Abstract
The previous identification of 2,5-dimethyl-3-(3-methylbutyl)pyrazine as the mandibular alarm pheromone of the little fire ant Wasmannia auropunctata (Roger), has been found to be incorrect. Gas chromatography–mass spectrometry (GC/MS) of ant extracts suggested the correct structure to be the regioisomer 2,5-dimethyl-3-(2-methylbutyl)pyrazine, which was confirmed by comparison with the synthetic pyrazine. GC/MS analysis also revealed the presence of an additional disubstituted alkylpyrazine which was identified as 3-methyl-2-(2-methylbutyl)pyrazine. Headspace sampling of confined ants with SPME and Porapak Q followed by GC/MS analysis showed 2,5-dimethyl-3-(2-methylbutyl)pyrazine as the major volatile released by W. auropunctata workers while 3-methyl-2-(2-methylbutyl)pyrazine was only detected in trace amounts. In laboratory bioassays, W. auropunctata workers were attracted and arrested by both pyrazines, although the results were not always consistent. Synthetic pyrazines generally attracted as many W. auropunctata workers as were attracted to a single crushed ant. However, higher numbers of W. auropunctata were arrested by crushed ant treatments than by synthetic pyrazines in all bioassays but one.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insects such as ants rely heavily on exocrine gland-produced pheromones for communication. Pheromones mediate many important and common behaviors such as alarm responses. Alarm pheromones, released by stressed ants, typically induce attraction, increased rates of locomotion, arrestment, and/or repulsion in nestmates. However, a strict definition of alarm behavior is difficult, as it is sometimes highly complex (Hölldobler and Wilson, 1990), and “alarm pheromones” may facilitate other group operations such as large prey capture (Dejean et al., 2007) and aggregation after disturbance (Feitosa, 2007). Many ants also use trail pheromones, which are generally laid down by successful scouts to indicate paths between food sources and the nest site. These chemical communication mechanisms are important in facilitating synchronization of group behaviors.
The success of invasive ants rests on biological traits, such as social organization, that increase competitiveness and result in their ecological dominance (Passera, 1994; Holway et al., 1998; Giraud et al., 2002). An example of this competitive success can be seen in Wasmannia auropunctata (Roger), an important invasive ant in tropical and subtropical regions of the world (Wetterer and Porter, 2003). This diminutive myrmicine possesses a number of ‘tramp ant’ characteristics, such as polygyny, budding of new nests, unicoloniality, opportunistic utilization of food sources and nest locations, and rapid nest relocation after disturbance, which facilitate its colonization of disturbed environments (Passera, 1994; Wetterer and Porter, 2003). One of the worst exotic pest ants (Lowe et al., 2000), W. auropunctata has a nearly pantropical distribution, with greenhouse infestations reported in temperate areas as far north as Canada and the UK (Jourdan et al., 2002; Wetterer and Porter, 2003), and it is a growing threat to biodiversity and agriculture in colonized areas (Le Breton et al., 2003; Wetterer and Porter, 2003; Walker, 2006). The ecological impact of W. auropunctata is not limited to the displacement of native ants (Le Breton et al., 2003; Walker, 2006), as it can reduce native invertebrate populations (Ulloa-Chacón et al., 1991) and stress vertebrates as well (Wetterer, 1997; Wetterer et al., 1999). In sharp contrast to its success as an invasive, W. auropunctata does not appear to be a dominant species in its native neotropical range (Wetterer and Porter, 2003).
A number of chemical defense and communication mechanisms may contribute to the competitive success of W. auropunctata. These include a powerful venom, interspecific aggression (Le Breton et al., 2004; Errard et al., 2005; Kirschenbaum and Grace, 2007), and effective mass recruitment (Howard et al., 1982). Although W. auropunctata workers are diminutive in size (approx. 1.5 mm in length) and are relatively slow-moving and non-aggressive toward humans, the venom from their stings can be quite painful. Many of the common names for this species, such as “little fire ant” and “electric ant”, are derived from this intense sting. W. auropunctata stings and their potent venom are thought to cause blindness and other negative effects in a number of vertebrate species (Wetterer and Porter, 2003), and they have become a major deterrent to laborers harvesting infested crops (Smith, 1965; Conant, 2000).
Interspecific aggressiveness and nestmate recognition are thought to contribute to the success of W. auropunctata in displacing native ants (Le Breton et al., 2002; Kirschenbaum and Grace, 2007). This has been studied by analysis of cuticular hydrocarbons (Errard et al., 2005). Lower variation in cuticular hydrocarbons in introduced populations relative to native populations may explain the unicoloniality and interspecific aggression behavior observed in invaded areas (Errard et al., 2005). Investigation of W. auropunctata recruitment resulted in the identification of an alkylpyrazine mandibular alarm pheromone (Howard et al., 1982). The pyrazine was identified as 2,5-dimethyl-3-(3-methylbutyl)pyrazine (3-MeBu-diMePy) by gas chromatography comparison with known pyrazines and was found to be present in up to 200 ng per ant. Howard et al. (1982) also conducted attraction/arrestment bioassays and found that 3-MeBu-diMePy at one ant equivalent was approximately half as active as ant head extracts. This attraction was not improved by 10–20 times ant equivalent doses and 3-MeBu-diMePy was never more than two-thirds as attractive as a single ant head extract. This led to the suggestion by Howard et al. that other less volatile compounds might also be involved in the observed behavior. The W. auropunctata pyrazine pheromone has also been indicated in the aggregation of workers observed after colony disturbance (Feitosa, 2007). Congregating ants hold their mandibles wide open, possibly releasing the alarm pheromone which may facilitate aggregation (Feitosa, 2007).
Our interest in W. auropunctata alarm pheromones stems from their potential use as attractants for detection and control methods. We report here that the identification of the W. auropunctata alarm pheromone as 3-MeBu-diMePy is incorrect and that the true structure is the regioisomer 2,5-dimethyl-3-(2-methylbutyl)pyrazine (2-MeBu-diMePy). We also report the isolation and characterization of a second mandibular pyrazine, 3-methyl-2-(2-methylbutyl)pyrazine (2-MeBu-MePy), which is novel in insects. Bioassays showed both compounds significantly attracted and arrested W. auropunctata workers.
Materials and methods
Insects
Wasmannia auropunctata workers and alates were collected from a shade house at the Agricultural Farm Laboratory of the University of Hawaii at Hilo (GPS coordinates: 19.650668, −155.050505) and shipped overnight in 25 ml vials to Eastern Mennonite University, Harrisonburg, VA. Ants were housed in 1.2-L plastic containers (13 × 13 × 7 cm high), which contained the vial in which the ants were shipped covered in foil to provide a retreat, a foraging area containing minced insects, and 4 ml cotton-topped vials of water and a 50% honey solution. Two colony containers were housed in a larger 7.6-L outer container to prevent escape. All containers were vented with mesh (7 cm2 per lid). Insect-a-Slip (Fluon, PTFE-30 BioQuip Products, Inc., Rancho Dominguez, CA) was applied to the sides of all containers to prevent escape. Ants were kept at ambient temperature (near 24°C) on a 16:8 h light/dark cycle.
Extraction and headspace sampling
Wasmannia auropunctata workers (approx. 200) were frozen at −70°C and extracted for 5 min in CH2Cl2. Trisected ants (heads, thoraxes, and gasters) were also separately extracted in the same manner. Extracts were transferred into conical glass tubes, concentrated under a purified nitrogen stream, and refrigerated until analysis by GC/MS.
Headspace samples were collected using a solid phase microextraction (SPME) fiber coated with polydimethylsiloxane (PDMS; film thickness 100 μm: Supleco Inc., Bellefonte, PA) with both live and crushed ants. Live workers (approx. 100) were transferred to a clean glass container and allowed to settle for 15 min. The SPME fiber was inserted through the lid of the container and exposed for 15 min. Collections from crushed ants were conducted as described by Di Tullio et al. (2003). Whole ants, separated head or gasters (5–10 per extract) were placed at the bottom of glass melting point tubes (FP-1, Mettler-Toledo Inc., Columbus, OH) cut to be 50 mm long. Samples were crushed with a wire before the fiber was inserted in the tube, which was then sealed with Teflon tape. Extractions were performed at 100°C for 30 min.
Headspace sampling was also performed with Porapak Q (50–80 mesh bulk packing material, Supleco). Absorbent (1 g) was packed between glass wool plugs in a Pasteur pipette, washed with CH2Cl2, and dried with purified nitrogen. Purified air was pumped through a glass tube containing workers (approx. 500) for 50 min, with volatiles collecting on the Porapak Q column. Absorbent was washed with CH2Cl2 (1 ml), which was concentrated under a purified nitrogen stream and refrigerated until analysis by GC/MS.
Gas chromatography–mass spectrometry
Ant extracts, headspace collections, and synthetic pyrazines were analyzed by gas chromatography–mass spectrometry (GC/MS) using two instruments. The first, located in Hawaii, consisted of an Agilent Technologies 6890N gas chromatograph interfaced to a Hewlett Packard 5973 Mass Selective Detector equipped with HP-5MS column (30 m × 0.25 mm ID, 0.25 μm film thickness). The standard temperature program used was 80–220°C at 10°C/min with a 1 min start delay. The injector temperature was set at 250°C and helium was used as a carrier gas (1.1 ml/min). The second instrument, located in Virginia, was a Hewlett Packard G1800A GCD system equipped with one of the following columns: ZB-5 (30 m × 0.25 mm ID, 0.25 μm film thickness); Rtx-1 (60 m × 0.32 mm ID, 1.0 μm film thickness); DB-225MS (30 m × 0.25 mm ID, 0.25 μm film thickness); Rt-BDEXm (30 m × 0.25 mm ID, 0.25 μm film thickness). The temperature program used was 60–250°C at 10°C/min with a 1 min start delay. The injector temperature set at 250°C and helium was used as a carrier gas (1.0 ml/min). Mass spectral data were analyzed using a NIST 98 mass spectral database.
Nuclear magnetic resonance and infrared spectroscopy
1H NMR spectra were obtained with a Bruker DRX-400 FT-NMR spectrometer equipped with a broadband gradient probe. All spectra were recorded in deuterated chloroform with 1% TMS as an internal standard. Fourier transform IR (FT-IR) spectra were collected with a Thermo Nicolet Magna-IR 560 Spectrometer. Liquid samples were analyzed using KBr plates.
Synthesis
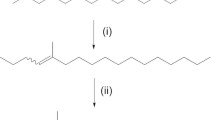
Pyrazines of interest were synthesized by an alkyl-zinc cross-coupling reaction modified from Sato and Matsuura (1996) (Fig. 1). All solvents and reagent compounds were purchased from Sigma–Aldrich, Inc., Saint Louis, MO, or Fisher Scientific, Rochester, NY, unless otherwise noted. The alkyl-zinc reagents were prepared in two steps from either 1-bromo-3-methylbutane (Sigma–Aldrich) or racemic 1-bromo-2-methylbutane (Frinton Laboratories, Inc., Vineland, NJ) via a Grignard reaction followed by addition of zinc bromide. These alkyl-zinc reagents were added to either 3-chloro-2,5-dimethylpyrazine (Sigma–Aldrich), 2-chloro-3-methylpyrazine (Pyrazine Specialties, Inc. Atlanta, GA) or a mixture of isomers 2-chloro-(3),(5),(6)-methylpyrazine (Pyrazine Specialties, Inc. Atlanta, GA), in the presence of the [1,2-bis(diphenylphosphino)-propane] dichloronickel catalyst in MeTHF at rt. After 2.5 h the reaction was quenched with ice water, filtered, and extracted. The compounds were purified by flash chromatography to yield a viscous yellow liquid for all products: 2,5-dimethyl-3-(3-methylbutyl)pyrazine (3-MeBu-diMePy), GC/MS (EI, 70 eV) m/z (relative intensity) 177 [M-1]+ (1), 163 (8), 149 (1), 135 (14), 122 (100), 107 (3), 80 (4), 53 (7); 1H NMR (CDCl3, 400 MHz) δ 0.990 (6H, d), 1.557 (2H, m), 1.690 (1H, m), 2.498 (3H, s), 2.537 (1H, s), 2.778 (3H, m), 8.158 (1H, s). Racemic 2,5-dimethyl-3-(2-methylbutyl)pyrazine (2-MeBu-diMePy), (95% purity; including both enantiomers); GC/MS (EI, 70 eV) m/z (relative intensity) 177 [M − 1]+ (1), 163 (6), 149 (4), 135 (2), 122 (100), 107 (2), 80 (4), 53 (8); 1H NMR (CDCl3, 400 MHz) δ 0.89 (3H, d), 0.94 (3H, t), 1.27 (2H, m). 1.44 (1H, m), 2.50 (3H, s), 2.53 (3H, s), 2.80 (2H, dd), 8.16 (1H, s); IR wavenumber, cm−1 (percent transmittance) 3,041 (66), 2,960 (49), 2927 (51), 2,874 (56), 2,856 (59), 1451 (55), 1,374 (58), 1168 (65), 1076 (68). Racemic 3-methyl-2-(2-methylbutyl)pyrazine (2-MeBu-MePy), (98% purity; including both enantiomers); GC/MS (EI, 70 eV) m/z (relative intensity) 163 [M − 1]+ (1), 149 (6), 135 (5), 121 (2), 108 (8), 93 (3), 67 (7), 53 (5); 1H NMR (CDCl3, 400 MHz) δ 0.91 (3H, d), 0.95 (3H, t), 1.27 (2H, m), 1.44 (1H, m), 2.58 (3H, s), 2.85 (2H, dd), 8.29 (1H, s), 8.34 (1H, s); IR wavenumber, cm−1 (percent transmittance) 3,045 (70), 2,961 (18), 2,928 (27), 2,875 (37), 1,234 (80), 1,460 (39), 1,405 (22), 1,378 (50), 1,168 (46).
Scheme modified from Sato and Matsuura (1996) for the synthesis of alkylpyrazines
Bioassays
Bioassays used to measure activity, attraction, and arrestment of W. auropunctata in response to synthetic pheromone components were modified from Fujiwara-Tsujii et al. (2006), Howard et al. (1982), and Vander Meer et al. (1988). Racemic 2-MeBu-diMePy and 2-MeBu-MePy, as prepared above, were used in all bioassays.
Experiments 1 and 2
The first set of bioassays accessed W. auropunctata responses to the synthetic pyrazines either singly or in blends. These assays were conducted in Petri dishes (85 mm ID × 45 mm high) with 25 mm diameter circles drawn on the underside of each dish. Ten ants were placed in each dish and left undisturbed for 2 min. Each test was initiated by placing a treated filter paper disk (5 mm diameter) at the center of the marked circle. Total number of ants crossing (into or out of) the center circle was recorded over 5 min. Arrestments, the number of ants in contact with the disk, were recorded every minute for 5 min. An ant from the colony being tested was crushed between filter paper disks and served as a positive control. The negative control was a filter paper disk treated with CH2Cl2. Petri dishes were cleaned with ethanol (95%) between replicates.
Experiment 1 was conducted to assess the relative activity and synergy of the synthetic alkylpyrazines. Three treatments were assayed in the manner described above: 200 ng of 2-MeBu-diMePy, 200 ng of 2-MeBu-MePy, and 200 ng of both pyrazines (all applied in CH2Cl2). The 200 ng dose was chosen to reflect the maximum amount of alkylpyrazine pheromone present in individual ants as estimated by Howard et al. (1982).
Experiment 2 was conducted to assess ant responses to varying concentrations of synthetic pyrazines. GC analysis of ant extracts showed 2-MeBu-diMePy/2-MeBu-MePy ratios ranging from 4.5:1 to >100:1, with the most common ratio being in the 100:1 range. Therefore, the following treatments of 100:1 2-MeBu-diMePy/2-MeBu-MePy were used: 200 ng:2 ng, 20 ng:200 pg, and 2 ng:20 pg. Additionally a 2 μg:200 ng (10:1) treatment was tested.
Experiments 3 and 4
A second type of bioassay was conducted by placing treated filter paper on a small inverted watch-glass (25 mm diameter, 3 mm center height, 1 mm thickness/height at edge) within the foraging area of the colony containers. This approach eliminated the disruption caused by transferring ants, and the height of the watch-glass provided an obstacle to decrease random foraging in the recording area. Three treatments were assayed: 200 ng of 2-MeBu-diMePy; 200 ng of 2-MeBu-MePy; and 200 ng of both pyrazines (all applied in CH2Cl2). Each treatment was run in every colony to control for differences in colony size, health, and activity. Arrestments were recorded as in Experiments 1 and 2. Crossings were recorded as onto or off of the watch-glass each minute for 5 min.
In Experiment 3, an ant from the colony being tested was crushed between filter paper disks and served as a positive control. The negative control was a filter paper disk treated with CH2Cl2. In Experiment 4, a separated ant head from the colony being tested, rather than a whole ant, was crushed between filter paper disks and served as a positive control. The negative control was a filter paper disk treated with CH2Cl2.
Experiment 5
This bioassay was conducted in order to determine the particular body segment of a crushed ant that produced the observed attractive effect. Ants were trisected into head, thorax, and gaster. Each of these sections was crushed between filter paper and introduced on a watch-glass to the foraging area as in Experiments 3 and 4. Crossings and arrestments were again recorded as in Experiments 3 and 4.
Analysis
Results of bioassays were analyzed using ANOVA followed by Tukey’s HSD test (alpha = 0.05) to compare means. Arrestment data were normalized by log transformation before analysis. All analyses of significance were made at the P < 0.05 level. All statistical analyses were performed using SPSS version 15.0 (SPSS, Inc. Chicago, IL).
Results
Structure elucidation
Analyses by coupled gas chromatography/mass spectrometry of ant extracts revealed an alkylated dimethyl pyrazine (base peak of the mass spectrum m/z 122) and an alkylated monomethyl pyrazine (base peak of the mass spectrum m/z 108), in ratios ranging from 4.5:1 to greater than 100:1, respectively (Fig. 2). Extracts from separated ant heads, thoraxes, and gasters confirmed the trisubstituted pyrazine to be the main volatile component in the head, as found by Howard et al. (1982). Other volatiles, including a number of sesquiterpenoids, were present in the gaster.
3-MeBu-diMePy, the compound reported by Howard et al. (1982), was prepared according to methods described by Sato and Matsuura (1996) and its structure was confirmed by NMR and MS (Fig. 1). Coinjection of 3-MeBu-diMePy with an extract of W. auropunctata on GC produced two distinct chromatographic peaks, indicating that the trisubstituted ant pyrazine was not 3-MeBu-diMePy (Fig. 3). Comparison of the trisubstituted ant pyrazine mass spectrum with database mass spectra suggested the structure 2-MeBu-diMePy (Fig. 4). Key differences in the mass spectra of 3-MeBu-diMePy and 2-MeBu-diMePy occur with the cleavage of the alkyl side chain between C-2 and C-3, which are reflected in the relative intensities of the fragmentation ion peaks, M-29 (m/z 149) and M-43 (m/z 135) (Fig. 4). Cleavage at this position yields a prominent M-29 peak and a reduced M-43 for 2-MeBu-diMePy while the reverse is seen with 3-MeBu-diMePy. This identification was confirmed by the synthesis of 2-MeBu-diMePy and its sequent retention time comparison to the trisubstituted ant pyrazine on several GC columns (Table 1). Coinjection of 2-MeBu-diMePy with an extract of W. auropunctata on GC produced no additional chromatographic peak.
Electron impact mass spectra (EIMS; 70 eV) of alkylpyrazines from CH2Cl2 extracts of W. auropunctata. Numbering system indicated for alkyl side chain. 2-MeBu-diMePy and 3-MeBu-diMePy alkyl side chain fragmentation between C-2 and C-3 are indicated. 2-MeBu-MePy shows a base peak at m/z 108, consistent with the formation of a McLafferty type rearrangement product, and is in good agreement with the mass spec data given by Friedel et al. (1971)
Mass spectrometry analysis suggested the disubstituted ant pyrazine to be a single methyl analog of 2-MeBu-diMePy (Fig. 4), of which three regioisomers are possible. A mixture of these isomers was synthesized from their corresponding chloropyrazine mixture. Isomer identifications were assigned based on elution order: 5-, 6-, and 3-methyl-2-(2-methylbutyl)pyrazine, as per Hwang et al. (1993) and Hwang et al. (1995) on a similar nonpolar column. Retention time comparison suggested 2-MeBu-MePy as the isomer present in ant extracts. This was confirmed by synthesis of pure 2-MeBu-MePy and its sequent retention time comparison to the disubstituted ant pyrazine on several GC columns (Table 1). Coinjection of 2-MeBu-MePy with an extract of W. auropunctata on GC produced no additional chromatographic peak. Headspace analyses with SPME and Porapak Q also showed 2-MeBu-diMePy to be the major released volatile component from both live and crushed ants. 2-MeBu-diMePy constituted >98% of the detected volatiles in SPME collections of separated ants heads. Small amounts of 2-MeBu-MePy were also detected by both headspace analysis techniques.
Both 2-MeBu-diMePy and 2-MeBu-MePy are chiral and an attempt to assign the absolute configurations of the alkylpyrazines present in W. auropunctata was made using a chiral GC column (Rt-BDEXm). Synthetic racemic 2-MeBu-diMePy and 2-MeBu-MePy, as well as W. auropunctata extracts, were analyzed. Although multiple temperature profiles, sampling and injection techniques were tried, no separations of enantiomers were achieved, thus we were unable to assign the absolute configurations of either natural alkylpyrazine.
Bioassays
In Experiment 1, ant responses to synthetic alkylpyrazines at a 200 ng dose, the maximum amount of alkylpyrazine pheromone present in individual ants as estimated by Howard et al. (1982), were tested (Fig. 5). Arrestments of ants by whole crushed ant, 2-MeBu-MePy, and the combination of both pyrazines were not significantly different, but these treatments all arrested more ants than 2-MeBu-diMePy or the negative control (ANOVA: F = 9.60, df = 4, P < 0.001). The number of crossings induced by 2-MeBu-MePy and by the combination of both pyrazines was significantly greater than the negative control (ANOVA: F = 5.79, df = 4, P = 0.002). Behaviors such as biting, carrying, or dragging the filter paper disks to another part of the arena were observed with the crushed ant and occasionally with synthetic pyrazines, but never with the negative control.
Numbers (mean ± SE) of W. auropunctata crossing circle perimeter and arresting on filter paper treatments in Experiment 1. Synthetic components were tested alone and at a 1:1 ratio combination. Letters represent significant differences (P < 0.05) between arrestments and crossings of different treatments (ANOVA, followed by Tukey’s HSD)
Experiment 2 did not show a significant dose response for the range tested (2 μg/200 ng–2 ng/20 pg). The synthetic pyrazine dilution series had significantly fewer arrestments than the crushed ant positive control for all concentrations tested (ANOVA: F = 12.508, df = 5, P < 0.001), while crossings did not significantly differ (ANOVA: F = 1.619, df = 5, P = 0.185).
Bioassays conducted in the foraging area within colonies produced data that conflicted with the previous experiment regarding the most active pyrazine (Fig. 6). In Experiment 3, arrestments were not significantly different between 2-MeBu-diMePy and whole crushed ant treatments, while both were significantly different from the negative control (ANOVA: F = 12.21, df = 4, P < 0.001). Synthetic pyrazines did not produce significantly different numbers of crossings compared with the negative control (ANOVA: F = 4.17, df = 4, P = 0.003).
Numbers (mean ± SE) of W. auropunctata crossing inverted watch-glass and arresting on filter paper treatment in Experiments 3 and 4. a In Experiment 3, positive control is one whole ant crushed between two filter paper disks. b In Experiment 4, positive control is one ant head crushed between two filter paper disks. Within each experiment, letters indicate significant differences (P < 0.05) between arrestments and crossings of different treatments (ANOVA, followed by Tukey’s HSD)
In Experiment 4, however, arrestments in the 2-MeBu-diMePy treatment were significantly lower than in the crushed ant head treatment, but still higher than in the 2-MeBu-MePy treatment and in the control (ANOVA: F = 34.53, df = 4, P < 0.001). Crossings in the 2-MeBu-diMePy treatment were not significantly different from the ant head treatment, but they were significantly higher than in the 2-MeBu-MePy treatment and in the control (ANOVA: F = 13.36, df = 4, P < 0.001). Treatments of combined pyrazines did not increase attraction in either Experiment 3 or 4.
Experiment 5 compared ant responses when exposed to trisected ant body parts (Fig. 7). The crushed head caused significantly more arrestments than either the crushed thorax or gaster (ANOVA: F = 36.67, df = 2, P < 0.001). The crushed head induced significantly more crossings than the crushed thorax (ANOVA: F = 4.38, df = 2, P < 0.019).
Numbers (mean ± SE) of W. auropunctata crossing inverted watch-glass and arresting on filter paper treatment. Experiment 5 tested attractiveness of the three ant segments. Letters represent significant differences (P < 0.05) between arrestments and crossings of different treatments (ANOVA, followed by Tukey’s HSD)
Discussion
GC coinjection of synthetic 3-MeBu-diMePy with extracts of W. auropunctata showed the previous identification of this compound as the alarm pheromone component by Howard et al. (1982) to be incorrect. Mass spectrometry suggested the true alarm pheromone structure to be 2-MeBu-diMePy, which was confirmed by synthesis and GC analysis. Separation and identification of these two pyrazines, based on retention times and mass spectrometry has been reported in a study of cephalic compounds in the ant Rhytidoponera metallica (Smith) showing that separation of the compounds is possible (Tecle et al., 1987).
The finding by Howard et al. (1982) that the pheromone components were confined to the mandibular glands is supported by our analysis of trisected ants. Additionally, headspace collections using SPME and Porapak Q showed that 2-MeBu-diMePy and 2-MeBu-MePy are released unaltered as active pheromone components and are not biological precursors, with 2-MeBu-diMePy being the most abundant volatile released.
In addition to being found in W. auropunctata, 2-MeBu-diMePy is secreted from the mandibular glands of the ants Dinoponera australis Emery (Oldham et al., 1994), Odontomachus bauri Emery (Morgan et al., 1999), Rhytidoponera metallica (Smith) (Tecle et al., 1987), Rhytidoponera victoriae (Andre) (Brophy, 1989), a Calomyrmex sp. (Brown and Moore, 1979), and an Ectatomma sp. (Morgan et al., 1999). Consistent with our analysis, 2-MeBu-diMePy in ants is found only in the head and has been described as an alarm pheromone in some cases. Similar alkylpyrazines, including 3-MeBu-diMePy, have been found as mandibular secretions of a phylogenetically diverse group of ants including Myrmeciinae, Myrmicinae, Formicinae and Dolichoderinae, and often as trail pheromones from Dufour’s gland (Attygalle and Morgan, 1984; Morgan et al., 1999; Tentschert et al., 2000; Hölldobler et al., 2001). Despite its structural similarity to known pheromone components, 2-MeBu-MePy is novel in insects, while it has been reported as a Maillard reaction-produced component of roasted coffee volatiles (Friedel et al., 1971).
While 2-MeBu-diMePy and 2-MeBu-MePy are chiral, no enantiomeric separations were achieved using a Rt-BDEXm chiral GC column. Consequently, the absolute configurations of neither natural alkylpyrazine were assigned. Enantiomeric separation of a closely related pyrazine, 2-methoxy-3-(1′-methylpropyl)pyrazine, have been reported (Bungert et al., 2001). The inability to separate the enantiomers of the alkylpyrazines in the current study may be due to differences in column stationary phases or functional group differences.
Behaviors induced by alarm pheromones are often difficult to quantify (Hölldobler and Wilson, 1990). While various bioassay types exist, many focus on arrestments, attraction, increased mobility, and repulsion. To test the alarm pheromone behaviors of W. auropunctata elicited by racemic 2-MeBu-diMePy and 2-MeBu-MePy, we chose a circle bioassay modified from Howard et al. (1982), Vander Meer et al. (1988), and Fujiwara-Tsujii et al. (2006). This assay allowed quantification of arrestment, attraction, and increased mobility behaviors.
Higher numbers of crossings in the bioassays indicate a combination of increased ant mobility and attraction, although the assays could not distinguish between these two characteristics of alarm behavior. Bioassay results generally showed that the numbers of crossings induced by synthetic pyrazines were significantly greater than those induced by the negative control, while they were not significantly different from the positive control. These behaviors are consistent with the assignment of 2-MeBu-diMePy and 2-MeBu-MePy as alarm pheromone components.
Compared to crossings, arrestment data showed greater variation both among treatments and between bioassay types. Importantly, crushed ant treatments produced significantly higher arrestment numbers than did synthetic pyrazine treatments in all bioassays except Experiment 1. This observation could be due to as yet unidentified pheromone compounds in the ant and/or the method of pyrazine presentation in the bioassays. Howard et al. (1982) hypothesized that alkylpyrazines attract W. auropunctata, while less volatile compounds contribute to arrestment. Headspace analysis by SPME and Porapak Q found that 2-MeBu-diMePy is the major volatile component released by W. auropunctata workers, with 2-MeBu-MePy and other volatiles present in small amounts. In addition, observed trail following behavior and the presence of additional volatile compounds in whole-ant extracts could indicate the presence of other pheromones that might contribute to ant arrestment. In support of this idea, extracts of separated ant heads showed 2-MeBu-diMePy to be the major volatile component while other volatile compounds were found to be confined to the gaster extracts.
However, bioassay experiments suggested that pheromones from parts of the ant other than the head do not contribute to increased arrestment. Experiments 3, 4, and 5 confirmed that increased arrestment is due to a characteristic of the ant head, and not other body segments or their components: positive controls for Experiments 3 and 4 (whole ant and crushed head, respectively) produced similar results and in Experiment 5; the crushed head arrested significantly more ants than did the crushed thorax or crushed gaster. If yet unidentified compounds account for increased arrestment by ant heads, they are likely nonvolatile (i.e. cuticular hydrocarbons) or present in trace amounts.
Another possible explanation for high arrestment by crushed ant may be the presentation of pheromone components in an optimum concentration and/or synergistic formulation. Experiment 2 tested a dilution series of the biologically relevant 100:1 formulation of 2-MeBu-diMePy/2-MeBu-MePy but found no synergy or dose response at the concentrations and formulation tested.
Variations in behavioral responses may also be the result of bioassay differences between the smaller arena (Experiments 1 and 2) and the larger arena (Experiments 3, 4, and 5). Interestingly, the most active pyrazine appears to be bioassay dependent, with 2-MeBy-MePy most active in the smaller arena, while 2-MeBy-diMePy is most active in the larger arena. These differences may arise from a number of different factors. The smaller arena, used in Experiments 1 and 2, may be biased toward shorter-range attraction, as ants were removed from their colony areas and placed in a small container. In contrast, Experiments 3, 4, and 5 were conducted in the foraging area of the colony with little to no disturbance due to moving ants between containers. Furthermore, Experiments 3 and 4 were conducted with many more ants, perhaps exaggerating the differences in arrestments compared to Experiment 1.
Additionally, without assignment of the absolute configuration of the natural W. auropunctata alkylpyrazines, strict comparisons between the natural pheromone and the racemic synthetic pyrazines are complicated. Absolute configurations of pheromones can cause profound differences in bioactivity as illustrated with the leaf-cutting ant, Atta texana (Riley et al., 1974). The principal alarm pheromone of this ant is (S)-4-methyl-3-heptanone, which was shown to be ~100 times more active than its corresponding (R)-isomer, demonstrating for the first time the dependence of pheromone bioactivity on absolute configuration. Additionally, the (R)-isomer did not inhibit responses to the (S)-isomer at the ratios tested. Illustration of the complex relationship between pheromone chirality and bioactivity are demonstrated by examples such as inhibition by the wrong enantiomers, blends of enantiomers (including racemic pheromones) and differential responses between males and females (Mori, 2007). Establishing what effect, if any, chirality has in the alarm signaling of W. auropunctata will require assigning the absolute configurations of both ant alkylpyrazines, and further bioassays with the enantiomers of 2-MeBy-diMePy and 2-MeBy-MePy.
Studies have shown a preference exhibited by W. auropunctata for peanut butter over other food sources, including honey, pineapple juice, and tuna oil (Williams and Whelan, 1992). Consequently, peanut butter is a commonly used bait for detection of W. auropunctata (Causton et al., 2005; Kirschenbaum and Grace, 2007). It is interesting to note that along with containing sugars, lipids, and proteins which make it a desirable food source, peanut butter also releases a number of volatile alkylpyrazines, including 2,5-dimethylpyrazine and 2,5-dimethyl-3-ethylpyrazine (Joo and Ho, 1997). The structural similarity of these pyrazines to the little fire ant alarm pheromone, along with the feeding preference shown for peanut butter, warrants an investigation into the attractiveness of peanut butter volatiles to W. auropunctata.
The attractiveness of 2-MeBu-diMePy and 2-MeBu-MePy makes these compounds good candidates for use in the detection and control of W. auropunctata infestations. For detection, pyrazine lures may have advantages over the commonly used peanut butter baits in that they may be more species-specific, longer lasting, more easily packaged, mess-free in application, and less prone to spoil. Potential control applications of W. auropunctata pheromones include use in insecticidal baits to increase feeding (Hughes et al., 2002) and direct disruption of ant behavior, similar in concept to pheromone mating disruption (Suckling et al., 2008). Further testing in the field will be required to assess the potential of these possible applications.
References
Attygalle A.B. and Morgan E.D. 1984. Chemicals from the glands of ants. Chem. Soc. Rev. 13: 245-278
Brophy J.J. 1989. Pyrazines obtained from insects: Their source, identification, synthesis and function. In: Studies in Natural Products Chemistry: Structures and Elucidation (Atta-ur-Rahman, Ed). Elsevier, Amsterdam, pp 221-273
Brown W.V. and Moore B.P. 1979. Volatile secretory products of an Australian formicine ant of the genus Calomyrmex (Hymenoptera: Formicidae). Insect Biochem. 9: 451-460
Bungert M., Jahns T. and Becker H. 2001. 2-Methoxy-3-(1′-methylpropyl)pyrazine, pea odour, from the marine bacterium Halomonas venusta. Flavour Fragr. J. 16: 329-333
Causton C.E., Sevilla C.R. and Porter S.D. 2005. Eradication of the little fire ant, Wasmannia auropunctata (Hymenoptera: Formicidae), from Marchena Island, Galapagos: On the edge of success? Fla. Entomol. 88: 159-168
Conant P. 2000. Wasmannia auropunctata (Hymenoptera: Formicidae): established on the Island of Hawai’i. Bishop Mus. Occas. Pap. 64: 21
Dejean A., Moreau C.S., Uzac P., Le Breton J. and Kenne M. 2007. The predatory behavior of Pheidole megacephala. C.R. Biol. 330: 701-709
Di Tullio A., De Angelis F., Reale S., Grasso D.A., Visicchio R., Castracani C., Mori A. and Le Moli F. 2003. Investigation by solid-phase microextraction and gas chromatography/mass spectrometry of trail pheromones in ants. Rapid Commun. Mass Spectrom. 17: 2071-2074
Errard C., Delabie J., Jourdan H. and Hefetz A. 2005. Intercontinental variation in the invasive ant Wasmannia auropunctata (Roger) (Hymenoptera Formicidae): a key to the invasive success of a tramp species. Naturwissenschaften 92: 319-323
Feitosa R.M. 2007. Aggregation and adult transport in disturbed colonies of Wasmannia auropunctata Roger (Hymenoptera Formicidae). Insect. Soc. 54: 200-201
Friedel P., Krampl V., Radford T., Renner J.A., Shephard F.W. and Gianturco M.A. 1971. Some constituents of the aroma complex of coffee. J. Agr. Food Chem. 19: 530-532
Fujiwara-Tsujii N., Yamagata N., Takeda T., Mizunami M. and Yamaoka R. 2006. Behavioral responses to the alarm pheromone of the ant Camponotus obscuripes (Hymenoptera: Formicidae). Zool. Science 23: 353-358
Giraud T., Pedersen J.S. and Keller L. 2002. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA 99: 6075-6079
Hölldobler B. and Wilson E.O. 1990. The Ants. Harvard University Press Cambridge, Mass. 732 pp
Hölldobler B., Morgan E.D., Oldham N.J. and Liebig J. 2001. Recruitment pheromone in the harvester ant genus Pogonomyrmex. J. Insect Physiol. 47: 369-374
Holway D.A., Suarez A.V. and Case T.J. 1998. Loss of intraspecific aggression in the success of a widespread invasive social insect. Science 282: 949-952
Howard D.F., Blum M.S., Jones T.H. and Tomalski M.D. 1982. Behavioral responses to an alkylpyrazine from the mandibular gland of the ant Wasmannia auropunctata. Insect. Soc. 29: 369-374
Hughes W.O.H., Howse P.E., Vilela E.F., Knapp J.J. and Goulson D. 2002. Field evaluation of potential of alarm pheromone compounds to enhance baits for control of grass-cutting ants (Hymenoptera: Formicidae). J. Econ. Entomol. 95: 537-543
Hwang H.-I., Hartman T.G. and Ho C.-T. 1995. Relative reactivities of amino acids in pyrazine formation. J. Agr. Food Chem. 43: 179-184
Hwang H.-I., Hartman T.G., Rosen R.T. and Ho C.-T. 1993. Formation of pyrazines from the Maillard reaction of glucose and glutamine-amide-15N. J. Agr. Food Chem. 41: 2112-2115
Joo K. and Ho C.-T. 1997. Quantitative analysis of alkylpyrazines in regular- and low-fat commercial peanut butter preparations. Biosci. Biotech. Biochem. 61: 171-173
Jourdan H., Bonnet de Larbogne L. and Chazeau J. 2002. The recent introduction of the neotropical ant Wasmannia auropunctata (Roger) into Vanuatu archipelago (Southwest Pacific). Sociobiology 40: 483-509
Kirschenbaum R. and Grace J.K. 2007. Dominant ant species in four habitats in Hawaii (Hymenoptera: Formicidae). Sociobiology 50: 1069-1073
Le Breton J., Chazeau J. and Dejean A. 2002. Field experiments to assess the use of repellent substances by Wasmannia auropunctata (Formicidae: Myrmicinae) during food exploitation. Sociobiology 40: 437-442
Le Breton J., Chazeau J. and Jourdan H. 2003. Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on native litter ant fauna in a New Caledonian rainforest. J. Aust. Ecol. 28 204-209
Le Breton J., Delabie J.H.C., Chazeau J., Dejean A. and Jourdan H. 2004. Experimental evidence of large-scale unicoloniality in the tramp ant Wasmannia auropunctata (Roger). J. Insect Behav. 17: 263-271
Lowe S., Browne M. and Boudjelas S. 2000. 100 of the world’s worst invasive alien species. Aliens 12: S1-S12
Morgan E.D., do Nascimento R.R., Keegans S.J. and Billen J. 1999. Comparative study of mandibular gland secretions of workers of Ponerine ants. J. Chem. Ecol. 25 1395-1409
Mori K. 2007. Significance of chirality in pheromone science. Bioorg. Med. Chem. 15: 7505-7523
Oldham N.J., Keegans S.J. and Morgan E.D. 1994. Mandibular gland contents of a colony of the queenless ponerine ant Dinoponera australis. Naturwissenschaften 81: 313-316
Passera L. 1994. Characteristics of tramp species. In: Exotic Ants: Biology, Impact, and Control of Introduced Species (D.F. Williams, Ed). Westview Press, Boulder, pp 23-43
Riley R.G., Silverstein R.M. and Moser J.C. 1974. Biological responses of Atta texana to its alarm pheromone and the enantiomer of the pheromone. Science 183: 760-762
Sato N. and Matsuura T. 1996. Studies on pyrazines. Part 32. Synthesis of trisubstituted and tetrasubstituted pyrazines as ant pheromones. J. Chem. Soc., Perkin Trans. 1: 2345-2350
Smith M.R. 1965. House-infesting ants of the eastern United States. Their recognition, biology, and economic importance. U.S. Department of Agriculture Technical Bulletin, pp 1-105
Suckling D.M., Peck R.W., Manning L.M., Stringer L.D., Cappadonna J. and El-Sayed A.M. 2008. Pheromone disruption of Argentine ant trail integrity. J. Chem. Ecol. 34: 1602-1609
Tecle B., Sun C.-M., Brophy J.J. and Toia R.F. 1987. Novel pyrazines from the head of Austrailian ponerine ant Rhytidoponera metallica. J. Chem. Ecol. 13: 1811-1822
Tentschert J., Bestmann H.-J., Hölldobler B. and Heinze J. 2000. 2,3-dimethyl-5-(3-methylpropyl)pyrazine, a trail pheromone component of Eutetramorium mocquerysi Emery (1899) (Hymenoptera: Formicidae). Naturwissenschaften 87: 377-380
Ulloa-Chacón P., Cherix D. and Meier R. 1991. Bibliografía de la hormiga colorada Wasmannia auropunctata (Roger) (Hymenoptera: Formicidae). Notic. Galápagos 50: 8-12
Vander Meer R.K., Alvarez F. and Lofgren C.S. 1988. Isolation of the trail recruitment pheromone of Solenopsis invicta. J. Chem. Ecol. 14: 825-838
Walker K.L. 2006. Impact of the little fire ant, Wasmannia auropunctata, on native forest ants in Gabon. Biotropica 38: 666-673
Wetterer J.K. 1997. Alien ants of the Pacific islands. Aliens 6: 3-4
Wetterer J.K. and Porter S.D. 2003. The little fire ant, Wasmannia auropunctata: Distribution, impact and control. Sociobiology 41: 1-41
Wetterer J.K., Walsh P.D. and White L.J.T. 1999. Wasmannia auropunctata (Roger) (Hymenoptera: Formicidae), a destructive tramp-ant, in wildlife refuges of Gabon. African Entomol. 7: 1-3
Williams D.F. and Whelan P.M. 1992. Bait attraction of the introduced pest ant Wasmannia auropunctata (Hymenoptera: Formicidae) in the Galapagos Islands. J. Entomol. Sci. 27: 29-34
Acknowledgments
We would like to thank Esther Schneider and Janice Nagata for assistance in conducting field experiments and collecting ants; The Shenandoah Valley NMR Consortium housed at James Madison University for use of 1H NMR; Nathan Derstine and two anonymous reviewers for comments and suggestions that improved the manuscript. This project was funded in part by grants from the Hawaii Invasive Species Council Research and Technology Grant Program (Agreement # 58148) and from the Thomas F. and Kate Miller Jeffress Memorial Trust Research Grants Program (Agreement # J-880).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Showalter, D.N., Troyer, E.J., Aklu, M. et al. Alkylpyrazines: alarm pheromone components of the little fire ant, Wasmannia auropunctata (Roger) (Hymenoptera, Formicidae). Insect. Soc. 57, 223–232 (2010). https://doi.org/10.1007/s00040-010-0075-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-010-0075-4