org 2023). Larvae feed on Malpighiaceae and have been found associated with E. tuberculatum on leaves of Stygmaphyllon sp. (Malpighiaceae) in Ecuador (De Vries et al. 1992).
Parvospila lucianus (Pandemina): The Lucianus Metalmark, is distributed from Costa Rica (La Cusinga) to Peru (Madre de Dios) and Brazil (Pará) and in Trinidad and Tobago and Guadalupe (Hall 2018). Larvae feed on Malpighiaceae, and caterpillars are facultatively tended by several ant species. A female butterfly was photographed on August 28, 2021, at Diego Martin, Trinidad, close to an E. tuberculatum worker carrying nectar between its mandibles and apparently showing no aggressive behavior toward the butterfly (Ramdeen 2021).
Stalachtis phlegia (Stalachtina): The White-dotted Mimic-mark or Dotted Prince is distributed from Venezuela (Merida) to the state of Mato Grosso (central zone) in Brazil (Magaldi et al. 2021). It has been found in association with various ant species of Camponotus, Crematogaster, and Cephalotes, and has been photographed with an unidentified species of Ectatomma (different from E. tuberculatum and E. brunneum) in Brazil on a leaf of Simaba sp. (Simaroubaceae) on November 29, 2017 (Guerra 2017) (see also L.A. Kaminski pers. obs. in Pérez-Lachaud et al. 2021 Suppl. Mat.). A photograph taken in Brazil in 2013 (Parque Ecológico Buritirana, Peri Mirim, Maranhão) by Lucas P. Martins (Fig. 8) shows several last-instar caterpillars of S. phlegia attended by workers of E. brunneum.
Synargis abaris (Lemoniadina): The Cinnamon-rimmed Lemmark, is known from Colombia (Meta, Cundimarca), Venezuela (Bolivar), Trinidad and Tobago, Guyana, and Suriname to Peru (Madre de Dios) and Brazil (Rondônia and Pará) (GBIF.org 2023). Larvae are polyphagous and caterpillars are facultatively tended by several ant species (DeVries et al. 1992; Kaminski 2021a). Larvae were found associated with E. tuberculatum on leaves of Acalypha sp. in Ecuador (DeVries et al. 1992). An unidentified caterpillar, probably cf. S. abaris (referred to as Juditha molpe), has been reported to be associated with E. tuberculatum on Passiflora auriculata (Passifloraceae) (Smiley 2013) and photographed (Fig. 9) while drinking from an EFN alongside an E. tuberculatum worker also attracted by the EFNs of this plant (Smiley, unpubl.).
Synargis calyce (Lemoniadina): The White-fringed Lemmark or Variable Lemmark or False Nymphidium is distributed from Mexico (Jalisco and Veracruz) to Peru, Colombia, Brazil (Santa Catarina) and Argentina (Misiones) (GBIF.org 2023). Larvae of S. calyce are generalist in terms of host plants and tending ants (Ceballos et al. 2024). Larvae have been found in association with E. tuberculatum on the EFN-bearing shrub Banisteriopsis malifolia (Alves-Silva et al. 2018) in Brazilian savanna. Caterpillars respond to antennation by E. tuberculatum foragers, everting the tentacle organs at the end of their abdomen. However, the interaction is apparently not stable, and the ants occasionally abandoned the caterpillars to forage elsewhere on the plant. No more than two individuals of E. tuberculatum have been reported attending a larva simultaneously (Alves-Silva et al. 2018). A photograph taken on November 12, 2002, in Trinidad and Tobago (Brasso Seco, Tunapuna-Piarco) (Deacon 2022) shows an association on a Fabaceae between a last-instar larva of S. calyce and a worker of E. tuberculatum standing nearby in an apparent protective posture typical of the species. Furthermore, a series of 12 photographs taken on September 23, 2023, at Sangre Grande, also in Trinidad and Tobago (Kaminski 2023d) illustrates a similar association with two E. tuberculatum workers, one of the photographs clearly showing the eversion of the tentacle organs under stimulation by Ectatomma. This same caterpillar was tended by Camponotus ants during the night, a daily rotation that is commonly observed in S. calyce. Two other series of five photographs each taken on March 21 and 23, 2023 in Brazil (Campus Universidad Federal de Alagoas, Maceió, Alagoas) illustrate the same association (Fig. 10), where both species, the ant and the caterpillar, simultaneously tend to the iceryine scale Crypticerya multicicatrices (Hemiptera: Monophlebidae) (Kaminski 2023c). However, this interaction was not stable, and the ants did not attach any importance to the caterpillar as, on other days, the same caterpillars were being attended to by two different species of Camponotus during the day and night. Interestingly, there is still no record of S. calyce everting ATOs during interactions with Ectatomma ants.
Synargis galena (Lemoniadina): The Galena Metalmark is distributed from French Guiana to Brazil (Minas Gerais) (GBIF.org 2023). This species occurs in open habitats and rocky outcrops, being abundant in the Brazilian “cerrado” savanna (Fig. 11a). Several observations of this species interacting with Ectatomma ants were made in the “cerrado”. An oviposition event was observed on old leaves of Bauhinia (Fabaceae) (Fig. 11b), on a plant occupied by E. brunneum. Another record of oviposition in Byrsonima sp. (Malpighiaceae) inflorescences close to E. tuberculatum ants was made on October 25, 2021 (Parque Mirante das Copaíbas, Brasilia, Distrito Federal, Brazil) (Fischer 2021) (see also Fig. 11c showing an egg of S. galena close to an E. tuberculatum worker). Larvae feed on Fabaceae, Malpighiaceae, Vochysiaceae, and Euphorbiaceae and have been found in Brazil in association with E. brunneum and E. tuberculatum (L.A. Kaminski pers. obs. in Pérez-Lachaud et al. 2021 Suppl. Mat., and Kaminski 2017, respectively). Three series of photographs taken in Brazil on March 30, 2013 (Goiás, Goiás) (Carreira 2024; Fig. 11d), on November 24, 2013 (Parque Nacional Chapada do Guimarães, Cuiaba, Mato Grosso) on an orchid tree (Bauhinia sp.) (Kaminski 2017, 2021b; Fig. 11f), and on November 26, 2013 (Diamantino, Mato Grosso) (Kaminski 2021c) illustrate different instars of S. galena caterpillars attended by E. tuberculatum workers (Fig. 11d, f) clearly feeding on fluids secreted by their TNOs, and their ATOs also were everted during interactions with ants (Fig. 11e). In the central Amazon (Coari, Amazonas, Brazil), in a clearing area, a probable cf. S. galena caterpillar attended by E. brunneum was recorded on a Dilleniaceae on June 28, 2018 (Kaminski 2024b). All records of immatures (n = 26) and oviposition events (n = 2) in four localities in Mato Grosso and Goiás, in the Brazilian “cerrado”, indicate that S. galena has obligate interactions with Ectatomma ants.
Habitat and life stages of Synargis galena in Brazilian “cerrado” savanna. a General view of the “cerrado rupestre” in Parque Nacional Chapada do Guimarães, Cuiaba, Mato Grosso, indicating places where interactions with Ectatomma tuberculatum (yellow stars) and Ectatomma brunneum (red star) were found. b Female egg laying on Bauhinia; note abdomen tip curved (arrow). c Egg close to an E. tuberculatum worker. d Last instar tended by workers of E. tuberculatum licking their TNOs in Brazil (Goiás, Goiás, March 30, 2013). e Last instar tended by E. brunneum. f Last instar tended by E. tuberculatum. Photos Lucas Kaminski (a–f) and Junia Carreira (d)
Synargis gela (Lemoniadina): The Hewitson’s Lemmark is found in Costa Rica (Puntarenas), Venezuela (Bolivar), Peru (Madre de Dios), and Brazil (Amazonas, Distrito Federal, Rondônia, north Mato Grosso, Alagoas) (GBIF.org 2023). Adults were found on edges and clearings of lowland secondary forests. Larvae feed on Stigmaphyllon spp. and Acalypha sp. in Costa Rica (DeVries 1997). In Ecuador, larvae have been found associated with E. tuberculatum on the leaves of Inga sp. (DeVries et al. 1992). A series of seven photographs taken on November 18, 2017, in Brazil (Alta Floresta, Mato Grosso) illustrate a fourth-instar larva of S. gela tended by E. tuberculatum on Inga sp.; this caterpillar was observed with tending ants during the day and night (Kaminski 2021d; Fig. 12e). Similarly, in southern Amazonia (Estação Ecológica Serra das Araras, Porto Estrela, Mato Grosso), an intermediate and a last instar were observed tended by E. tuberculatum on Inga sp. (Fig. 12a, b). This same last-instar caterpillar was observed in the prepupal phase at the base of the plant hosting the ant nest (Fig. 12c). Another series of three photographs taken on January 22, 2023, in French Guiana (Trésor Regional Nature Reserve, Roura, Cayenne) shows two workers of E. tuberculatum tending a larva (Fig. 12d), one of them collecting nectar from the TNOs (Park 2023). All records of caterpillars made during the day and night in different localities in Brazil, Ecuador, and French Guiana indicate that S. gela has obligate interactions with E. tuberculatum ants.
Interactions between Synargis gela and Ectatomma tuberculatum ants. a–c Caterpillars and ants on Inga sp. (Estação Ecológica Serra das Araras, Porto Estrela, Mato Grosso). a Two workers tending a third instar (arrow). b Last instar tended by four workers. c Last instar tended by one worker. d Two workers tending a fourth-instar caterpillar (Natural Regional Reserve ‘Trésor’, Roura, Cayenne, French Guyana, January 22, 2023. e Three workers tending a last-instar caterpillar, note everted tentacle nectary organ (arrow) (Cristalino Lodge, Alta Floresta, Mato Grosso, Brazil, November 19, 2017. Photos Lucas Kaminski (a–c, e) and Jonghyun Park (d)
Synargis mycone (Lemoniadina): Known as the Rusty Metalmark, this species is distributed from Mexico (Colima, Morelos, Nayarit, Oaxaca, Jalisco, Veracruz) to Ecuador (Loja) and Peru (Pirua). Larvae feed on Dilleniaceae, Caesalpiniaceae, Fabaceae, Sapindaceae, Euphorbiaceae, Polygalaceae, Polygonaceae, and Bignoniaceae and caterpillars are facultatively tended by several ant species (DeVries et al. 1992; DeVries 1997). Larvae were found associated with E. ruidum on the leaves of Pithecellobium sp. (Fabaceae), Paullinia fibrigera (Sapindaceae), Securidaca diversifolia (Polygalaceae), Phryganocydia corymbosa and Pachyptera kere (Asteraceae), Doliocarpus sp. and Tetracera sp. (Dilleniaceae) in Panama, and with E. tuberculatum on the leaves of Cassia fruticosa (Fabaceae) in Costa Rica and Panama, and leaves of Tetracera sp. and Omphalea diandra (Euphorbiaceae) in Panama (De Vries et al. 1992).
Thisbe irenea (Lemoniadina): The Sailor’s Lemmark is distributed from Mexico (Oaxaca, Veracruz) and Costa Rica (Alajuela) to Colombia, Brazil (Amazonas, Espírito Santo, Mato Grosso), and Trinidad. Larvae feed on Euphorbiaceae. Facultative mutualism with E. ruidum and E. tuberculatum on leaves of Croton billbergianus (Euphorbiaceae) has been reported in Panama where T. irenea larvae (third to fifth instars) appease their host using larval ant organs (VP, TNOs and ATOs), while the ants protect larvae against predatory vespid wasps but not against tachinid parasitoids (DeVries 1988, 1991a). Associations on the leaves of Croton spp. with E. ruidum have also been reported in Costa Rica, and with E. tuberculatum in Belize and Ecuador (DeVries et al. 1992). A pair of photographs (Fig. 13) taken on February 24, 2024, in French Guiana (Regional Nature Park of French Guiana, Roura, Cayenne) show three E. tuberculatum workers tending a larva of an unidentified species of Thisbe (probably T. irenea) on Croton sp. (Greene 2024).
Nymphalidae (Danainae, Ithomiini)
Forbestra olivencia (Mechanitina): The Olivencia Tigerwing or Olivencia Eyemark is found in shaded lowland rainforest between sea level and about 500 m. It is distributed from the northeastern province of Sucumbios in Ecuador to the Cochabamba department in Bolivia, and Mato Grosso do Sul province in Brazil (Hill 2006; GBIF.org 2023). Adults visit bird droppings and both Solanaceae and Asteraceae plants which are commonly patrolled by ants from several genera. Crematogaster spp. have been observed attacking and carrying away the lepidopteran eggs, while the presence of other ant species such as an unidentified Ectatomma sp. seems to favor female oviposition, or at least does not disturb it (Hill 2006). Eggs are laid singly, or in small clusters (2–4).
Mechanitis polymnia isthmia (Mechanitina): The Orange-spotted Tiger Clearwing or Disturbed Tigerwing is found in wet and dry forests from southern California in USA to Pichincha province in Ecuador, Bolivia, and Trinidad and Tobago (GBIF.org 2023). Females deposit large clusters of eggs (30–60) as a single mass on the upper side of a large leaf and larvae rest and feed gregariously (Young and Moffett 1979). The larvae feed on Solanum lancaeifolium, S. ochraceo-ferrugineum, S. hispidum, and S. jamaicense in Costa Rica and Panama (Young and Moffett 1979; Robbins and Aiello 1982). In Costa Rica, several E. ruidum workers have been observed patrolling for three consecutive days over stems and leaves where a group of first-instar M. polymnia isthmia larvae were present and engaged in a form of mutualistic interaction (Young 1978). The larvae were not attacked by the ants, but occasionally, E. ruidum workers approached a feeding larva and stroked it with their antennae, triggering the larva to momentarily withdraw from the leaf edge. The ants appeared to take advantage of the cuts made in the leaf by the lepidopteran larvae and the liquids exuding from the damaged tissue as they generally began feeding on the edge of a cut leaf almost immediately after the caterpillars had moved away or even pushed the larvae with their heads to dislodge them. However, no apparent protection is provided by E. ruidum against predatory Polybia wasps (Young 1978).
Discussion
Despite the small size of most Ectatomma colonies, their aggressiveness, and the fact that all the available information concerns only five of the 18 species of the genus (E. ruidum sp. 1 and sp. 2, E. tuberculatum, E. brunneum, and E. edentatum), we found numerous associations with lepidopteran species. However, all these associations occurred outside Ectatomma nests and no strictly obligate lepidopteran myrmecophiles, spending variable periods of their lives within ant nests, were recorded even if some species of Synargis (S. galena, S. gela) seem to have obligate interactions with Ectatomma ants. At least for the five species we researched, and for which we collected complete colonies, the fact that we have never found a single lepidopteran larva inside the nests could provide partial evidence that there is no social parasitic species of lepidopterans associated with these species. The hypothesis that Aricoris domina caterpillars are aphytophagous and complete their cycle inside Ectatomma ant nests needs to be confirmed (Robbins and Aiello 1982; Hall and Harvey 2002). Nonetheless, it remains true that no information exists for the other 13 species of the genus and that the presence of myrmecophilous lepidopterans in Ectatomma nests may have been deeply underestimated. In addition, the example of the association between Pseudonymphidia agave (Riodinidae) and its ponerine ant host Neoponera villosa (Pérez-Lachaud et al. 2021) has shown that, in some cases, a very large sampling effort is required to find a caterpillar inside an ant nest (only one specimen found in a colony out of 82 colonies collected).
A total of 29 lepidopteran species from 19 genera belonging to four tribes in three subfamilies and three families were found to be involved in at least 41 reliable associations (Table 1). Although very aggressive, several characteristics of the five Ectatomma species involved in these associations, such as their ecological dominance, local abundance, and wide distribution, may have led to the evolution of a variety of associations with other organisms, although their colonies are of medium size. Furthermore, as host–plant phenology and extrafloral nectar production can directly and indirectly mediate the abundance of facultative associations between ants and lepidopterans (e.g., Calixto et al. 2021), the known attraction of most Ectatomma species to EFNs (Lachaud 2021) may have been an important facilitating factor for the emergence of such associations (DeVries 1991a). Indeed, it seems quite significant that, unlike most ground-feeding Ectatomma ants, the five species involved in all the associations with lepidopterans reported here generally visit liquid food sources on foliage.
According to Pierce and Dankowicz (2022), around 17.6% of lycaenids and 4.5% of riodinids are reliably documented as obligate or facultative ant associates; however, based on the similarity of life histories between closely related taxa of the same genus, these authors proposed that the actual number of associations with ants could be at least 70% and 20%, respectively. Unsurprisingly, associations of Ectatomma species with these two lepidopteran families were also by far the most frequent, but the proportions were virtually reversed with 36.6% of associations with lycaenids and 58.5% with riodinids which, like Ectatomma, are mainly neotropical. Only two associations were found with butterflies of the Nymphalidae family. Although rare, these two associations seem particularly relevant since very few cases of symbiotic interactions between ants and butterflies are known outside the Lycaenidae and Riodinidae families. One of the few documented interactions involving Ectatomma and a Nymphalidae (Freitas and Oliveira 1992) describes a predator–prey type interaction, where Eunica bechina larvae are attacked and bitten by E. tuberculatum. The larvae of non-myrmecophilous butterflies are frequently preyed upon or removed from host plants by foraging ants (Freitas and Oliveira 1992), but even in groups known to establish close relationships with ants, such as lycaenid and riodinid butterflies, various examples of attacks by Ectatomma workers have been recorded (Ross 1966; Bächtold and Alves-Silva 2013; Kaminski 2024c). Although they do not produce any rewards for ants, the caterpillars of Forbestra olivencia and Mechanitis polymnia isthmia display certain morphological and behavioral traits that could allow co-occurrence with Ectatomma ants. The body of first-instar larvae is sparsely covered with setae in F. olivencia (Hill 2006) and Mechanitis caterpillars are known to overcome plant defenses through social facilitation and silk trails production (Despland 2019), which may also protect them from ant attack. Furthermore, it has been demonstrated that Mechanitis lysimnia caterpillars use chemical camouflage as a defense against ants (Portugal and Trigo 2005). Bleeding and regurgitation reflex or the ability to drag lines to escape may make it easier for older caterpillars to occupy plants visited by Ectatomma ants. In addition, older caterpillars have conspicuous warning color patterns that may deter ants as some Ectatomma are known to learn cues associated with risky prey (Hénaut et al. 2014). Mimicry is suspected in F. olivencia (Willmott et al. 2011) while adults of this species and those of Mechanitis follow army ants and feed on bird droppings (Ray and Andrews 1980; Hill 2006). According to Hill (2006), the association involving F. olivencia resembles a case of oviposition facilitation in adult females. In contrast, the association involving M. polymnia isthmia and E. ruidum appears to be much more complex, with direct interactions between ants and butterfly larvae, and a contact-behavioral response exchange suggesting a preliminary step toward a true mutualistic interaction (Young 1978). To our knowledge, no similar interaction between other ant species and Nymphalidae caterpillars has ever been reported.
A least 25 of the 29 lepidopteran species found to be associated with Ectatomma ants (86.2%) (Table 1) exhibit several behavioral and/or morphological adaptations related to myrmecophily (presence of specialized organs producing exudates and specialized setae, ability to produce sounds or vibrations). In numerous cases, reports found in the literature or photographs available on the Internet only provide us with sufficient information to note the co-occurrence of a lepidopteran larva with one or several Ectatomma workers, without providing details on the type of interactions between the two organisms (see for example the associations with Tmolus, Nymphidium, Parvospila, Stalachtis). However, with the possible exception of the observations concerning E. tuberculatum and both Parvospila lucianus (Ramdeen 2021) and Calycopis sp. (Sanz-Veiga et al. 2017), where the association is not well established and could represent only to a momentary, occasional proximity between two insects, the other cases clearly involve Ectatomma workers that do not display aggressive behavior toward lepidopterans. Instead, they explore and walk on the eggs or larvae or ignore them completely. In some cases, Ectatomma workers exhibit typical protective behavior, as observed in the association between E. tuberculatum and Synargis calyce (Deacon 2022). In this case, the worker adopts a characteristic posture (front legs extended, head and thorax raised, antennae and mandibles held wide open) similar to that of guards defending the nest or foragers protecting a food source (Dejean and Lachaud 1992; Sanz-Veiga et al. 2017). Nevertheless, in 13 cases involving 9 lepidopteran species (A. lincoides, P. polibetes, R. palegon, T. terentia, E. elvina, S. calyce, S. galena, S. gela, T. irenea), a reliable facultative mutualistic association could be established between butterfly caterpillars and Ectatomma workers based on the secretion of attractive fluids through their nectary organs (DNO or TNOs). Some associations (around 15%) where no direct symbiotic interaction was observed in the field (A. strophius and A. domina with E. tuberculatum, S. galena with E. brunneum and E. tuberculatum, and likely T. echion, A. domina, and F. olivencia with an unidentified Ectatomma species) involve only a form of oviposition facilitation (cues for enemy-free space) without direct interaction with ants as ant presence correlates with an increase in the number of eggs found in branches patrolled by Ectatomma workers. Finally, in A. separata and M. polymnia isthmia, there is no direct protection of the caterpillars by the ants, but the association allows the ants to feed on the liquids exuding from the damaged tissues of leaves cut by the caterpillars.
The majority of new data presented in this review –covering new associations, such as E. tuberculatum with A. separata and P. lucianus, E. brunneum with P. polibetes, and E. ruidum with N. caricae, as well as new information on direct mutualistic interaction involving E. tuberculatum with S. calyce, S. galena, and S. gela—come from unpublished records in online photographic databases, especially iNaturalist. The use of citizen science projects (community science) as an inexpensive and effective way of studying large areas is becoming increasingly important as more data is generated. Although unstructured citizen science data has several drawbacks and biases compared to semi-structured data (e.g., Caley et al. 2020; Callaghan et al. 2021; Geurst et al. 2023; Skvarla and Fisher 2023), datasets comprising species observations or biological records are an immensely valuable source of information for tracking trends in insect distributions, for example (Rousselet et al. 2013, Suzuki-Ohno et al. 2017, Hochmair et al. 2020, and Cull 2022), and helping to depict trophic interactions. A bias toward diurnal ants both in research studies and in data generated by community science has certainly influenced our literature and online surveys. With the exception of E. tuberculatum (McCluskey 1987; Valenzuela et al. 1995), Ectatomma ants are predominantly diurnal foragers, although active diurnal and nocturnal foragers are also known in E. ruidum (Lachaud 1990; Esch et al. 2017), E. edentatum, and E. brunneum (Cogni et al. 2000). Species that forage during the day are more likely to be recorded than those that are active at night, and large species are more readily perceived by the layman (most interactions in iNaturalist involve E. tuberculatum, for example). Other mutualistic networks, for example those formed by protective ants and plants, may also be underestimated by daytime sampling alone (Dáttilo et al. 2014).
Myrmecophily has arisen multiple times across the Lepidoptera (Espeland et al. 2018) and co-evolution with ants is known in both true butterflies and moths (e.g., Dejean et al. 2016, 2017), but we have found no records of moth families, or their caterpillars, associated with Ectatomma ants. There is an urgent need to intensify field observation efforts including nocturnal sampling and diversification of the ant species studied to gain a broader overview of the diversity of organisms associated with a given genus.
Data availability
Not applicable.
References
Aguilar-Velasco RG, Poteaux C, Meza-Lázaro R, Lachaud J-P, Dubovikoff D, Zaldívar-Riverón A (2016) Uncovering species boundaries in the neotropical ant complex Ectatomma ruidum (Ectatomminae) under the presence of nuclear mitochondrial paralogues. Zool J Linn Soc 178(2):226–240
Als TD, Vila R, Kandul NP, Nash DR, Yen S-H, Hsu Y-F, Mignault AA, Boomsma JJ, Pierce NE (2004) The evolution of alternative parasitic life histories in large blue butterflies. Nature 432(7015):386–390
Alves-Silva E, Bächtold A, Del-Claro K (2018) Florivorous myrmecophilous caterpillars exploit an ant–plant mutualism and distract ants from extrafloral nectaries. Austral Ecol 43(6):643–650
Antonialli WF Jr, Giannotti E (2001) Nest architecture and population dynamics of the ponerine ant Ectatomma edentatum (Hymenoptera: Formicidae). Sociobiology 38(3A):475–486
Antonialli-Junior WF, Giannotti E (1997) Nest architecture and populations dynamics of the ponerine ant, Ectatomma opaciventre Roger (Hymenoptera: Formicidae). J Adv Zool 18(2):64–71
Atsatt PR (1981a) Ant-dependent food plant selection by the mistletoe butterfly Ogyris amaryllis (Lycaenidae). Oecologia 48(1):60–63
Atsatt PR (1981b) Lycaenid butterflies and ants: selection for enemy-free space. Am Nat 118(5):638–654
Bächtold A, Alves-Silva E (2013) Behavioral strategy of a lycaenid (Lepidoptera) caterpillar against aggressive ants in a Brazilian savanna. Acta Ethol 16(2):83–90
Bächtold A, Alves-Silva E, Kaminski LA, Del-Claro K (2014) The role of tending ants in host plant selection and egg parasitism of two facultative myrmecophilous butterflies. Naturwissenschaften 101(11):913–919
Bächtold A, Alves-Silva E, Del-Claro K (2016) Ants, plant characteristics and habitat conservation status affect the occurrence of myrmecophilous butterflies on an extrafloral nectaried Malpighiaceae. Stud Neotrop Fauna Environ 51(2):112–120
Bächtold A, Alves-Silva E, Del-Claro K (2017) Ant-related oviposition is not associated to low parasitism of he myrmecophilous butterfly Allosmaitia strophius in an extrafloral nectaried shrub. Acta Oecol 83(1):15–21
Ballmer GR, Pratt GF (1991) Quantification of ant attendance (myrmecophily) of Lycaenid larvae. J Res Lepid 30(1–2):95–112
Blüthgen N, Fiedler K (2004) Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. J Anim Ecol 73(1):155–166
Brévignon C, Gallard J-Y (1999) Inventaire des Riodinidae de Guyane Française. VI- Riodinidae: Nymphidini Stalachtini. Description de nouveaux genres. Lambillionea 99(2):277–290
Caley P, Welvaert M, Barry SC (2020) Crowd surveillance: estimating citizen science reporting probabilities for insects of biosecurity concern. J Pest Sci 93(1):543–550
Calixto ES, Novaes LR, dos Santos DFB, Lange D, Moreira X, Del-Claro K (2021) Climate seasonality drives ant–plant–herbivore interactions via plant phenology in an extrafloral nectary-bearing plant community. J Ecol 109(2):639–651
Callaghan CJ (2001) New riodinids from the Central Brazilian plateau (Lepidoptera, Riodinidae). Rev Bras Zool 18(3):765–778
Callaghan CT, Poore AG, Hofmann M, Roberts CJ, Pereira HM (2021) Large-bodied birds are over-represented in unstructured citizen science data. Sci Rep 11:19073
Carreira JYO (2024) iNaturalist observation: https://www.inaturalist.org/observations/205532324. Accessed on 9 April 2024
Casacci LP, Bonelli S, Balletto E, Barbero F (2019) Multimodal signaling in myrmecophilous butterflies. Front Ecol Evol 7:454
Ceballos-González AV, da Silva RC, Lima LD, Kaminski LA, Turatti ICC, Lopes NP, do Nascimento FS (2024) Influence of host plants and tending ants on the cuticular hydrocarbon profile of a generalist myrmecophilous caterpillar. J Chem Ecol 50(5–6):222–236
Cogni R, Raimundo RLG, Freitas AVL (2000) Daily activity of ants associated with the extrafloral nectaries of Turnera ulmifolia L. (Turneraceae) in a suburban area in southeast Brazil. Entomol Month Mag 136:141–147
Cottrell CB (1984) Aphytophagy in butterflies: its relationship to myrmecophily. Zool J Linn Soc 80(1):1–57
Cull B (2022) Monitoring trends in distribution and seasonality of medically important ticks in North America using online crowdsource records from iNaturalist. Insects 13:404
Dáttilo W, Fagundes R, Gurka CAQ, Silva MSA, Vieira MCL, Izzo TJ, Díaz-Castelazo C, Del-Claro K, Rico-Gray V (2014) Individual-based ant–plant networks: diurnal-nocturnal structure and species-area relationship. PLoS One 9(6):e99838
De Vries PJ (1988) The larval ant-organs of Thisbe irenea (Lepidoptera: Riodinidae) and their effects upon attending ants. Zool J Linn Soc 94(4):379–393
De Vries PJ (1990) Enhancement of symbioses between butterfly caterpillars and ants by vibrational communication. Science 248(4959):1104–1106
De Vries PJ (1991a) Mutualism between Thisbe irenea butterflies and ants, and the role of ant ecology in the evolution of larval-ant associations. Biol J Linn Soc 43(3):179–195
De Vries PJ (1991b) Call production by myrmecophilous riodinid and lycaenid butterfly caterpillars (Lepidoptera): morphological, acoustical, functional, and evolutionary patterns. Am Mus Novit 26(3025):1–23
De Vries PJ (1997) The butterflies of Costa Rica and their natural history. Vol II: Riodinidae. Princeton University Press, Princeton
De Vries PJ, Baker I (1989) Butterfly exploitation of an ant–plant mutualism: adding insult to herbivory. J NY Entomol Soc 97(3):332–340
De Vries PJ, Chacon IA, Murray D (1992) Toward a better understanding of host use and biodiversity in riodinid butterflies (Lepidoptera). J Res Lepid 31(1–2):103–126
Deacon A (2022) iNaturalist observation: https://www.inaturalist.org/observations/141948034. Accessed on 9 April 2024
Dejean A, Lachaud J-P (1992) Growth-related changes in predation behavior in incipient colonies of the ponerine ant Ectatomma tuberculatum (Olivier). Insectes Soc 39(2):129–143
Dejean A, Orivel J, Azémar F, Hérault B, Corbara B (2016) A cuckoo-like parasitic moth leads African wearver ants to their ruin. Sci Rep 6:23778
Dejean A, Azémar F, Libert M, Compin A, Hérault B, Orivel J, Bouyer T, Corbara B (2017) Ant–lepidopteran associations along African forest edges. Sci Nat 104:7
Despland E (2019) Caterpillars cooperate to overcome plant glandular trichome defenses. Front Ecol Evol 7:232
Dresch F (2021) História natural e morfologia dos estágios imaturos da borboleta formigueira-zebrada Arawacus separata (Lathy, 1926) (Lepidoptera: Lycaenidae). Bachelor dissertation, Universidade Federal do Rio Grande do Sul, Porte Alegre, Brazil
Duarte M, Robbins RK (2009) Immature stages of Calycopis bellera (Hewitson) and C. janeirica (Felder) (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): taxonomic significance and new evidence for detritivory. Zootaxa 2325(1):39–61
Duarte M, Robbins RK, Mielke OHH (2005) Immature stages of Calycopis caulonia (Hewitson, 1877) (Lepidoptera, Lycaenidae, Theclinae, Eumaeini), with notes on rearing detritivorous hairstreaks on artificial diet. Zootaxa 1063(1):1–31
Esch C, Jimenez JP, Peretz C, Uno H, O’Donnell S (2017) Thermal tolerances differ between diurnal and nocturnal foragers in the ant Ectatomma ruidum. Insect Soc 64(3):439–444
Espadaler X, Pérez Hidalgo N, Villalobos Muller W (2012) Ant–aphid relations in Costa-Rica, Central America (Hymenoptera: Formicidae; Hemiptera: Aphididae). Sociobiology 59(3):959–970
Espeland M, Breinholt J, Willmott KR, Warren AD, Vila R, Toussaint EFA, Maunsell SC, Aduse-Poku K, Talavera G, Eastwood R, Jarzyna MA, Guralnick R, Lohman DJ, Pierce NE, Kawahara AY (2018) A comprehensive and dated phylogenomic analysis of butterflies. Curr Biol 28:770–778
Feitosa RM, Hora RR, Delabie JHC, Valenzuela J, Fresneau D (2008) A new social parasite in the ant genus Ectatomma F. Smith (Hymenoptera, Formicidae, Ectatomminae). Zootaxa 1713(1):47–52
Fiedler K (1991) Systematic, evolutionary, and ecological implications of myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papilionoidea). Bonn Zool Monogr 31:1–210
Fiedler K (1998) Lycaenid-ant interactions of the Maculinea type: tracing their historical roots in a comparative framework. J Insect Conserv 2(1):3–14
Fiedler K (2012) The host genera of ant-parasitic Lycaenidae butterflies: a review. Psyche 2012:153975
Fiedler K (2021) The ant associates of Lycaenidae butterfly caterpillars—revisited. Nota Lepid 44:159–174
Fierro-Minda A (2022) iNaturalist observation https://www.inaturalist.org/observations/119078163. Accessed on 10 July 2024
Fischer GA (2021) iNaturalist observation https://www.inaturalist.org/observations/99410619. Accessed on 10 July 2024
Freitas AVL, Oliveira PS (1992) Biology and behavior of the neotropical butterfly Eunica bechina (Nymphalidae) with special reference to larval defense against ant predation. J Res Lepid 31(1–2):1–11
GBIF.org (2023) GBIF Home page. Available at https://www.gbif.org. last accessed [09/12/2023]
Geurts EM, Reynolds JD, Starzomski BM (2023) Not all who wander are lost: trail bias in community science. PLoS One 18(6):e0287150
Glassberg J, Robbins RK (2005) Cafe con leche in a Mexican forest: a rare hairstreak makes a guest appearance in Mexico. Am Butterflies 13(3):47
Greene A (2024) iNaturalist observation: https://www.inaturalist.org/observations/200582687. Accessed on 9 April 2024
Guerra R (2017) Stalachtis plegia (Cramer, 1779). https://www.flickr.com/photos/142712970@N03/38713147222. Accessed on 10 July 2024
Hall JPW (2018) A monograph of the Nymphidiina (Lepidoptera: Riodinidae: Nymphidiini): phylogeny, taxonomy, biology, and biogeography. Entomological Society of Washington, Washington
Hall JPW, Harvey DJ (2002) Basal subtribes of the Nymphidiini (Lepidoptera: Riodinidae): phylogeny and myrmecophily. Cladistics 18(6):539–569
Hénaut Y, Machkour-M’Rabet S, Lachaud J-P (2014) The role of learning in risk-avoidance strategies during spider-ant interactions. Anim Cogn 17(2):185–195
Henning SF (1983) Biological groups within the Lycaenidae (Lepidoptera). J Entomol Soc South Afr 46(1):65–85
Hill RI (2006) Life history and biology of Forbestra olivencia (Bates, 1862) (Nymphalidae, Ithomiinae). J Lepid Soc 60(4):203–210
Hill DS (2008) Pests of crops in warmer climates and their control. Springer, Dordrecht
Hochmair HH, Scheffrahn RH, Basille M, Boone M (2020) Evaluating the data quality of iNaturalist termite records. PLoS One 15(5):e0226534
Hölldobler B, Kwapich CL (2022) The guests of ants: how myrmecophiles interact with their hosts. Harvard University Press, Cambridge
Hooks CR, Pandey RR, Johnson MW (2003) Impact of avian and arthropod predation on lepidopteran caterpillar densities and plant productivity in an ephemeral agroecosystem. Ecol Entomol 28(5):522–532
Horvitz CC, Turnbull C, Harvey DJ (1987) Biology of immature Eurybia elvina (Lepidoptera: Riodinidae), a myrmecophilous metalmark butterfly. Ann Entomol Soc Am 80(4):513–519
Ibarra-Isassi J, Oliveira PS (2018) Indirect effects of mutualism: ant–treehopper associations deter pollinators and reduce reproduction in a tropical shrub. Oecologia 186(3):691–701
Ibarra-Núñez G, García JA, López JA, Lachaud J-P (2001) Prey analysis in the diet of some ponerine ants (Hymenoptera: Formicidae) and web-building spiders (Araneae) in coffee plantations in Chiapas, Mexico. Sociobiology 37(3B):723–755
Kaminski LA (2017) Formigas, besouros e lepidópteros. In: Diehl E (org) Interações das formigas com outros organismos: diversidade ecológica e evolutiva. Oikos, São Leopoldo, RS, Brasil, pp 51–65
Kaminski LA (2021a) Ant–butterfly interactions - Borboletas formigueiras. iNaturalist. https://www.inaturalist.org/projects/ant-butterfly-interactions-borboletas-formigueiras. Accessed on 10 July 2024
Kaminski LA (2021b) iNaturalist observation: https://www.inaturalist.org/observations/73228802. Accessed on 9 April 2024
Kaminski LA (2021c) iNaturalist observation: https://www.inaturalist.org/observations/73234265. Accessed on 9 April 2024
Kaminski LA (2021d) iNaturalist observation: https://www.inaturalist.org/observations/78139820. Accessed on 9 April 2024
Kaminski LA (2023a) iNaturalist observation: https://www.inaturalist.org/observations/166232155. Accessed on 9 April 2024
Kaminski LA (2023b) iNaturalist observation: https://www.inaturalist.org/observations/185775910. Accessed on 9 April 2024 and https://www.inaturalist.org/observations/228438840. Accessed on 10 July 2024
Kaminski LA (2023c) iNaturalist observations: https://www.inaturalist.org/observations/151942976 and https://www.inaturalist.org/observations/152067077. Accessed on 9 April 2024
Kaminski LA (2023d) iNaturalist observation: https://www.inaturalist.org/observations/184572788. Accessed on 9 April 2024
Kaminski LA (2024a) iNaturalist observation: https://www.inaturalist.org/observations/205707238#identification-7b4478a6-233e-4e57-a2ca-5d79440e3973. Accessed on 9 April 2024
Kaminski LA (2024b) iNaturalist observation: https://www.inaturalist.org/observations/228381796. Accessed on 10 July 2024
Kaminski LA (2024c) iNaturalist observation https://www.inaturalist.org/observations/228136123. Accessed on 10 July 2024
Kaminski LA, Freitas AVL (2010) Natural history and morphology of immature stages of the butterfly Allosmaitia strophius (Godart) (Lepidoptera: Lycaenidae) on flower buds of Malpighiaceae. Stud Neotrop Fauna Environ 45(1):11–19
Kaminski LA, Carvalho-Filho FS (2012) Life history of Aricoris propitia (Lepidoptera: Riodinidae)—A myrmecophilous butterfly obligately associated with fire ants. Psyche 2012:126876
Kaminski LA, Freitas AVL, Oliveira PS (2010) Interaction between mutualisms: ant-tended butterflies exploit enemy-free space provided by ant–treehopper associations. Am Nat 176(3):322–334
Kaminski LA, Rodrigues D, Freitas AVL (2012) Immature stages of Parrhasius polibetes (Lepidoptera: Lycaenidae): host plants, tending ants, natural enemies and morphology. J Nat Hist 46(11–12):645–667
Kaminski LA, Volkmann L, Callaghan CJ, DeVries PJ, Vila R (2021) The first known riodinid ‘cuckoo’ butterfly reveals deep-time convergence and parallelism in ant social parasites. Zool J Linn Soc 193(3):860–879
Katayama N, Hembry DH, Hojo MK, Suzuki N (2013) Why do ants shift their foraging from extrafloral nectar to aphid honeydew? Ecol Res 28(5):919–926
Kawakami K (2022) iNaturalist observation: https://www.inaturalist.org/observations/111006435. Accessed on 9 April 2024
Kistner DH (1982) The social insects’ bestiary. In: Hermann HR (ed) Social insects, vol 3. Academic Press, New York, pp 1–244
Kronauer DJC (2004) Trophic parasitism of a wasp (Hymenoptera: Ampulicidae: Ampulex sp.) on the ant Ectatomma ruidum (Roger, 1860) (Hymenoptera: Formicidae). Myrmecol News 6:77–78
Kugler C, Brown WL Jr (1982) Revisionary and other studies on the ant genus Ectatomma, including the descriptions of two new species. Search Agric Ithaca 24:1–8
Kursar TA, Wolfe BT, Epps MJ, Coley PD (2006) Food quality, competition, and parasitism influence feeding preference in a neotropical lepidopteran. Ecology 87(12):3058–3069
Lachaud J-P (1990) Foraging activity and diet in some neotropical ponerine ants. I. Ectatomma ruidum Roger (Hymenoptera, Formicidae). Folia Entomol Mex 78:241–256
Lachaud J-P (2021) Ectatomma. In: Starr C (ed) Encyclopedia of social insects. Springer, Cham, pp 358–365
Lachaud J-P, Pérez-Lachaud G (2012) Diversity of species and behavior of hymenopteran parasitoids of ants: a review. Psyche 2012:134746
Lachaud J-P, Pérez-Lachaud G (2015) Ectaheteromorph ants also host highly diverse parasitic communities: a review of parasitoids of the Neotropical genus Ectatomma. Insectes Soc 62(2):121–132
Lachaud J-P, Lenoir A, Witte V (eds) (2012) Ants and their parasites. Psyche special issue. Hindawi Publishing Corporation, New York
Lachaud J-P, Lenoir A, Hughes DP (eds) (2013) Ants and their parasites 2013. Psyche special issue. Hindawi Publishing Corporation, New York
Lapèze J (2021) Guide Illustré des Membracides de Guyane. Version 3, Avril 2021. Inventaire National du Patrimoine Naturel
Leal IR, Fischer E, Kost C, Tabarelli M, Wirth R (2006) Ant protection against herbivores and nectar thieves in Passiflora coccinea flowers. Ecoscience 13(4):431–438
Leighton GRM, Hugo PS, Roulin A, Amar A (2016) Just Google it: assessing the use of Google Images to describe geographical variation traits of organisms. Meth Ecol Evol 7(9):1060–1070
Lima LD, Trigo JR, Kaminski LA (2021) Chemical convergence between a guild of facultative myrmecophilous caterpillars and host plants. Ecol Entomol 46(1):66–75
Magaldi LM, Kaminski LA, Seraphim N, Azeredo-Espin AM, Silva-Brandão KL, Freitas AVL (2021) Hidden in the wing dots: disentangling mimetic sister species of butterflies (Riodinidae: Stalachtis) with an integrative approach. Zool Anz 294:92–99
Majer JD, Delabie JHC, Smith MRB (1994) Arboreal ant community patterns in Brazilian cocoa farms. Biotropica 26(1):73–83
Malicky H (1969) Versuch einer Analyse der ökologischen Beziehungen zwischen Lycaeniden (Lepidoptera) und Formiciden (Hymenoptera). Tijdschr Entomol 112(8):213–298
Malicky H (1970) New aspects of the association between lycaenid larvae (Lycaenidae) and ants (Formicidae, Hymenoptera). J Lepid Soc 24(3):190–202
McCluskey ES (1987) Cyrcadian rhythm in the tropical ant Ectatomma (Hymenoptera: Formicidae). Psyche 94(3–4):245–251
Melati BG, Leal LC (2018) Aggressive bodyguards are not always the best: preferential interaction with more aggressive ant species reduces reproductive success of plant bearing extrafloral nectaries. PLoS One 13(6):e199764
Mexzón RG, Chinchilla CM (2000) Especies vegetales atrayentes de la entomofauna benéfica en plantaciones de palma aceitera (Elaeis guineensis Jacq.) en Costa Rica. ASD Oil Palm Pap (Costa Rica) 19:23–39
Meza-Lázaro RN, Poteaux C, Bayona-Vásquez NJ, Branstetter MG, Zaldívar-Riverón A (2018) Extensive mitochondrial heteroplasmy in the neotropical ants of the Ectatomma ruidum complex (Formicidae: Ectatomminae). Mitochond DNA Part A 29(8):1203–1214
Meza-Lázaro RN, Peña-Carrillo KI, Poteaux C, Lorenzi MC, Wetterer JK, Zaldívar-Riverón A (2022) Genome and cuticular hydrocarbon-based species delimitation shed light on potential drivers of speciation in a Neotropical ant species complex. Ecol Evol 12(3):e8704
Monteiro RF (1991) Cryptic larval polychromatism in Rekoa marius Lucas and R. palegon Cramer (Lycaenidae: Theclinae). J Res Lepid 29(1–2):77–84
Monteiro RF (2000) Coloração críptica e padrão de uso de plantas hospedeiras em larvas de duas epécies mirmecófilas de Rekoa Kaye (Lepidoptera, Lycaenidae). In: Martins RP, Lewinsohn TM, Barbeitos MS (eds), Ecologia e comportamento de Insetos. Ser. Oecologia Brasiliensis, vol. 8, PPGE-UFRJ, Rio de Janeiro, Brasil, pp 259–280
Mota LL, Oliveira PS (2016) Myrmecophilous butterflies utilise ant–treehopper associations as visual cues for oviposition. Ecol Entomol 41(3):338–343
Nettel-Hernanz A, Lachaud J-P, Fresneau D, López-Muñoz RA, Poteaux C (2015) Biogeography, cryptic diversity, and queen dimorphism evolution of the Neotropical ant genus Ectatomma Smith, 1958 (Formicidae, Ectatomminae). Org Divers Evol 15(3):543–553
Nicolay SS (1979) A review of the Hubnerian genus Parrhasius and description of a new genus Michaelus (Lycaenidae: Eumaeini). Bull Allyn Mus 56:1–51
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
Nyffeler M, Şekercioğlu ÇH, Whelan CJ (2018) Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci Nat 105:47
Oliveira PS, Del-Claro K (2005) Multitrophic interactions in a neotropical savanna: ant–hemipteran systems, associated insect herbivores and a host plant. In: Burslem DFRP, Pinard MA, Hartley SE (eds) Biotic interactions in the tropics: their role in the maintenance of species diversity. Cambridge University Press, Cambridge, pp 414–438
Park J (2023) iNaturalist observation: https://www.inaturalist.org/observations/190974469 Accessed on 9 April 2024
Parmentier T, Dekoninck W, Wenseleers T (2014) A highly diverse microcosm in a hostile world: a review on the associates of red wood ants (Formica rufa group). Insectes Soc 61(3):229–237
Peña-Carrillo KI, Poteaux C, Leroy C, Meza-Lázaro RN, Lachaud J-P, Zaldívar-Riverón A, Lorenzi MC (2021) Highly divergent cuticular hydrocarbon profiles in the cleptobiotic ants of the Ectatomma ruidum species complex. Chemoecology 31(2):125–135
Peña Carrillo KI, Lorenzi MC, Brault M, Devienne P, Lachaud J-P, Pavan G, Poteaux C (2022) A new putative species in the Ectatomma ruidum complex (Formicidae: Ectatomminae) produces a species-specific distress call. Bioacoustics 31(3):332–347
Pérez-Lachaud G, Klompen H, Poteaux C, Santamaría C, Armbrecht I, Beugnon G, Lachaud J-P (2019) Context dependent life-history shift in Macrodinychus sellnicki mites attacking a native ant host in Colombia. Sci Rep 9:8394
Pérez-Lachaud G, Rocha FH, Pozo C, Kaminski LA, Seraphim N, Lachaud J-P (2021) A new ant–butterfly symbiosis in the forest canopy fills an evolutionary gap. Sci Rep 11:20770
Perfecto I (1990) Indirect and direct effects in a tropical agroecosystem: the maize-pest-ant system in Nicaragua. Ecology 71(6):2125–2134
Pierce NE (1995) Predatory and parasitic Lepidoptera: carnivores living on plants. J Lepid Soc 49(4):412–453
Pierce NE, Dankowicz E (2022) Behavioral, ecological and evolutionary mechanisms underlying caterpillar-ant symbioses. Curr Opin Insect Sci 52:100898
Pierce NE, Elgar MA (1985) The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous lycaenid butterfly. Behav Ecol Sociobiol 16(3):209–222
Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771
Pomerantz A (2015) Mystery of the yellow bulbs: Discovery of a new caterpillar–ant-parasitic plant relationship. Entomol Today. https://entomologytoday.org/2015/12/07/caterpillar-depends-on-parasitic-plants-and-nectar-drinking-ants/ Accessed on 9 April 2024
Portugal AHA, Trigo JR (2005) Similarity of cuticular lipids between a caterpillar and its host plant: a way to make prey undetectable for predatory ants? J Chem Ecol 31(11):2551–2561
Quicke DLJ (2017) Mimicry, crypsis, masquerade and other adaptive resemblances. Wiley-Blackwell, Hoboken
Ramdeen B (2021) iNaturalist observation: https://www.inaturalist.org/observations/92856871 Accessed on 9 April 2024
Ray TS, Andrews CC (1980) Antbutterflies: butterflies that follow army ants to feed on antbird droppings. Science 210(4474):1147–1148
Rettenmeyer CW, Rettenmeyer ME, Joseph J, Berghoff SM (2011) The largest animal association centered on one species: the army ant Eciton burchellii and its more than 300 associates. Insectes Soc 58(3):281–292
Robbins RK (1991a) Cost and evolution of facultative mutualism between ants and lycaenid larvae (Lepidoptera). Oikos 62(3):363–369
Robbins RK (1991b) Evolution, comparative morphology, and identification of the eumaeine butterfly genus Rekoa Kaye (Lyceaenidae: Theclinae). Smithson Contrib Zool 498:1–64
Robbins RK, Aiello A (1982) Foodplant and oviposition records for Panamanian Lycaenidae and Riodinidae. J Lepid Soc 36(2):65–75
Robbins RK, Cong Q, Zhang J, Shen J, Busby RC, Faynel C, Duarte M, Martins ARP, Prieto C, Lamas G, Grishin NV (2022) Genomics-based higher classification of the species-rich hairstreaks (Lepidoptera: Lycaenidae: Eumaeini). Syst Entomol 47(3):445–469
Roberts CJ, Vergés A, Callaghan CT, Poore AGB (2022) Many cameras make light work: opportunistic photographs of rare species in iNaturalist complemented structured surveys of reef fish to better understand species richness. Biodivers Conserv 31(4):1407–1425
Ross GN (1966) Life-history studies on Mexican butterflies. IV. The ecology and ethology of Anatole rossi, a myrmecophilous metalmark (Lepidoptera: Riodinidae). Ann Entomol Soc Am 59(5):985–1004
Rousselet J, Imbert C-E, Dekri A, Garcia J, Goussard F, Vincent B, Denux O, Robinet C, Dorkeld F, Roques A, Rossi J-P (2013) Assessing species distribution using Google Street View: a pilot study with the pine processionary moth. PLoS One 8(10):e74918
Santos-Murgas A, Cambra TRA, Lanuza-Garay A, Cobos-Hernández RM, Osorio-Arenas MA (2020) Observaciones biológicas de larvas y pupas de Rekoa marius (Lucas) (Lepidoptera: Lycaenidae) en Panamá. Rev Chil Entomol 46(4):653–660
Sanz-Veiga PA, Ré Jorge L, Benitez-Vieyra S, Amorim FW (2017) Pericarpial nectary-visiting ants do not provide fruit protection against pre-dispersal seed predators regardless of an species composition and resource availability. PLoS One 12(12):e0188445
Schatz B, Lachaud J-P (2008) Effect of high nest density on spatial relationships in two dominant ectatommine ants (Hymenoptera: Formicidae). Sociobiology 51(3):623–643
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Schönrogge K, Barbero F, Casacci LP, Settele J, Thomas JA (2017) Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim Behav 134:249–256
Sepp J (1848) Surinaamsche vlinders. Papillons de Surinam. Amsterdam, Sepp JC en zoon [1848–1852]
Seraphim N, Kaminski LA, DeVries PJ, Penz C, Callaghan C, Wahlberg N, Silva-Brandão KL, Freitas AVL (2018) Molecular phylogeny and higher systematics of the metalmark butterflies (Lepidoptera: Riodinidae). Syst Entomol 43(2):407–425
Siddiqui SA, Ngah N, Eddy-Doh AM, Ucak I, Afreen M, Fernando I, Singh S, Shah MA, Povetkin SN, Castro-Muños R (2023) Edible Lepidoptera as human foods—a comprehensive review. J Insects Food Feed 10(1):25–49
Silva NAP, Duarte M, Diniz IR, Morais HC (2011) Host plants of Lycaenidae on inflorescences in central Brazilian cerrado. J Res Lepid 44:95–105
Silva NAP, Duarte M, Araújo EB, Morais HC (2014) Larval biology of anthophagous Eumaeini (Lepidoptera: Lycaenidae, Theclinae) in the cerrado of Central Brazil. J Insect Sci 14:184
Skvarla MJ, Fisher JR (2023) Online community photo-sharing in entomology: a large-scale review with suggestions on best pratices. Ann Entomol Soc Am 116(5):276–304
Smiley JT (2013) Color in the rainforest: passionflower flea beetles and butterflies. http://lsfleabeetles.blogspot.com/2013/11/back-at-la-selva-its-nice-to-be-back.html Accessed on 9 April 2024
Smiley JT (unpubl) Passiflora auriculata. http://johnterahsmiley.com/heliconius-passiflora-flea%20beetle/passiflora/auriculata/auriculata.html Accessed on 9 April 2024
Suzuki-Ohno Y, Yokoyama J, Nakashizuka T, Kawata M (2017) Utilization of photographs taken by citizens for estimating bumblebee distributions. Sci Rep 7:11215
Szentivanyi T, Vincze O (2022) Tracking wildlife diseases using community science: an example through toad myasis. Eur J Wildl Res 68:74
Torréns J (2013) A review of the biology of Eucharitidae (Hymenoptera: Chalcidoidea) from Argentina. Psyche 2013:926572
Torres PJ, Pomerantz AF (2016) Butterfly kleptoparasitism and first account of immature stages, myrmecophily, and bamboo host plant of the metalmark Adelotypa annulifera (Riodinidae). J Lepid Soc 70(2):130–138
Tran S (2023) iNaturalist observation https://www.inaturalist.org/observations/152279614 Accessed on 10 July 2024
Valenzuela-González J, López-Méndez A, Lachaud J-P (1995) Activity patterns and foraging activity in nests of Ectatomma tuberculatum (Hymenoptera: Formicidae) in cacao plantations. Southwest Entomol 20(4):507–515
Vieira AS, Antoniali-Junior WF, Fernandes WD (2007) Modelo arquitetônico de ninhos da formiga Ectatomma vizottoi Almeida (Hymenoptera, Formicidae). Rev Bras Entomol 51(4):489–493
Villaseñor Ríos JL, Espinosa García FJ (1998) Catálogo de malezas de México. Universidad Nacional Autónoma de México. Consejo Nacional Consultivo Fitosanitario. Fondo de Cultura Económica. México, DF
Wagner WL, Robinson H (2001) Lipochaeta and Melanthera (Asteraceae: Heliantheae subtribe Ecliptinae): establishing their natural limits and a synopsis. Brittonia 53(4):539–561
Walton RE, Sayer CD, Bennion H, Axmacher JC (2020) Nocturnal pollinators strongly contribute top ollen transport of wild flowers in an agricultural landscape. Biol Lett 16:20190877
Warren AD, Davis KJ, Stangeland EM, Pelham JP, Willmott KR, Grishin NV (2024) Illustrated list of American butterflies (North and South America) [9-III-2024] https://www.butterfliesofamerica.com/L/Neotropical.htm
Willmer P (2011) Pollination by butterflies and moths. Pollination and floral ecology. Princeton University Press, Princeton, pp 322–336
Willmott KR, Elias M, Sourakov A (2011) Two possible caterpillar mimicry complexes in Neotropical danaine butterflies (Lepidoptera: Nymphalidae). Ann Entomol Soc Am 104(6):1108–1118
Witek M, Barbero F, Markó B (2014) Myrmica ants host highly diverse parasitic communities: from social parasites to microbes. Insectes Soc 61(4):307–323
Wood K (1984) Life history patterns of tropical membracids (Homoptera: Membracidae). Sociobiology 8(3):299–344
Young AM (1978) Possible evolution of mutualism between Mechanitis caterpillars and an ant in Northeastern Costa Rica. Biotropica 10(1):77–78
Young AM, Moffet MW (1979) Studies of the population biology of the tropical butterfly Mechanitis isthmia in Costa Rica. Am Midl Nat 101(2):309–319
Zhang B-C (1994) Index of Economically Important Lepidoptera. CAB International, Wallingford
Acknowledgements
We would like to thank Junia Yasmin Carreira, Alexander Greene, Kozue Kawakami, Lucas Pereira Martins, Jonghyun Park, and John Smiley for permission to use their photos and for their comments. We would also like to thank Bret J. Sinclair and Nathan Rank for helping with contacts, José Antonio López Méndez, José Álvaro Garcia Ballinas, Carmelino Salvador, and Don Emilio Hernández Colomo for their help in collecting hundreds of Ectatomma nests, Julian Flavell for improving the English, and two anonymous reviewers for their valuable comments and editing efforts. LAK thanks Luan D. Lima and Rafael Dell’Erba for help during fieldwork in Mato Grosso; Conselho Nacional de Pesquisa (CNPq 140183/2006-0), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 10/51340-8), National Geographic Society (WW-224R-17), Lindblad-National Geographic (LEX-NG) Visiting Scientist program, and Programa Nacional de Pós-Doutorado (PNPD) Coordenação de Aperfeicoamento de Pessoal de Nivel Superior (CAPES Finance Code 001).
Funding
The funding has been eceived from Programa Nacional de Pós-Doutorado (PNPD) Coordenação de Aperfeicoamento de Pessoal de Nivel Superior with Grant no. CAPES Finance Code 001, Conselho Nacional de Pesquisa with Grant no. CNPq 140183/2006-0, Fundação de Amparo à Pesquisa do Estado de São Paulo with Grant no. FAPESP 10/51340-8, National Geographic Society with Grant no. WW-224R-17, Lindblad-National Geographic with Grant no. (LEX-NG) Visiting Scientist program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lachaud, JP., Kaminski, L.A. & Pérez-Lachaud, G. Diversity of butterfly–ant symbioses in the neotropical genus Ectatomma (Formicidae: Ectatomminae). Insect. Soc. (2024). https://doi.org/10.1007/s00040-024-00996-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00040-024-00996-x