Abstract
Four species of Tetramorium pavement ants are known to guide foraging activities of nestmates via trail pheromones secreted from the poison gland of worker ants, but the trail pheromone of T. immigrans is unknown. Our objectives were to (1) determine whether poison gland extract of T. immigrans workers induces trail-following behavior of nestmates, (2) identify the trail pheromone, and (3) test whether synthetic trail pheromone induces trail-following behavior of workers. In laboratory no-choice bioassays, ants followed poison-gland-extract trails farther than they followed whole-body-extract trails or solvent-control trails. Gas chromatographic-electroantennographic detection (GC-EAD) analyses of poison gland extract revealed a single candidate pheromone component (CPC) that elicited responses from worker ant antennae. The CPC mass spectrum indicated, and an authentic standard confirmed, that the CPC was methyl 2-methoxy-6-methylbenzoate (MMMB). In further laboratory no-choice bioassays, ants followed poison-gland-extract trails (tested at 1 ant equivalent) and synthetic MMMB trails (tested at 0.35 ant equivalents) equally far, indicating that MMMB is the single-component trail pheromone of T. immigrans. Moreover, in laboratory two-choice bioassays, ants followed MMMB trails ~ 21-times farther than solvent-control trails. In field settings, when T. immigrans colonies were offered a choice between two paper strips treated with a synthetic MMMB trail or a solvent-control trail, each leading to an apple bait, the MMMB trails efficiently recruited nestmates to baits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Except for polar regions, ants occupy every terrestrial habitat (Hölldobler and Wilson 1992). Pavement ants thrive in urban settings, where they nest beneath and forage along sidewalks and driveways (Buczkowski and Richmond 2012; Penick et al. 2015; JMC, pers. obs.). Tetramorium immigrans is the most common pavement ant in North America (Flucher et al. 2021; Wagner et al. 2017). It is native to the western Palaearctic and was likely introduced to North America in the 1800s (Brown 1957; Flucher et al. 2021). It is now found in British Columbia (BC), Ontario, and Quebec (Guénard 2017), and in both urban and agricultural environments in the U.S.A. (Zhang et al. 2019; Helms et al. 2021).
Like many other ants, Tetramorium colonies recruit nestmates to food sources via trail pheromones that foraging ants deposit as chemical orientation guidelines for nestmates (Beckers et al. 1990; Morgan 2009; Czaczkes et al. 2015). To date, trail pheromones are known for four Tetramorium species: T. caespitum (3-ethyl-2,5-dimethylpyrazine, 2,5-dimethylpyrazine; Attygalle and Morgan 1983), T. impurum (methyl 2-hydroxy-6-methylbenzoate; Morgan and Ollett 1987), T. meridionale (indole, methylpyrazine, 2,5-dimethylpyrazine, trimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine; Jackson et al. 1990), and T. tsushimae (methyl 2-hydroxy-6-methylbenzoate; Nakamura et al. 2019). In all four Tetramorium species, the poison gland produces and secretes the trail pheromone (Attygalle and Morgan 1983; Morgan and Ollett 1987; Jackson et al. 1990; Nakamura et al. 2019), as has been shown for more than 30 myrmicine ant species (Morgan 2009; Cerdá et al. 2014).
Our objectives were to (1) determine whether poison gland extract of T. immigrans workers induces trail-following behavior of worker ants, (2) identify the trail pheromone, and (3) test whether synthetic trail pheromone induces trail-following behavior of worker ants in both laboratory and field settings.
Methods and Materials
Experimental Insects
Laboratory colonies of T. immigrans were established between July and September 2019 from natural nests located within the Iona Island Causeway (Latitude: 49°12′41″ N, Longitude: 123°12′08″ W; Richmond, BC, Canada). From various nest entrances that were separated by at least 1 m, > 200 worker ants were collected and placed in bins (41 × 29 × 24 cm or 58 × 43 × 31 cm) that were housed in the Science Research Annex on the Burnaby campus (49°16′33″ N, 122°54′55″ W) of Simon Fraser University. The room temperature was set to 25 °C and the photoperiod to 12L:12D. The bins contained a 5–10-cm thick layer of the same substrate (sand, rocks, wood) in which field nests resided from which the ants were collected. Three times per week, each nest was provisioned with food consisting of ambrosia apples as well as euthanized mealworms, Tenebrio molitor, and German cockroaches, Blattella germanica. Water was provided in a test tube (10 mL) fitted with a plug of cotton which was replaced when it became dry. Twice per week, the bins were sprayed with water to ensure adequate moisture in the nest substrate and foraging area.
Preparation of Poison Gland Extract
To obtain poison gland extract, each of 60 worker ants was cold-euthanized on dry ice and submerged in distilled water. Using fine hard-tipped forceps, the stinger—with the poison gland and the Dufour’s gland attached—was gently pulled out so that it floated to the water surface. The two glands were identified by reference to figures from Hölldobler and Wilson (1992) and were separated using an insect pin, forceps, and dissection scissors. The poison glands of 30 workers were placed in a vial kept on dry ice and filled with 800 µL of dichloromethane (DCM; EMD Millipore Corp., Billerica, MA, U.S.A.). Gland tissues were macerated, using a glass stirring rod and a vortex mixer (Labnet International Inc., Edison, NJ, U.S.A.), and were then filtered through a ~ 10-mg glass wool plug (5 × 3 mm diam.) in a glass pipette to obtain ‘tissue-free’ poison gland extract. Two samples of 30 glands each that had been obtained on different days were combined in a single poison gland extract, and aliquots of 1 ant equivalent (1 AE) in 25 µL of DCM were tested in trail-following experiments (see below).
Preparation of Whole-Body Extract
To account for the (unlikely) possibility that the trail pheromone of T. immigrans is produced by a gland other the poison gland, and not knowing the location of this gland, we also obtained a ‘whole-body extract’ of worker ants. To this end, the severed heads, thoraces, and abdomens of 30 cold-euthanized ants were placed in three separate (tagma-specific) vials kept on dry ice and filled with 1 mL of DCM. After macerating the body sections with a glass rod, syringe plunger, and a vortex mixer (Labnet International Inc., Edison, NJ, U.S.A.), each of the three samples was filtered through a glass wool plug (see above) to obtain ‘tissue-free’ head, thorax, and abdomen extracts, respectively. After taking subsamples of these extracts for chemical analyses (not reported here), the extracts were combined in a single whole-body extract, and aliquots of 1 AE in 25 µL of DCM were tested in trail-following experiments (see below).
Circular Trail-following Experiment 1 (Laboratory Setting)
The experimental design was slightly modified from previous descriptions (Chalissery et al. 2019; Renyard et al. 2019). Briefly, to standardize visual cues during bioassays, all bioassays were run under a metal scaffold (123 × 57 × 36 cm) enclosed in black fabric, lit from above with two lights (one plant light and one daylight fluorescent light, each 48″ 32 W F32T8; Phillips, Amsterdam, NL), and fitted with a video camera (Sony HDR CX210, Sony, Tokyo, JP) to record the ants’ behavior. Using a micro-syringe, a test stimulus was applied as a continuous 25-cm long trail 5 mm from the edge of a circular Whatman filter paper (90 mm diam.; Sigma-Aldrich), with the circumference marked with a pencil in 1-cm intervals. This filter paper was then placed in a Pyrex glass Petri dish arena (150 mm diam.; Fig. 1a).
Graphic and photographic illustrations of experimental designs (drawings not to scale). a Design components of circular trail-following experiments, consisting of (i) a Pyrex petri dish arena fitted with a circular filter paper marked in 1-cm intervals along the edge; (ii) a holding tube from which a single ant enters the arena; and (iii) a 25-cm long stimulus trail (dotted line). b Design components of the choice-of-trail laboratory experiment, consisting of (i) a Plexiglass arena fitted with a sheet of paper marked with pencil lines in V-shape (dotted lines), with each line being divided into 1-cm intervals and treated with a test stimulus; and (ii) a holding tube from which a single ant enters the arena. c Design components of the choice-of-trail field experiment, consisting of (i) two paper strips placed at 0° and 180° from the nest entrance (denoted by arrow) and treated with a test stimulus; (ii) nails securing the strips to the ground; and (iii) an apple bait at the distant end of strips. Note the number of ants on the apple bait with a pheromone trail leading to it
Bioassay ants were isolated singly in 1.5-mL micro-tubes (Axygen™ MaxyClear Snaplock; Thermo Fisher Scientific, Waltham, MA, U.S.A.) and allowed 5 min to acclimate in the scaffold enclosure (see above). To initiate a bioassay, a micro-tube was placed in the Petri dish arena so that its exit hole was ~ 1 cm away from the edge of the filter paper (Fig. 1a), and the ant was given 5 min to leave the tube. Once an ant had exited the micro-tube, her behavior was video recorded for 5 min. Video footage was analyzed using VLC Media Player (Version 2.2.6) or QuickTime Player (Version 10.4) to count the number of 1-cm intervals a trail-following ant had crossed during a bioassay, allowing us to determine the total distance she had covered as a measure of orientation in response to the test stimulus (Morgan 2009; Chalissery et al. 2019; Renyard et al. 2019). Ants that did not exit the micro-tube within 5 min were considered non-responders and were excluded from statistical analyses. For each replicate testing a single ant, a new filter paper was used.
Experiment 1 (n = 10) tested three treatments: (1) poison gland extract (1 AE in 25 μl DCM), (2) whole-body extract (1 AE in 25 μl DCM), and (3) a solvent control (25 μl DCM). The same number of replicates was run for each treatment on any bioassay day. Between bioassays, preparative surfaces and the Petri dish arenas were cleaned with 70% ethanol and hexane, and the room was aired out for 5–10 min by opening an exterior door.
Gas Chromatographic-Electroantennographic Detection (GC-EAD) and GC-Mass Spectrometric (MS) Analyses of Poison Gland Extract
With evidence that ants follow poison-gland-extract trails significantly farther than solvent-control trails (see Results), the poison gland extract was analyzed by GC-EAD and GC–MS in splitless mode (1–2 µL injection volume), using procedures and equipment modified from previous reports (Gries et al. 2002). Briefly, the GC-EAD setup employed a Hewlett-Packard (HP) 5890 gas chromatograph (GC) fitted with a DB-5 GC column (30 m × 0.32 mm I.D., film thickness 0.25 µm; J & W Scientific, Folsom, CA, U.S.A.). Helium served as carrier gas (35 cm · s−1) with the following oven program: 100 °C for 1 min., then 20 °C · min−1 to 280 °C. The injector port and flame ionization detector (FID) were set at 260 °C. For GC-EAD recordings (N = 2), an antenna was carefully dislodged from the head of a worker ant and suspended between two glass capillary electrodes [1.0 mm outer diameter (0.58 mm inner diameter) × 100 mm; A-M Systems, Carlsborg, WA, U.S.A.] adapted to accommodate the ant antenna (~ 1 mm in length) and filled with saline (Staddon and Everton 1980). Antennal responses to compounds in the column effluvium, that was directly released into a stream of medical air (250 mL/min flow) continuously passing over the electrode-suspended antenna, were amplified with a custom-built amplifier and recorded on an HP 3392A integrator. The compound in the poison gland extract that elicited a response from ant antennae (see Results) was considered a candidate pheromone component (CPC) and was analyzed by GC–MS in full-scan electron ionization mode, using an Agilent 7890B GC coupled to a 5977A MSD (Agilent Technologies Inc., Santa Clara, CA, U.S.A.) and fitted with a DB-5 GC–MS column (30 m × 0.25 mm ID, film thickness 0.25 µm). The injector port was set to 250 °C, the MS source to 230 °C, and the MS quadrupole to 150 °C. Helium was used as a carrier gas at a flow rate of 35 cm s−1 with the following temperature program: 50 °C held for 5 min, 10 °C min−1 to 280 °C (held for 10 min).
To determine the structure of the CPC, its mass spectrum and retention index (relative to aliphatic alkanes; Van Den Dool and Kratz 1963) were compared with those of authentic standards that were synthesized in our laboratory (see below). To quantify the amount of CPC in the poison gland extract, its GC peak area was compared with that of a synthetic standard prepared at 1 ng/μl.
Syntheses of Candidate Pheromone Components
Methyl 2-methoxy-6-methylbenzoate
2-Hydroxy-6-methylbenzoic acid (1 mmol, 152 mg), iodomethane (6 mmol, 850 mg), and K2CO3 (7.5 mmol, 1036 mg) were suspended in 5 mL of dimethylformamide (DMF) and heated to 70 °C for 16 h. After cooling to room temperature (RT), the solution was diluted in water and extracted with ethyl acetate. After drying over Na2SO4, the organic phase was evaporated and the resulting oil was purified by flash chromatography (silica gel, hexane/ethyl acetate 3:1), affording methyl 2-methoxy-6-methylbenzoate (126 mg, 70% yield) as a yellow oil. The NMR data were in agreement with those previously reported (Baur et al. 2013).
Methyl 2-hydroxy-4,6-dimethylbenzoate
4,6-Dimethylsalicylic acid (1 mmol, 166 mg) in MeOH (5 mL) was treated at RT with 0.1 mL of sulfuric acid (95%) and 0.1 g molecular sieves (0.4 nm). After heating the reaction mixture at reflux for 72 h, it was cooled to RT, filtered, and the residue was washed with MeOH. The filtrate was evaporated, and the residue was dissolved in CH2Cl2, poured into crushed ice, and extracted twice with CH2Cl2. The organic phases were washed with water, dried over magnesium sulfate, filtered, and concentrated. The residue was purified by flash column chromatography (hexane/EtOAc 4:1), affording methyl 2-hydroxy-4,6-dimethylbenzoate (72 mg, 40% yield) as a colorless solid. The NMR data were in agreement with those previously reported (Reim et al. 2009).
Circular Trail-Following Experiment 2 (Laboratory Setting)
Following the identification of methyl 2-methoxy-6-methylbenzoate (MMMB) as the candidate trail pheromone component (see Results), experiment 2 (n = 20) compared trail-following behaviour of ants in response to (1) synthetic MMMB (2.5 ng; 0.35 AE in 25 μl DCM), (2) poison gland extract (1 AE in 25 μl DCM), and (3) a solvent control stimulus (25 μl DCM). Because we assumed that ants secrete only a portion of their poison gland pheromone content when marking trails, we kept the amount of synthetic MMMB, conservatively, about threefold lower than the amount measured in 1 AE of extract. The protocol of experiment 2 closely followed that of experiment 1 (see above).
Choice-of-Trail Experiment (Laboratory Setting)
With data revealing, in circular trail-following experiment 2, that ants follow synthetic pheromone trails significantly farther than solvent-control trails (see Results), we wanted to ascertain that they did not just follow the edge of the circular filter paper. Therefore, we adopted the design of a ‘choice-of-trail’ experiment (n = 18) previously described (Renyard et al. 2019). Briefly, a plexiglass arena (64 × 44 × 10 cm) was fitted with a sheet of white printer paper (22 × 28 cm) marked with two pencil lines in a V-shape (45° angle), with each line divided into 25 1-cm intervals (Fig. 1b). For each replicate, the treatment stimulus (synthetic MMMB (2.5 ng) at 0.35 AE in 25 µL DCM) and the control stimulus (25 µL DCM) were randomly assigned to one of the two lines and applied with a micro-syringe. To initiate a bioassay replicate, the micro-tube containing a single ant was placed into the arena such that the exit hole of the tube pointed towards the intersection of the “V”. For each ant, we recorded the distance she walked along either line. Ants that did not exit the micro-tube within 5 min were considered non-responders and were excluded from statistical analyses. For each replicate testing a single ant, a new filter paper was used, and all surfaces were cleaned with 70% ethanol and hexane.
Choice-of-Trail Experiment (Field Setting)
To determine whether synthetic trail pheromone affected nestmate recruitment in field settings, a choice-of-trail experiment was run in Surrey (49°10′20″N 122°42′09″W), BC, CA in June 2020, and Burnaby (49°12′29″N 122°59′22″W), BC, CA in June 2021 (n = 11). Ant colonies with more than 20 ants near the nest entrance at the time of assessment, or with more than 50 foragers recruited within 30 min to an apple-slice bait placed 25 cm from the nest entrance, were deemed to have high foraging activity, whereas all others were considered colonies of low foraging activity.
For each replicate, two strips of filter paper (25 × 2 cm each) were placed at 0º and 180º as close as possible to the entrance of a T. immigrans nest (Fig. 1c), taking care not to disrupt debris near the entrance. To minimize the likelihood of recruiting the same foragers in various replicates, only nests with entrances separated by at least 0.5 m were selected for testing. To prevent wind from moving strips, they were weighed down with several nails placed along the edges (but not center) of strips. The distant end of each strip was baited with a small cylinder (~ 0.5 × 2 cm) of an ambrosia apple presented on a piece of paper (4.5 × 4.5 cm) to prevent ant access from below. After placing apple baits, the treatment stimulus (synthetic MMMB (2.5 ng) at 0.35 AE in 25 µL DCM) and the control stimulus (25 µL DCM) were randomly assigned to one of the two strips and applied with a micro syringe. To determine whether synthetic MMMB expedited recruitment of nestmates to baits, photos of ants at food baits were taken at 5-min intervals for 50 min.
Statistical Analyses
We analyzed all data with R 3.6.3 using packages “multcomp”, “plotrix”, “plyr”, “nlme”, and “emmeans”. Data (distance followed) of circular trail-following experiments were analyzed using a generalized linear model (GLM; quasi-Poisson distribution). The mean and standard error for distance followed by ants in response to test stimuli were analyzed by an analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) tests. Data (proportional distance walked by ants on pheromone trails or solvent-control trails) of the choice-of-trail experiment (laboratory setting) were compared against a theoretical 50:50 distribution using a χ2-test. The mean and standard error for distance walked by ants in response to test stimuli were analyzed by a paired t-test. For the choice-of-trail experiment (field setting), we analyzed the number of foraging ants on food baits at the end of either a pheromone trail or solvent-control trail over 10 5 min time points using a linear mixed-effects model. The mean and standard error for number of foraging ants in response to treatment and time point (with colony and location as random variables) were analyzed by a repeated measures analysis of variance (ANOVA).
Results
Circular Trail-Following Experiment 1 (Laboratory Setting)
The distances worker ants followed trails of (i) poison gland extract, (ii) whole-body extract or (iii) a solvent control significantly differed (ANOVA, F(2, 27) = 11.6, P < 0.001; Fig. 2a). Ants followed poison-gland-extract trails farther than whole-body-extract trails (z = 3.24, P = 0.00325) or solvent-control trails (z = 3.52, P = 0.00177), but followed whole-body-extract trails and solvent-control trails equally far (z = 0.669, P = 0.777).
Distances that worker ants of Tetramorium immigrans followed trails in circular trail-following experiments (design in Fig. 1a). Grey and black symbols show the distance that each ant, and 10 ants (a) or 20 ants (b) on average (mean ± standard error), followed trails. Test stimuli consisted of (a) a solvent control (circles), whole-body extract [WBE, 1 ant equivalent; squares] and poison gland extract [PGE, 1 ant equivalent; triangles] of T. immigrans workers, or (b) a solvent control [circles], poison gland extract [PGE, 1 ant equivalent; triangles] of T. immigrans workers, and synthetic methyl 2-methoxy-6-methylbenzoate [MMMB (2.5 ng = 0.35 ant equivalents); squares]. In each subpanel, means associated with different letters are statistically different (Tukey’s HSD test, P < 0.01)
GC-EAD and GC–MS Analyses of Poison Gland Extract
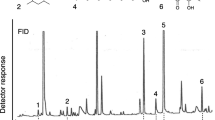
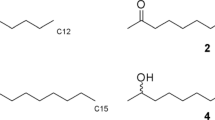
GC-EAD analyses of T. immigrans poison gland extract revealed a single candidate pheromone component (marked as ‘CPC’ in Fig. 3a) that consistently elicited responses from worker ant antennae. The mass spectrum of CPC (Fig. 3b) indicated a nominal mass of 180 daltons, and fragment ions indicative of an aromatic ring (m/z 77) and a methyl branch on the ring (m/z 91). Overall, the CPC mass spectrum resembled that of methyl 2-hydroxy-6-methylbenzoate (MW 166), but the CPC had one additional carbon. We hypothesized that the CPC had a second methyl branch on the aromatic ring or a methoxy group, instead a hydroxyl group, at C2. Keeping the methyl branch conservatively at C6, we first synthesized methyl 2-hydroxy-4,6-dimethylbenzoate, which eluted later than the CPC by GC. We then prepared methyl 2-methoxy-6-methylbenzoate (MMMB) which had a mass spectrum and a GC retention index consistent with those of the CPC. With this information in hand, we then determined that the poison gland of an individual ant contained about 7 ng of MMMB.
a Representative recording (one of two replicates) of the responses of a gas chromatographic flame ionization detector (FID) and an electroantennographic detector (EAD: antenna of a Tetramorium immigrans worker ant) to compounds present in poison gland extract of T. immigrans worker ants. CPC denotes methyl 2-methoxy-6-methylbenzoate; the first large peak (eluting between 5.5 and 6.0 min) was pentadecane, the second large peak (eluting between 6.0 and 6.5 min) is unknown. b Mass spectrum of methyl 2-methoxy-6-methylbenzoate
Circular Trail-Following Experiment 2 (Laboratory Setting)
The distances worker ants followed trails of (i) poison gland extract, (ii) synthetic MMMB, and (iii) a solvent control significantly differed (ANOVA, F(2, 57) = 19.8, P < 0.001; Fig. 2b). Ants followed poison-gland-extract trails and synthetic MMMB trails equally far (z = − 0.647, P = 0.787) but followed trails of either stimulus farther than solvent-control trails (poison gland extract: z = 4.80, P < 0.001; synthetic MMMB: z = 4.42, P < 0.001).
Choice-of-Trail Experiment (Laboratory Setting)
When presented with a choice between two pencil lines drawn in a V-shape and treated with either synthetic MMMB or a solvent control, the proportion of distance that ants followed either line differed from a theoretical 50:50 distribution (χ2 = 16.8, df = 1, P < 0.001). Ants followed the pheromone trail ~ 21-times farther than the solvent-control trail (Paired t-test: t = − 8.70, df = 17, P < 0.001; Fig. 4).
Distances worker ants of Tetramorium immigrans followed V-shaped trails in a choice-of-trail laboratory experiment (design in Fig. 1b). Grey and black symbols show the distance that each ant and 18 ants on average (mean ± standard errors), followed trails. Test stimuli consisted of a solvent control (circles) and synthetic methyl 2-methoxy-6-methylbenzoate [MMMB (2.5 ng = 0.35 ant equivalents); squares]. Means associated with different letters are statistically different (Tukey’s HSD test, P < 0.01)
Choice-of-Trail Experiment (Field Setting)
When T. immigrans colonies with low and high foraging activities of ants (random variable) were offered a choice between two paper strips placed at 0° and 180° from a nest entrance and treated with either a synthetic MMMB trail or a solvent-control trail each leading to an apple bait (Fig. 1c), there was a significant effect of treatment (pheromone vs control) and time point (5–50 min in 5-min intervals) on the number of ants recruited to the apple bait (repeated measures ANOVA: treatment, F(1,17) = 48.4, P < 0.001; time point, F(9,189) = 14.4, P < 0.001; Fig. 5).
Mean numbers (solid and dotted lines; standard errors = shaded areas around lines) of Tetramorium immigrans worker ants recruited to apple baits when offered a choice between two paper strips placed at 0° and 180° from a nest entrance and treated with either a synthetic pheromone trail [methyl 2-methoxy-6-methylbenzoate (2.5 ng = 0.35 ant equivalents)] or a solvent-control trail (design in Fig. 1c). Analyses of all replicates (with activity level as a random variable) revealed a significant effect of treatment (pheromone vs control) and time point on the number of ants recruited to apple baits (repeated measures ANOVA: treatment, P < 0.001; time point, P < 0.001). Note the pronounced effect of the trail pheromone on ant recruitment in settings of low ant foraging activity
Discussion
Our data support the conclusions that (1) methyl 2-methoxy-6-methylbenzoate (MMMB) is the major and possibly the only component of the T. immigrans trail pheromone, (2) MMMB originates from the poison gland, and (3) synthetic MMMB deployed in field settings expedites recruitment of nestmates to food sources.
Drawing on the literature that trail pheromones of myrmicine ants often originate from the poison gland (Morgan 2009; Cerdá et al. 2014), we excised the poison gland from the abdomen of ants, extracted the glands in DCM, and bioassayed poison gland extract for trail-following behavior (Fig. 2a). To address the possibility that the trail pheromone of T. immigrans might originate from a gland other than the poison gland, we also prepared extracts of the abdomen, thorax and head, and combined aliquots of these three separate extracts in a whole-body extract for trail-following bioassays. As predicted, ants followed poison-gland-extract trails tested at 1 ant equivalent significantly farther than they followed solvent-control trails (Fig. 2a). However, whole-body-extract trails (which contained the trail pheromone) were not followed any farther than solvent-control trails (Fig. 2a). As the poison gland extract and the (recombined) whole-body extract contained trail pheromone in similar amounts, this suggests that the whole-body extract contained other pheromones that function in diverse contexts, and thus may have presented a “mixed” message to ants, prompting no trail-following response. For example, the whole-body extract might contain an alarm pheromone that may originate from an exocrine gland in the head (Blum 1969; Pasteels et al. 1980). Analyzing head, thorax, and abdomen extracts separately by GC–EAD and GC–MS in a future study, and testing synthetic compounds in behavioral bioassays, should reveal the pheromone(s) that interfered with trail-following behavior by T. immigrans ants when they were presented with a whole-body extract.
With convincing evidence that the trail pheromone of T. immigrans originates from the poison gland (Fig. 2), we analyzed poison gland extract by GC–EAD and GC–MS. The single compound that consistently elicited responses from ant antennae (Fig. 3a) had a mass spectrum that resembled that of methyl 2-hydroxy-6-methylbenzoate [the trail pheromone of T. impurum (Morgan et al. 1990)] but indicated one additional carbon (Fig. 3b). Of the two most likely structures, methyl 2-hydroxy-4,6-dimethylbenzoate and methyl 2-methoxy-6-methylbenzoate (MMMB), only the latter had spectrometric and chromatographic characteristics entirely consistent with the ant-produced compound.
Based on this result, we prepared synthetic MMMB for circular trail-following bioassays (Fig. 1A), testing it at 0.35 ant equivalents. Even at this low dose, ants followed MMMB trails for about the same distance as they followed poison-gland-extract trails tested at 1 ant equivalent (Fig. 2b). These bioassay data, coupled with electrophysiological data showing that ant antennae sense only MMMB of all the compounds present in poison gland extracts (Fig. 3a), support the conclusion that MMMB is the major and possibly only component of the T. immigrans trail pheromone. MMMB is also a trace component in the poison gland of T. impurum and may enhance the effect of the major pheromone component methyl 2-hydroxy-6-methylbenzoate (Morgan et al. 1990), but no quantitative data on component interactions have been reported.
To address the possibility that T. immigrans ants followed MMMB trails, in part, because these trails were presented along the edge of a circular filter paper which may have served as a physical guide, we also tested synthetic MMMB in a choice-of-trail experiment, as previously described for trail pheromone testing with western carpenter ants, Camponotus modoc (Renyard et al. 2019). In this type of bioassay, single ants exit a holding tube and face a choice between two trails presented in V-shape without a physical edge in the center of plain paper (Fig. 1b). When tested in this choice-of-trail bioassay, worker ants of T. immigrans followed synthetic MMMB trails ~ 21-times farther than they followed solvent-control trails, indicating that a physical guide, such as the filter paper edge in circular trail-following bioassays, is not needed for trail-following responses of T. immigrans workers. In contrast, the amount of trail pheromone deployed could have a strong effect on trail-following behavior, but it is not necessarily the largest amount that elicits the strongest trail-following response (Renyard et al. 2019; and references therein). The optimal dose of MMMB for trail-following of T. immigrans workers has yet to be determined.
With convincing data that synthetic MMMB elicited trail-following behavior by T. immigrans in laboratory bioassays (Figs. 2, 4), we then tested synthetic MMMB in field settings, using a choice-of-trail experiment with multiple T. immigrans nests. For each nest, we placed two paper strips at 0° and 180° from the nest entrance (Fig. 1c), baited the distant end of each strip with a piece of apple, and applied synthetic MMMB or a solvent-control stimulus to each strip. As trail pheromones serve to recruit nestmates to food sources (Beckers et al. 1990; Morgan 2009; Czaczkes et al. 2015), we recorded the number of nestmates recruited to apple baits, rather than distance followed, as the response criterion. Our field data show that trails of synthetic MMMB expedite recruitment of nestmates to apple baits (Fig. 5). The recruitment effect of synthetic MMMB was clearly apparent in settings of both low and high ant foraging activity, but the effect was particularly pronounced in low ant activity settings (Fig. 5a). There, pheromone trails prompted expeditious recruitment of nestmates to apple baits. While workers in high ant activity settings quickly located the pheromone trails and apple baits, the number of ants recruited to baits plateaued early (Fig. 5b), thereby preventing trail and resource ‘overcrowding’. Both data sets reflect an optimal foraging strategy, as reported for many ants (Detrain and Deneubourg 2008; Czaczkes et al. 2015).
Synthetic trail pheromones have been field-tested for trail-following behavior of ants in only one other study, dating back 40 years (Tumlinson et al. 1972). In this study, medium and large workers of the leaf-cutting ant, Atta texana, readily followed the trail pheromone methyl 4-methylpyrrole-2-carboxylate when applied as a 2.7-pg/cm trail on a cardboard strip placed across an “erased” section of the natural pheromone trail. According to this study, the ants also responded to a synthetic pheromone trail that was dribbled on sand at a 45° angle from the natural trail.
Other studies have tested the synthetic trail pheromone ((Z)-9-hexadecenal) of Argentine ants as a means of enhancing the attractiveness and consumption of lethal baits (Greenberg and Klotz 2000; Welzel and Choe 2016) or of disrupting foraging activities (Sisk et al. 1996; Nishisue et al. 2010, 2020; Suckling et al. 2010, 2011; Sunamura et al. 2011). For example, permeating the environment with synthetic pheromone suppressed foraging activities of Argentine ants in pheromone treatment plots (Nishisue et al. 2020). However, we know of no field study which has explored the ability of trails of synthetic pheromone as a means of leading ants to a (lethal) food bait. The reasons for this are not known but may include (i) incomplete knowledge of the entire trail pheromone blend, (ii) lack of effective trail pheromone formulations, (iii) expectations that ants eventually find lethal food baits irrespective of trail pheromone deployment, and (iv) no prospect of commercializing synthetic trail pheromone lures for individual ant species. While further feasibility studies are needed, our field data suggest that the deployment of synthetic trail pheromone may have potential as a means of enhancing recruitment of ants to food baits treated with a toxicant. This tactic may be most effective at early stages of new ant invasions when population densities are still low or immediately following population knockdowns through various other control measures.
In conclusion, our data indicate that methyl 2-methoxy-6-methylbenzoate (MMMB) is the major and possibly only component of the T. immigrans trail pheromone, and that synthetic MMMB deployed in field settings expedites the recruitment of nestmates to baits. Our field data provide the basis for further investigations to determine whether synthetic trail pheromones can be developed for control of pest ants by enhancing recruitment to lethal baits that result in the demise of nests (e.g., Hoefele et al. 2021). This type of management tactic might be particularly useful for control of economically important invasive ant pests such as the red imported fire ant, Solenopsis invicta, and the tawny crazy ant, Nylanderia fulva. Commercial viability of this tactic may be increased if trail pheromones of multiple species can be combined into a single formulation, with potential synergism and no antagonism between pheromone components (Chalissery et al. 2019).
Data Availability
All data are presented in the main body of the manuscript.
Code Availability
Not applicable.
References
Attygalle AB, Morgan ED (1983) Identification of trail pheromone of the ant Tetramorium caespitum L. (Hymenoptera: Myrmicinae). Naturwissenschaften 70:364–365. https://doi.org/10.1007/BF00990315
Baur B, Storch K, Martz KE, Goettert MI, Richters A, Rauh D, Laufer SA (2013) Metabolically stable dibenzo[b,e]oxepin-11(6H)-ones as highly selective p38 MAP kinase inhibitors: optimizing anti-cytokine activity in human whole blood. J Med Chem 56:8561–8578. https://doi.org/10.1021/jm401276h
Beckers R, Deneubourg JL, Goss S, Pasteels JM (1990) Collective decision making through food recruitment. Insectes Soc 37:258–267. https://doi.org/10.1007/BF02224053
Blum MS (1969) Alarm pheromones. Annu Rev Entomol 14:57–80. https://doi.org/10.1146/annurev.en.14.010169.000421
Brown WLJ (1957) Is the ant genus Tetramorium native in North America? Breviora 72:1–8
Buczkowski G, Richmond DS (2012) The effect of urbanization on ant abundance and diversity: a temporal examination of factors affecting biodiversity. PLoS ONE 7:e41729. https://doi.org/10.1371/journal.pone.0041729
Cerdá X, van Oudenhove L, Bernstein C, Boulay RR (2014) A list of and some comments about the trail pheromones of ants. Nat Prod Commun 9:1934578X1400900. https://doi.org/10.1177/1934578X1400900813
Chalissery JM, Renyard A, Gries R, Hoefele D, Alamsetti SK, Gries G (2019) Ants sense, and follow, trail pheromones of ant community members. Insects 10:383. https://doi.org/10.3390/insects10110383
Czaczkes TJ, Grüter C, Ratnieks FLW (2015) Trail pheromones: an integrative view of their role in social insect colony organization. Annu Rev Entomol 60:581–599. https://doi.org/10.1146/annurev-ento-010814-020627
Detrain C, Deneubourg J-L (2008) Collective decision-making and foraging patterns in ants and honeybees. Adv Insect Physiol 35:123–173. https://doi.org/10.1016/S0065-2806(08)00002-7
Flucher SM, Krapf P, Arthofer W, Suarez AV, Crozier RH, Steiner FM, Schlick-Steiner BC (2021) Effect of social structure and introduction history on genetic diversity and differentiation. Mol Ecol mec. https://doi.org/10.1111/mec.15911
Greenberg L, Klotz JH (2000) Argentine ant (Hymenoptera: Formicidae) trail pheromone enhances consumption of liquid sucrose solution. J Econ Entomol 93:119–122. https://doi.org/10.1603/0022-0493-93.1.119
Gries R, Khaskin G, Gries G, Bennett RG, King GGS, Morewood PS, Slessor KN, Morewood D (2002) (Z,Z)-4,7-Tridecadien-(S)-2-yl acetate: sex pheromone of Douglas-fir cone gall midge, Contarinia oregonensis. J Chem Ecol 28:2283–2297. https://doi.org/10.1023/A:1021005517389
Guénard B (2017) The global ant biodiversity informatics (GABI) database: synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae). Myrmecol News 24:83–89. https://doi.org/10.25849/myrmecol.news_024:083
Helms JA, Roeder KA, Ijelu SE, Ratcliff I, Haddad NM (2021) Bioenergy landscapes drive trophic shifts in generalist ants. J Anim Ecol 90:738–750. https://doi.org/10.1111/1365-2656.13407
Hoefele D, Chalissery JM, Renyard A, Gries G (2021) Experimentally guided development of a food bait for European fire ants. Entomol Exp Appl 169:780–791. https://doi.org/10.1111/eea.13053
Hölldobler B, Wilson EO (1992) The ants. The Belknap Press of Harvard University Press, Cambridge
Jackson BD, Keegans SJ, Morgan ED et al (1990) Trail pheromone of the ant Tetramorium meridionale. Naturwissenschaften 77:294–296
Morgan ED (2009) Trail pheromones of ants. Physiol Entomol 34:1–17. https://doi.org/10.1111/j.1365-3032.2008.00658.x
Morgan ED, Ollett DG (1987) Methyl 6-methylsalicylate, trail pheromone of the ant Tetramorium impurum. Naturwissenschaften 74:596–597. https://doi.org/10.1007/BF00368520
Morgan ED, Jackson BD, Ollett DG, Sales GW (1990) Trail pheromone of the ant Tetramorium impurum and model compounds: structure-activity comparisons. J Chem Ecol 16:3493–3510. https://doi.org/10.1007/BF00982113
Nakamura T, Harada K, Akino T (2019) Identification of methyl 6-methylsalicylate as the trail pheromone of the Japanese pavement ant Tetramorium tsushimae (Hymenoptera: Formicidae). Appl Entomol Zool 54:297–305. https://doi.org/10.1007/s13355-019-00626-0
Nishisue K, Sunamura E, Tanaka Y, Sakamoto H, Suzuki S, Fukumoto T, Terayama M, Tatsuki S (2010) Long-term field trial to control the invasive Argentine ant (Hymenoptera: Formicidae) with synthetic trail pheromone. J Econ Entomol 103:1784–1789. https://doi.org/10.1603/EC10008
Nishisue K, Koyama S, Satoh T (2020) Identification of the Argentine ant Linepithema humile (Hymenoptera: Formicidae) using an artificially synthesized trail pheromone and its effects on native Japanese ants. Appl Entomol Zool 55:141–147. https://doi.org/10.1007/s13355-019-00663-9
Pasteels JM, Verhaeghe JC, Braekman JC, Daloze D, Tursch B (1980) Caste-dependent pheromones in the head of the ant Tetramorium caespitum. J Chem Ecol 6:467–472. https://doi.org/10.1007/BF01402923
Penick CA, Savage AM, Dunn RR (2015) Stable isotopes reveal links between human food inputs and urban ant diets. Proc R Soc B Biol Sci 282:20142608. https://doi.org/10.1098/rspb.2014.2608
Reim S, Lau M, Adeel M, Hussain I, Yawer MA, Riahi A, Ahmed Z, Fischer C, Reinke H, Langer P (2009) Synthesis of biaryls, fluorenones, cyclopenta[def]phenanthren-4-ones, and benzophenones based on formal [3+3] cyclocondensations of 1,3-bis(silyloxy)buta-1,3-dienes with 3-(silyloxy)-2-en-1-ones. Synthesis 3:445–463. https://doi.org/10.1055/s-0028-1083309
Renyard A, Alamsetti SK, Gries R, Munoz A, Gries G (2019) Identification of the trail pheromone of the carpenter ant Camponotus modoc. J Chem Ecol 45:901–913. https://doi.org/10.1007/s10886-019-01114-z
Sisk CB, Shorey HH, Gerber RG, Gaston LK (1996) Semiochemicals that disrupt foraging by the Argentine ant (Hymenoptera: Formicidae): laboratory bioassays. J Econ Entomol 89:381–385. https://doi.org/10.1093/jee/89.2.381
Staddon BW, Everton IJ (1980) Haemolymph of the milkweed bug Oncopeltus fasciatus (Heteroptera; lygaeidae): inorganic constituents and amino acids. Comp Biochem Physiol a: Physiol 65:371–374. https://doi.org/10.1016/0300-9629(80)90046-8
Suckling DM, Peck RW, Stringer LD, Snook K, Banko PC (2010) Trail pheromone disruption of Argentine ant trail formation and foraging. J Chem Ecol 36:122–128. https://doi.org/10.1007/s10886-009-9734-1
Suckling DM, Stringer LD, Corn JE (2011) Argentine ant trail pheromone disruption is mediated by trail concentration. J Chem Ecol 37:1143–1149. https://doi.org/10.1007/s10886-011-0019-0
Sunamura E, Suzuki S, Nishisue K, Sakamoto H, Otsuka M, Utsumi Y, Mochizuki F, Fukumoto T, Ishikawa Y, Terayama M, Tatsuki S (2011) Combined use of a synthetic trail pheromone and insecticidal bait provides effective control of an invasive ant. Pest Manag Sci 67:1230–1236. https://doi.org/10.1002/ps.2172
Tumlinson JH, Moser JC, Silverstein RM, Moser JC, Brownlee RG, Ruth JM (1972) A volatile trail pheromone of the leaf-cutting ant, Atta texana. J Insect Physiol 18:809–814. https://doi.org/10.1016/0022-1910(72)90018-2
Van Den Dool H, Kratz P (1963) A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A 11:463–471. https://doi.org/10.1016/S0021-9673(01)80947-X
Wagner HC, Arthofer W, Seifert B, Muster C, Steiner FM, Schlick-Steiner BC (2017) Light at the end of the tunnel: Integrative taxonomy delimits cryptic species in the Tetramorium caespitum complex (Hymenoptera: Formicidae). Myrmecol News 25:95–129
Welzel KF, Choe D-H (2016) Development of a pheromone-assisted baiting technique for Argentine ants (Hymenoptera: Formicidae). J Econ Entomol 109:1303–1309. https://doi.org/10.1093/jee/tow015
Zhang YM, Vitone TR, Storer CG, Payton AC, Dunn RR, Hulcr J, McDaniel SF, Lucky A (2019) From pavement to population genomics: Characterizing a long-established non-native ant in North America through citizen science and ddRADseq. Front Ecol Evol. https://doi.org/10.3389/fevo.2019.00453
Acknowledgements
We thank two anonymous reviewers for constructive comments, Devon Rai and his family for permission to run replicates of the field experiment on their property, Adam Blake and Asim Renyard for advice on statistical analyses and graphical illustrations, Asim Renyard and Sean McCann for help locating and identifying the ants that were collected for laboratory colonies, April Lin, Jasper Li, Kai Zhang, Kelvin Lau, Kristy Lok, Saif Nayani, and Srishti Kumar for help with ant colony maintenance, and Sharon Oliver for word processing and comments.
Funding
This research was supported by a MPM Graduate Entrance Scholarship, a Graduate Fellowship, and the Dr. H.R. MacCarthy Graduate Bursary from Simon Fraser University, and the Mildred Wells Scholarship from the BC Council of Garden Clubs, to JMC. The research was further supported by a Natural Sciences and Engineering Research Council of Canada (NSERC)—Industrial Research Chair to GG with BASF Canada Ltd. and Scotts Canada Inc. as the industrial sponsors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest or competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
About this article
Cite this article
Chalissery, J.M., Gries, R., Alamsetti, S.K. et al. Identification of the Trail Pheromone of the Pavement Ant Tetramorium immigrans (Hymenoptera: Formicidae). J Chem Ecol 48, 302–311 (2022). https://doi.org/10.1007/s10886-021-01317-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01317-3