Abstract
Social insect colonies are characterized by extensive interactions among individuals, exchanges that can also potentially transmit pathogens. The large majority of these social interactions in a honeybee colony result from food transfer among individuals. Since colony hunger is likely to have a significant influence on these interactions, we investigated its effect on the distribution of food within the colony. By pulsing two colonies having different amounts of stored food with a radioactive label, we found that a starved colony sent out a larger number of foragers, brought in more food, and stored more of it than the satiated colony. We also found that the food brought into a starved colony was distributed more uniformly within each age class than that in the satiated colony. The queen and the young individuals received the lowest exposure to the label even though the label entered different regions of the colony at the same rate. The satiation level of the colony did not influence the relative exposures of different age groups to the label but a higher amount of it was stored in the hungry colony. We discuss the significance of these results in terms of the role played by the organizational structure of the honeybee colony on the transmission dynamics of an infectious disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insect colonies provide an ideal environment for pathogens to persist, multiply, and spread due to the large number of susceptible hosts and the close contact among them, especially in terms of extensive social interactions. It has therefore been suggested that the social organization within a colony could have been shaped in part to resist the spread of pathogens, an idea referred to as organizational immunity (Naug and Camazine, 2002; Naug and Smith, 2007). Mouth-to-mouth food transfers known as trophallaxis constitute a large majority of the interactions in a social insect colony, and even though pathogens have been shown to spread via these interactions (Bailey and Gibbs, 1964; Higes et al., 2009), their role in the epidemiology of an infectious disease remain largely unknown).
Given the nature of trophallactic interactions, one could expect that they are likely to be significantly influenced by the food availability in the colony and lead to important changes in within-colony transmission patterns. Starvation is known to increase the frequency of behaviors related to food sharing (Wallis, 1962; Schulz et al., 2002) and the number of individuals participating in them (Howard and Tschinkel, 1980). Several studies have also shown that the number of foragers significantly increases when energy reserves are low either at the level of the individual (Schulz et al., 1998; Toth et al., 2005) or the colony (Seeley, 1989). Fewell and Winston (1996) have, however, shown that the amount of colony reserves only influences foraging for pollen but not for nectar, suggesting that the regulation of colony foraging could be more complex.
The distribution of food within a social insect colony can be studied with two general approaches, behavioral observations that offer descriptions of the transfer process and tracer experiments that provide measurements of the resulting patterns. While a number of studies have used radioactive tracers to investigate the food flow patterns in ant and termite colonies (Wilson and Eisner, 1957; McMahan, 1966; Spragg and Fox, 1974; Howard and Tschinkel, 1980; Suarez and Thorne, 2000), similar studies in honeybees are surprisingly rare. In an early study, Nixon and Ribbands (1952) showed that food transfer in a honeybee colony could be quantified with minimal colony disruption using 32P. Advances in techniques now allow us to measure radioactivity much more accurately and also follow its spread on a temporal and spatial scale.
Pulsing a honeybee colony with a radioactive label such as 32P by training foragers to a source containing the label mixed in sugar water, one can also simulate the introduction and spread of an infectious disease in the colony. Several honeybee pathogens are transmitted via food and the epidemiology of some of these diseases are known to be influenced by the availability of food in the environment. For example, the prevalence of European foulbrood and tracheal mites are known to increase with nectar flows (Bailey, 1958, 1960). However, no experimental study has addressed the mechanisms by which colony hunger can influence the within-colony transmission of a disease. In this paper, we take a first step toward understanding this by quantifying the distribution of labeled food in two colonies with different amounts of stored food.
Methods
Observation hive set-up
We used brood from four full-sized colonies to assemble the observation hives. Brood frames containing pupae within 1–2 day of eclosion were removed from these colonies and kept overnight in an incubator maintained at 32°C. The following morning we introduced 1250 newly emerged bees into a two-frame observation hive after applying a cohort-specific paint mark on the thorax of each individual. In order to create a typical social organization, four cohorts of bees were added on a weekly schedule although 2-week and 3-week-old bees were later combined into a single age class (“middle-aged bees”) for further analyses because there was no significant difference between them. For recording the radioactive pulse on a phosphoimaging screen, we replaced the glass pane of the observation hive with a wire screen and confined all bees to the front with wooden blocks placed inside the hive. We connected the entrance of the hive by a tube to a 10 × 10 × 10 m tent and trained foragers to collect 30% sugar water from a feeder located inside the tent. An additional paint mark was applied to foragers drinking from the feeder, allowing us to separate those bees that directly accessed the feeder from our analysis. The two treatments for the experiment consisted of: (1) a satiated colony, which had one frame of uncapped honey, and (2) a starved colony, which had an empty frame, on the day of the experiment. While the study consists of only one experimental treatment of each type, largely due to the logistical challenges of doing an experiment using radioactive labels at the colony level, the dataset is consistent with earlier experimental results (Naug and Smith, 2007; Naug, 2008).
Radioactive pulse
Our preliminary studies had determined that each bee must ingest a minimum of 0.01 μCu of P32 to be detected on a phosphoimage. With a null hypothesis that if 25 ml of sugar water were equally divided among the 5,000 bees in the hive, each bee can potentially receive 5 μl of it and thus potentially have an activity of 0.1 μCu, on the day of the experiment we filled the feeder with 25 ml of 30% sugar water that contained 500 μCu of 32P. During the entire time the foragers fed on the labeled sugar water and introduced the pulse into the colony, we took a phosphoimage of the hive every 30 min by placing a phosphoimaging plate (35 × 43 cm) on the front screen of the hive for 2 min. The images obtained were later developed on a STORM M840 phosphoimager. Eighteen hours from when the pulse began, we laid down the hive horizontally and poured liquid nitrogen on the colony through the wire mesh to instantly freeze-kill all the bees while maintaining their spatial positions. We separated all the bees according to their spatial positions by placing a 4 × 4 wire grid (each square measuring 10 × 12.5 cm) on the hive. Each square was assigned to one of three distances according to its radial diameter from the entrance. We also sampled the honey cells within each square by inserting a cotton swab into a single cell and removing its entire contents. Each individual bee and honey sample was placed in an 8 ml scintillation vial filled with scintillation fluid and the amount of 32P was measured using a liquid scintillation counter. Because the highest level of background activity measured in a blank sample was 138 counts per minute (CPM), any sample with a count lower than this was considered not to have any 32P.
Results
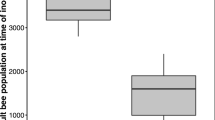
The starved colony took in the entire 25 ml of labeled sugar water via 30 foragers in 3 h while the satiated colony took in only about half that amount via 7 foragers in the same time. While every bee in both treatments contained the label, those in the starved colony had significantly more label on average than those in the satiated colony (F 1,3162 = 508.93, P < 0.001; Fig. 1). In order to compare the within-colony distribution of the label between the two treatments, the data were scaled by dividing the amount of label in each bee by the average amount of label held by a bee of its respective colony. All further comparisons between the two treatments were conducted using these scaled data. The scaled data show that the distribution of the label among the individuals was significantly influenced by the amount of food stored in a colony (Kolmogorov–Smirnov: Z = 7.11, P < 0.001; Fig. 2a), with the starved colony showing a more uniform distribution. Whether this difference in homogeneity is true for each age class was determined using an F-test. For each age class, the variance in the amount of label was significantly lower in the starved colony (Old: F 194,442 = 2.62, P < 0.001; Middle-Aged: F 639,752 = 3.08, P < 0.001; Young: F 563,569 = 4.88, P < 0.001; Fig. 2b).
a Distribution of 32P within a starved and a satiated colony. The amount of label within each bee was corrected for the total label in its colony (a bee with a score of 1 has an amount of label equal to the colony average, a bee with a score < 1 has an amount less than the colony average, and one with a score > 1 has an amount more than the colony average), coefficient of variation (CV) of the distributions: Starved = 1.19, Satiated = 2.11; Maximum: Starved = 10.08, Satiated = 24.84 and b Variance in the amount of label within each age class. Asterisks (*) represent a significant (P < 0.01) difference between the two treatments, and the number above each bar represents the number of bees in the group
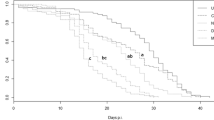
The effect of colony hunger on the mean amount of label received by bees of different ages was tested using a 2-way ANOVA with age and satiation level of the bees as the fixed factors. The amount of label in a bee was significantly influenced by its age (F 2,3159 = 36.25, P < 0.001; Fig. 3a), with young bees having significantly less label than either of the two older age classes (Tukey MSD: Young-Old = 0.61, P < 0.001; Young-Middle-aged = 0.44, P < 0.001; Middle-aged-Old = 0.17, P = 0.07). The amount of label in the queen was lower than that in each of the other age classes but the difference was not statistically compared due to the single data point for the queen. However, the amount of label received by the different age classes was not significantly influenced by the amount of food stored in the colony (F 1,3159 = 2.64, P = 0.10).
The effect of colony hunger on the mean amount of label received by bees at different distances was also tested using a 2-way ANOVA with distance and satiation level of the bees as the fixed factors. The amount of label in a bee was significantly influenced by its distance from the hive entrance (F 2,3159 = 4.24, P = 0.01; Fig. 3b), with the highest amount being present in bees closest to the entrance (Tukey MSD: Closest–Farthest = 0.37, P = 0.005; Closest–Intermediate = 0.28, P = 0.04; Intermediate–Farthest = 0.09, P = 0.28). The starvation level of the colony once again did not influence the amount of label received by bees at different regions of the colony (F 1,3159 = 0.002, P = 0.96). Using the software ImageJ, the phosphoimages were converted into numerical values according to their intensity (Abramoff et al., 2004). A regression analysis and a comparison of the slopes within each treatment showed that there was no significant difference in the rate at which the label penetrated the three spatial regions in either of the two treatments (Starved: F 2,9 = 1.52, P = 0.27; Satiated: F 2,9 = 0.61, P = 0.56).
Colony hunger influenced the distribution of label in the colony food stores, with a larger proportion of honey cells in the starved colony containing the label (G-test of independence: G = 44.59, N = 70, P < 0.001, Fig. 4).
Discussion
The amount of stored food had a significant influence on food flow into the colony with starvation increasing both the number of foragers and the frequency of trips, a result consistent with previous studies (Seeley, 1989; Schulz et al., 1998, 2002). Starvation also influenced the distribution pattern of food within the colony. A more even spread of food in the starved colony can probably be explained by the fact that foragers are more likely to donate a smaller amount of food during each trophallactic bout but have an increased number of such bouts with a larger number of receivers in such colonies (Wallis, 1962; Howard and Tschinkel, 1980).
Because bees are known to encounter pathogens at food sources (Durrer and Schmid-Hempel, 1994), increased foraging could result in a higher exposure to pathogens and consequently a higher level of infection in the colony. The uniform spread of a food-borne infection could also compromise the defense mechanisms that exist in social insect colonies to reduce the spread of diseases (Woodrow and Holst, 1942; Waddington and Rothenbuhler, 1976; Visscher, 1980; Arathi et al., 2000), all of which rely on targeting a few highly infected individuals. By this rationale, a starved colony is likely to be more susceptible to an epidemic since it will experience both a higher rate of pathogen invasion and a lower efficacy of the defensive mechanisms to combat the disease.
The observation that the queen and the youngest individuals receive less amount of the incoming pulse suggests that the most valuable members of the colony could be provided with some amount of immunity to an incoming pathogen due to the organizational structure of the colony. There are two alternate mechanisms that can provide this organizational immunity, a spatial segregation that keeps the youngest bees at the innermost regions of the colony or a behavioral segregation that directs the least number of interactions toward them. Although age-based spatial segregation has been demonstrated in a honeybee colony with the youngest bees at the center (Naug, 2008), the results from this study suggest that it may not be the sole cause for the reduced exposure of young bees. The fact that the youngest bees remained relatively insulated from the pulse, despite the phosphoimaging results showing that food entered all regions of the colony at the same rate, indicate that age-specific interaction patterns may transcend the observed spatial segregation and the respective role of these two factors in generating the organizational immunity needs further investigation.
The observation that the relative exposure of each age class was not significantly influenced by the two treatments suggests that the fundamental structure of the social organization in the colony is largely resilient to changes in food availability. This means that even if colonies at different nutritional states might face vastly different absolute risks in terms of contacting an infection, the organizational immunity is still likely to remain intact and ensure that the valuable members of the colony are afforded the same protection in terms of their relative risk of getting infected. However, the nutritional state of the colony would probably influence the status of its disease reservoir as suggested by the higher number of contaminated honey cells in the starved treatment. A number of honeybee diseases, such as American foulbrood, Chalkbrood, and Nosemosis, are caused by pathogens that are known to remain viable as spores for long periods of time (Bailey, 1981; Fenoy et al., 2009). Spores stored in the honey can initiate future within-colony transmission events and re-introduce a disease in an otherwise healthy colony. This could explain in part the seasonal outbreaks of diseases like Nosemosis in the winter, when bees are confined to the hive and sustain themselves with stored honey.
There are surprisingly few studies that have looked at the influence of food availability on the spread of disease in a honeybee colony and all of them deal with the food abundance in the environment without regard to the amount of stored food within the colony. For example, Bailey (1958, 1960) somewhat paradoxically found that the prevalence of some honeybee diseases reaches a peak during the early phase of a nectar-flow and then decreases rapidly. Our results suggest that the initial increase in prevalence can be explained by the large foraging effort triggered by the nectar flow while the subsequent decrease could result from the shift in colony nutritional state from starvation to satiation. This indicates that one must consider both the availability of food in the environment and the nutritional state of the colony itself in order to fully understand the introduction and the spread of a disease in a honeybee colony. In addition, recent results showing the role of disease in increasing the hunger level of an individual (Mayack and Naug, 2009; Naug and Gibbs, 2010) also suggest that individual nutritional status could interact with social hunger in complex ways.
References
Abramoff M.D., Magelhaes P.J. and Ram S.J. 2004. Image processing with ImageJ. Biophotonics Intl. 11: 36-42
Arathi H.S., Burns I. and Spivak M. 2000. Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of hygienic bees. Ethology 106: 365-379
Bailey L. 1958. The epidemiology of the infestation of the honeybee, Apis mellifera L., by the mite Acarapis-woodi rennie and the mortality of infested bees. Parasitology 48: 493-506
Bailey L. 1960. The epizootiology of European foulbrood of the larval honey bee, Apis mellifera Linnaeus. J. Insect Pathol. 2: 67-83
Bailey L. and Gibbs A.J. 1964. Acute infection of bees with paralysis virus. J. Insect Pathol. 6: 395-407
Bailey L. 1981. Honey Bee Pathology. Academic Press, London. 193 pp
Durrer S. and Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258: 299-302
Fenoy S., Rueda C., Higes M., Martín-Hernández R. and Aguila C. 2009. High resistance of Nosema ceranae, a parasite of honeybee, to temperature and desiccation. Appl. Environ. Microbiol. 75: 6886-6889
Fewell J.H. and Winston M.L. 1996. Regulation of nectar collection in relation to honey storage levels by honey bees, Apis mellifera. Behav. Ecol. 7: 286-291
Higes M., Martín-Hernández R., Garcíá-Palencia P., Marín P. and Meana A. 2009. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environmental Microbiology Reports 1: 495-498
Howard D.F. and Tschinkel W.R. 1980. The effects of colony size and starvation on food flow in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 7: 293-300
Mayack C. and Naug D. 2009. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invert. Pathol. 100: 185-188
McMahan E.A. 1966. Food transmission within the Cryptotermes brevis colony (Isoptera: Kalotermitidae). Ann. Entomol. Soc. Am. 59: 1131-1137
Naug D. and Camazine S. 2002. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 215: 427-439
Naug D. and Smith B.H. 2007. Experimentally induced change in infectious period affects transmission dynamics in a social group. Proc. R. Soc. Lond. B 274: 61-65
Naug D. 2008. Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav. Ecol. Sociobiol. 62: 1719-1725
Naug D. and Gibbs A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie (in press)
Nixon H.L. and Ribbands C.R. 1952. Food transmissin within the honeybee community. Proc. R. Soc. Lond. B 140: 43-50
Schulz D.J., Huang Z.Y. and Robinson G.E. 1998. Effects of colony food shortage on behavioral development in honey bees. Behav. Ecol. Sociobiol. 42: 295-303
Schulz D.J., Vermiglio M.J., Huang Z.Y. and Robinson G.E. 2002. Effects of colony food shortage on social interactions in honey bee colonies. Insect. Soc. 49: 50-55
Seeley T.D. 1989. Social foraging in honey bees: how nectar foragers assess their colony’s nutritional status. Behav. Ecol. Sociobiol. 24: 181-199
Spragg W.T. and Fox R.E. 1974. The use of radioactive tracer to study the nesting system of Mastotermes darwinensis Frogat. Insect. Soc. 21: 18-25
Suarez M.E. and Thorne B.L. 2000. Rate, amount and distribution pattern of alimentary fluid transfer via trophallaxis in three species of termites (Isoptera, Rhinotermitidae, Termopsidae). Annls Entomol. Soc. Am. 93: 145-155
Toth A.L., Kantarovich S., Meisel A.F. and Robinson G.E. 2005. Nutritional status influences socially regulated foraging ontogeny in honey bees. J. Exp. Biol. 208: 4641-4649
Visscher K. 1980. Adaptions of honey bees (Apis mellifera) to problems of nest hygiene. Sociobiology 5: 249-260
Waddington K.D. and Rothenbuhler W.C. 1976. Behaviour associated with hairless-black syndrome of adult honeybees. J. Apicult. Res. 15: 35-41
Wallis D.I. 1962. The relation between hunger, activity and worker function in an ant colony. Proc. Zool. Soc. Lond. 139: 589-605
Wilson E.O. and Eisner T. 1957. Quantitative studies of liquid food transmission in ants. Insect. Soc. 4: 157-166
Woodrow A.W. and Holst E.C. 1942. The mechanism of colony resistance to American foulbrood. J. Econ. Entomol. 35: 327-330
Acknowledgments
We would like to thank Salah Abdel-Ghany, Chris Cohu and Marinus Pilon for lending us their radioactive-isotope expertise and facilities. This study was supported by a NSF grant to D.N., who designed the experiment and wrote the paper with C.F. who collected and analyzed the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feigenbaum, C., Naug, D. The influence of social hunger on food distribution and its implications for disease transmission in a honeybee colony. Insect. Soc. 57, 217–222 (2010). https://doi.org/10.1007/s00040-010-0073-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-010-0073-6