Abstract
Social interactions with heterospecifics can yield important insights into the flexibility of behaviour and the role of learning in communication. Recently, the honeybee dance, a unique symbolic communication system to communicate positions in space, has been shown to involve learning. We asked if this communication system could potentially be learned by members of a species not normally using this communication system, the bumblebee(Bombus terrestris)—indicating that learning might have been at the origins of dance communication. We used mixed-species colonies of bumblebees and honeybees (Apis millefera) to investigate how the readiness to first establish contact with dancers might develop in uninformed bumblebee foragers. Over a month of observations, we recorded and classified a series of behavioural patterns in newly emerged honeybees introduced into queenright bumblebee colonies. A small subset of the introduced honeybees was able to establish in the nests and displayed their typical behavioural patterns, including homing, dance communication, trophallaxis, and social grooming. Remarkably, grooming and trophallaxis were also displayed to heterospecifics, and bumblebees accepted both, including food offered through trophallaxis, even though this behaviour is not normally used by bumblebees. However, bumblebees never attended honeybees’ waggle dances. Our results contribute to insights about bee social behaviour and cognition by providing a fascinating example of the adaptive use and modification of innate behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The honeybee (Apis) waggle dance is a sophisticated referential communication system used by successful foragers to provide spatial information about resources (von Frisch 1965). This signal encodes information on the direction, distance, and quality of a food resource as delivered by a dancer performing a sequence of stereotypical motor patterns (Seeley 1995; Dyer 2002). Nestmates that follow the dance (recruits) decode and memorise this information to locate the indicated food resource (Seeley 1995). The waggle dance recruitment signal requires two complementary components to work successfully, the stereotyped motor patterns of the dancer and the readiness of potential recruits to follow the dance. However, a long-standing question in the evolution of this signalling system is how both the ability of successful foragers to display a signal (dances) and the recruits’ readiness to respond to it could emerge in parallel.
Recent work has shown that social learning shapes the correct information encoding of the waggle dance (Dong et al. 2023). Similarly, different honeybee species have subtle variations in their dances’ distance code that can be learnt by heterospecifics (Su et al. 2008). This evidence highlights the influence of learning on the functional elements of the dance communication (Chittka and Rossi 2023).
Even though the waggle dance is unique to honeybee species, their extant relatives, the bumblebees, and stingless bees display excitatory motor patterns that serve to recruit nestmates to food sources (Lindauer and Kerr 1960; Dornhaus and Chittka 1999). Bumblebees, for example, use a rudimentary recruitment system in which successful foragers perform irregular runs whilst dispersing a pheromone to alert their colony about a food resource. Unlike the honeybee waggle dance, this recruitment system conveys no spatial information, but recruits obtain the scent of the advertised floral source from successful foragers (Dornhaus et al. 2003; Dornhaus and Chittka, 1999). It has been suggested that primitive forms of communication, akin to the bumblebees’ recruitment system, might be at the root of the evolution of the dance language of honeybees (Dornhaus and Chittka 1999).
The honeybee waggle dance is naturally restricted to the confines of the nest, where it remains imperceptible to heterospecifics (Seeley 1995). Yet, in experimental mixed-species colonies of honeybees, waggle dances are conspicuous to heterospecific nestmates, who eventually learn to decode the signal of another species (Su et al. 2008). Social information can also flow bidirectionally across honeybees and bumblebees spatially co-occurring in foraging contexts (Romero-González et al. 2020; Dawson and Chittka 2012). Likewise, it has been shown that stingless bees (Trigona) can learn to interpret heterospecific chemical signals (Slaa and Hughes 2009). If indeed the plasticity provided by learning was at the evolutionary root of deciphering the waggle dance, could it be possible that bumblebees exposed to the honeybee waggle dance might detect this signal as a relevant social cue and subsequently acquire the readiness to respond to it?
Here, we experimented with mixed-species colonies of bumblebees and honeybees since anecdotal evidence exists in such colonies for callow honeybees and host bumblebee workers having trophallactic contacts (Chittka, unpublished observations). This form of food transfer is common in honeybees (Seeley 1995) but inexistent in bumblebees. Thus, bumblebees’ preparedness to engage in trophallaxis might be a learned behaviour, perhaps facilitated by contacting the food regurgitated by honeybees whilst incidentally directing this behaviour towards bumblebee nestmates. We tested the hypothesis whether the exposure to waggle dances might result in a learning process in bumblebees, to detect and be attracted to nestmate honeybees performing the waggle dance.
2 Materials and methods
2.1 Study species
We performed experiments from September to October 2018 in a greenhouse facility at Queen Mary University of London. Two queenright bumblebee, Bombus terrestris, colonies were obtained from a commercial supplier (Biobest, Belgium N.V.). Newly emerged honeybee workers, Apis mellifera, were sourced either from a hive located on the rooftop of the Fogg Building of Queen Mary University, London, or a hive managed within an agricultural landscape at Rothamsted Research, Hertfordshire, UK.
2.2 Setting-up of mixed-species colonies
We artificially created two mixed-species colonies by introducing newly emerged honeybee workers into established bumblebee queenright colonies, containing approximately 50 workers. Before introducing honeybees, bumblebee colonies were given three days to adapt to housing conditions. Colonies were kept in bipartite wooden nest boxes (29.5 × 11.5 cm and 9.5 cm high). We placed a glass sheet (29.5 × 11.5 cm) atop these boxes to facilitate behavioural observations. Colonies were fed with 30% (w/w) sucrose solution through a gravity feeder placed at the front chamber of the nest boxes and provided with frozen pollen (Koppert B.V., The Netherlands) every other day.
To obtain newly emerged honeybee workers, we removed a frame of comb, with sealed brood about to emerge from one of the source hives. This frame was then transferred to an incubator at 35 °C and 50% relative humidity to let young adults emerge overnight. Emerged honeybees were collected in plastic jars, and a cohort of approximately 50 honeybees was introduced, within 24 h of their emergence, into each of the two bumblebee nests. In pilot experiments, introduced honeybees were promptly attacked by host bumblebees, even when we lightly sprayed the honeybees with a vanilla-scented sugar solution before introduction, which is a common practice to assist acceptance of honeybees introduced in host conspecific nests (Kolmes 1985b). We therefore assisted honeybees to acquire the colony chemical cues (Krasnec and Breed 2012) by allowing them to freely interact with the bumblebee queen and nest material whilst bumblebee workers remained isolated in the front chamber of the nest box. The bumblebee queen did not respond aggressively to honeybees during these interactions. We reintroduced all bumblebee workers into the nest 15 min after completing the introduction; thereafter aggressions towards honeybees ceased.
Once both mixed-species colonies were fully settled, we placed them along different (perpendicular) walls of the greenhouse. Colonies had free access to local floral resources outdoors via holes (2.6 cm Ø) drilled into the walls. Bumblebee foragers were then seen carrying out foraging activity. Yet, we supplemented the nest daily with grounded frozen pollen and sucrose solution at nighttime, so young adult honeybees could meet their requirements (Seeley 1995). During our daily observations, we noticed that the number of honeybees declined in both colonies; we thus introduced a second cohort of 30 newly emerged honeybees into each nest. We repeated the same procedure to introduce the new cohort after 12 days of the first introduction, in the evening corresponding to the fifth day of the observation period. Both colonies remained in darkness under an opaque cover except during observation periods.
2.3 Behavioural observations
Observations began once honeybees in the first cohort were 7 days old. This is the earliest age at which honeybees tend to initiate foraging in natural conditions (Toth and Robinson 2005). The observation period comprised 25 days for colony A and 32 days for colony B. We performed one to two daily observation sessions per colony, in the morning, and/or afternoon (between 0700 and 1700). Observations lasted 30–170 min (colony A, 69 ± 2.64 (SE) min; colony B, 66 ± 2.51 (SE) min). We stopped observations once we could only detect a maximum of five honeybees in the nests over at least three consecutive days.
Activity inside the nests was recorded from above the nest-boxes with two iPhones 6 (Apple, CA, USA) with a recording frame rate of 30 fps, under natural lighting conditions. One observer conducted all behavioural sampling from video recordings to minimise variability in behaviour discrimination (Perez and Johnson 2019; O’Donnell and Foster 2001). The observer registered focal honeybees’ in-nest behavioural activities via continuous recording for behavioural events (e.g. antennation with conspecific) and instantaneous sampling at 5-min intervals for behavioural states (e.g. stand) displayed by all visible honeybees (Bateson and Martin 2021). Further, censuses of honeybees in both colonies were taken daily by counting all visible honeybees at every 5-min instantaneous sampling during observation sessions. Censuses allowed us to record the maximum number of honeybees in each colony at a given day, which we then regarded as the daily population of honeybees.
We categorised honeybees’ activity in the nest by identifying and matching each observed behaviour to descriptions in previous studies (Seeley 1982; Winston and Punnett 1982; Kolmes 1985a; Robinson 1987). Given that our study sought to determine potential interspecific interactions occurring during honeybees’ dance communication, we focused on identifying honeybees’ foraging activity and social interactions with conspecifics and heterospecifics. We thus extended the social categories of our reference ethograms (e.g., antennate) to include interactions with heterospecific bumblebees.
In addition, preliminary observations allowed us to identify specific behavioural patterns that honeybees may display when inhabiting bumblebee nests. We integrated these behaviours in our ethogram: ‘manipulate wax’, ‘disperse attracting pheromone’, ‘fast walk’, ‘inspect storage pot’, and ‘inside storage pot’ (see Table I for descriptions).
2.4 Classification of behavioural activities
In total, 18 different behavioural activities were recorded (Table I). Three types of social interactions (antennate, groom, and trophallactic contacts) were registered as separate behaviours depending on the interacting counterpart (conspecific or heterospecific). Intra- and interspecific aggressive interactions were rare behaviours with a frequency lower than 0.05% of all behavioural acts performed by honeybees in both colonies; we therefore did not include these behaviours in our analysis.
2.5 Statistical analyses
We recorded 52 h of activity for colony A, including 583 instantaneous samples and 10,840 behavioural acts (2,954 states and 7,886 events), and 66 h of activity for colony B, with 698 instantaneous samples and 13,134 behavioural acts (4,587 states and 8,547 events). An average of 638.9 ± 56 (SE) honeybee behaviours were observed per day.
Data of colony A and colony B for both morning and afternoon sessions were pooled for analysis. The sample size comprised 105 observation sessions for all visible focal honeybees in both nests. Frequencies of all behaviours in our ethogram were calculated as both average frequency per observation session and relative frequency in proportion to all recorded behavioural acts per observation session.
To determine whether honeybees might discriminate the species of their nestmates, thus interacting with honeybees and bumblebees at a different rate, we compared, with a Wilcoxon signed rank test, the proportion of antennation and social grooming (the two most common social interactions) that was directed at conspecifics and heterospecifics, relative to the total occurrence of these behaviours.
Further, given that the queen in colony A died on the 10th day of the observation period (see Section 3), we compared, with a Wilcoxon rank sum test, the behaviour of honeybees in colony A and colony B (Online resource 1) as well as the behaviour of honeybees in colony A before and after the queen died (Online resource 2). This allowed us to evaluate whether the behaviour of honeybees in both colonies was consistent, and whether the behaviour of honeybees in colony A remained unaffected after losing the host queen.
3 Results
3.1 Honeybee introduction success
Unlike Apis mixed-species colonies, in which newly emerged young adult bees may be readily accepted by host colonies (Yang et al. 2010), we found difficulty in introducing newly emerged honeybees in bumblebee nests due to aggressive behaviour by hosts. However, we suppressed aggressions towards honeybees by allowing them to interact with the bumblebee queen and nest material in the absence of bumblebee workers. Honeybees may thus have acquired the odour cues of their host colony in this way. Once we successfully completed the introduction phase, an extended period of interspecific nest cohabitation followed, in which bumblebees’ behaviour had no noticeable changes with regards to the pre-introduction stage.
3.2 Foraging activity
In 75.2% of the observations, honeybees performed behaviours associated with foraging, yet these behaviours had the lowest occurrence, representing altogether 11.4% of all in-nest non-social activities. The most predominant behaviour in this category was entering and exiting the nest. We considered entering and exiting the nest as indicators of foraging activity based on previous studies (Seeley and Kolmes 1991), although these behaviours might on some occasions reflect honeybees leaving the nest for orientation or defecation flights (Seeley 1982). Even though we noticed honeybees performing orientation flights at the nest entrance (see Capaldi and Dyer 1999 for a description), we did not record this behaviour systematically, given that our observations centred on in-nest behaviours.
We detected that the honeybees left the nest for the first time 5 days after beginning observations, when bees in the first cohort were 12 days old and bees in the second cohort had not yet been introduced in the nest. After this, we consistently observed honeybees entering and exiting the nest during the complete observation period (Figure 1). However, returning bees usually disappeared rapidly amidst the brood clumps, making it not possible to discern whether they deposited any nectar into the nest honeypots or transferred it to conspecific honeybees. We noticed a few instances where honeybees bore pollen on their hind legs, but pollen foraging was uncommon (< 0.05% of all recorded behavioural acts) and thus not included in our analysis. This result indicates that honeybees may have predominantly collected nectar in their foraging trips.
Proportion of four behaviours indicative of foraging, relative to all behavioural acts per day in honeybees introduced in bumblebee nests during a 36-day observation period. Data for morning and afternoon observation sessions of both colony A and colony B are pooled. Behavioural acts were recorded via continuous recording.
3.3 Dance communication
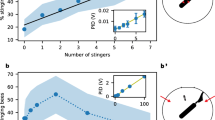
We observed waggle dances in a small proportion of the observations comprising a continuous 10-day period (Figure 1) starting 20 days after we began the observations, when bees in the first cohort were 27 days old and bees in the second cohort were 16 days old. That is, the age of bees performing waggle dances oscillated between 27–37 days and 16–26 days for the first and second cohort, respectively. Waggle dances always occurred over the brood clumps near the nest entrance and had a mean duration of 12 ± 2.3 (SE) waggle runs. In 87.5% of the dances, at least one conspecific followed the dancing honeybee (Online resource 3). Since bees were not individually marked, we could not establish whether dance followers eventually left the nest to locate the advertised resource. Also, no bumblebees ever reacted to honeybee dancers. In addition, honeybees displayed tremble dances earlier and more frequently than waggle dances (Table II and Figure 1). Tremble dances were observed for the first time on the 11th day of the observation period, when honeybees in the first and second cohort were, respectively, 18 and 7 days old. Unlike waggle dances that occurred in a defined period, tremble dances were distributed across the observation sessions (Figure 1). The occurrence of both dances in the same observation session took place only once for each colony.
3.4 Honeybees in-nest behavioural activity
Social interactions accounted for 41.6% of all honeybee’s behavioural acts recorded per observation session. Inspecting storage pot was honeybees’ most common non-social activity, followed by manipulating nest material and walking, altogether comprising 29.2% of all recorded behavioural acts. Table II lists the observed behavioural activities of honeybees, and their mean and relative frequencies per observation session.
3.5 Social interactions of honeybees
Figure 2 shows the social interactions held by honeybees during the observation period. The three most common social interactions were antennation with conspecific, antennation with heterospecific, and grooming heterospecific, corresponding to 84.7% of all recorded social interactions. All interspecific social interactions were initiated by honeybees. They directed social grooming towards both conspecifics and heterospecifics, but bumblebee workers, that remained stationary during the interaction (Figure 3), received significantly more grooming than conspecific honeybees per day of observation (Wilcoxon signed-rank: V = 0, N = 36, P < 0.001; Figure 2). This might at first indicate that honeybees groomed bumblebees more frequently because their population in the nest was larger, making them more ‘available’ to be groomed. However, honeybees held antennal contact much more frequently with conspecifics than with bumblebees per observation day (Wilcoxon signed-rank: V = 611, N = 36, P < 0.001; Figure 2). These results suggest that honeybees might have discriminated their nestmates’ species and interacted with them accordingly through specific behaviours.
Social interactions of honeybees in bumblebee nests. a Proportion of social interactions with conspecifics and heterospecifics relative to all behavioural acts per day in honeybees introduced in bumblebee nests during a 36-day observation period. Data for morning and afternoon observation sessions of both colony A and colony B are pooled. Behavioural acts were recorded via continuous recording. b Proportion of honeybees’ antennal contacts with both conspecifics and heterospecifics relative to all their antennal contacts (left panel) and proportion of social grooming directed towards both conspecifics and heterospecifics relative to of all social grooming performed by honeybees (right panel). Means are shown ± SE. *P < 0.05.
Interspecific social interactions of honeybees and bumblebees inside a bumblebee nest. a Honeybees groomed bumblebees who accepted the interaction while remaining stationary. b Honeybees initiated trophallactic contact with bumblebees that acted receptively during the interaction. Illustrations by Meredith G. Johnson.
Similarly, 96.5% of all honeybees’ trophallactic contacts were held with a conspecific. All interspecific trophallactic contacts were initiated by honeybees whilst bumblebees seemed receptive to the interactions (Figure 3, Online resource 4). The mean duration of interspecific trophallaxis was 8.73 ± 2.56 (SE) s, and its frequency represented only a small fraction of all recorded acts (Table II), occurring intermittently in a few observation sessions, mainly during the first half of the study (Figure 2). Further, no interspecific trophallaxis occurred in the context of the waggle dance.
We consistently observed honeybees attending the bumblebee queen during the observation period (Figure 2), but such social interactions represented only a small percentage of all behavioural acts (Table II).
3.6 Non-social activities
Standing still was frequently observed in honeybees over the course of the study (Table II and Figure 4) However, we observed that honeybees standing on the surface of storage pots and brood cells were not completely inactive, but they were usually interacting with the wax of these structures, using either their legs, mouthparts, or both. Sometimes, they simultaneously displayed intermittent abdominal movements against the nest surface on which they were standing. This behaviour matched the description by Riessberger and Crailsheim (1997), indicating that honeybees might have been either consuming or manipulating the wax. We assumed, but could not clearly establish, that honeybees were manipulating the wax of storage pots and brood cells. Wax manipulation was somewhat common, with a frequency of 9.75 ± 0.006 (SE) relative to all recorded behavioural acts.
In-nest non-social activities of honeybees introduced in bumblebee nests during a 36-day observation period. Proportion of behaviours performed by honeybees relative to all their behavioural acts per day. Data for morning and afternoon observation sessions of both colony A and colony B are pooled. Behavioural acts were recorded via continuous recording; behaviours marked with * were recorded using 5-min instantaneous sampling.
Walking across the nest had a relatively high mean frequency (Table II). We could not ascertain whether this was related to patrolling for work (Seeley 1982) or honeybees’ exploratory behaviour in the search for food within the nest. Likewise, the inspection of storage pots could be directly linked to both assessing the need to forage or merely searching/consuming food in the storage pots. This behaviour was prevalent during the complete observation period (Figure 4) and occurred at a similar rate as walking (Table II).
We were also able to recognise honeybees distinctively walking at a high pace indicating an active state (‘fast walk’). Honeybees performing this behaviour principally moved rapidly on the smooth surfaces of the nest box, like the wooden walls and the glass sheet covering the nest. Even though this fast walking occurred consistently during the observation period (Figure 4), its mean frequency was much lower than regular walking (Table II). During this active state, we did not see honeybees displaying concurrent behaviours, like tremble dances or pressing through other bees, as reported in other studies (Biesmeijer 2003; Seeley 1982).
Honeybees were regularly observed staying motionless with their entire body fitted into an empty storage pot, commonly facing the pot’s bottom and without moving their legs or mouthparts. Given the lack of movement, we considered that these honeybees were ‘resting’ (van der Blom 1993) rather than ‘cleaning’ the storage pots (Seeley 1982). However, this resting behaviour in storage pots had a low frequency (Table II, Figure 4).
Self-groom was a common behaviour across all the observation sessions (Figure 4) that had a mean relative frequency of 4.54 ± 0.003 (SE) of all acts (Table II). Other non-social activities in honeybees had a lower occurrence, including disperse attracting pheromone, lateral shake, and fan wings. The relative frequency of each of these behaviours did not surpass 2% of all recorded acts. We observed that honeybees within the nest exposed the Nasanov’s gland (upper surface of the abdomen) whilst simultaneously fanning the wings. This behaviour normally serves the purpose of dispersing a pheromone that attracts nestmates and naturally takes place outside the hive (Seeley 1995). Honeybees dispersed the attracting pheromone more frequently towards the second half of the observation period (Figure 4). Honeybees’ lateral shake was observed across all observations, but this behaviour never resulted in the shaking bee being groomed by a neighbouring conspecific (‘grooming dance’, see van der Blom 1993 and Land and Seeley 2004). We found that the relative frequency of fanning wings ranged from 0 to 2.31% of all recorded acts. Fanning wings is a collective thermoregulatory response to increases in temperature, for ventilation (when CO2 levels are high) or dispersing pheromones. During observation sessions, mean ambient temperatures oscillated between 11.1 and 22 °C, which is far lower than the temperatures that stimulate honeybees’ thermal fanning response threshold (Kaspar et al. 2018). Further, this behaviour was not performed collectively but only observed in 1–2 individuals per observation session. It is thus unlikely that fanning behaviour in our honeybees may indicate a thermoregulatory response.
3.7 Behavioural patterns over time
Our results reveal some changes over time in the proportional occurrence of honeybees’ behavioural activities. We found that the relative frequency of some behaviours (manipulate wax, inspect storage pot, and antennate with bumblebee) started high and had a steady decline across the 32 days of observation (Figures 2 and 4). For other behaviours (enter/exit nest, trophallactic contacts with honeybee, and disperse attracting pheromone), their relative frequency stayed low until rising slightly towards the second half of the observation period (Figures 1, 2, and 4). For one behaviour (attend queen), the relative frequency started high, then declined sharply and finally rose again towards the last days of observations (Figure 2). The relative frequency of other behaviours (walk, groom honeybee, groom bumblebee, self-groom, antennate with honeybee, fast walk, lateral shake, and inside storage pot) remained somewhat stable during the entire observation period (Figures 2 and 4). Other behaviours were either scarcely performed (waggle dance, attend waggle dance, tremble dance, trophallactic contacts with bumblebee) or their mean frequency did not have a defined pattern (fan wings) over the observation period.
3.8 Honeybee population in the nest
Figure 5 shows the honeybee population dynamics in both colony A and colony B over the observation period. We recorded a median of 5 honeybees for colony A (interquartile range (IQR) 3) and 6 honeybees for colony B (interquartile range (IQR) 3) at each 5-min instantaneous sampling during the daily observation sessions. On the first day of observations (7 days after introducing the first cohort of honeybees), we censused a maximum of 7 honeybees in each of the colonies (Figure 5). This drastic reduction in the honeybee population in the nests (approximately 85%) over a 7-day period may likely reflect mortality or drifting (Kolmes 1985a). Since aggression from bumblebees ceased after accomplishing the introduction phase, this is an unlikely cause of mortality. Further, other honeybee hives were unavoidably present in the surroundings (50 m away from our colonies); these included one of our source hives for frames of comb. Hence, drifting may be a more plausible explanation for the disappearance of honeybees. Yet, we did not determine whether our bees actually appeared in the neighbouring hives.
The population of honeybees introduced in two bumblebee nests. Data collected over 583 (colony A) and 698 (colony B) 5-min sampling intervals during an observation period of 25 days for colony A (left panel) and 32 days for colony B (right panel). A second cohort of honeybees was introduced in each colony the evening corresponding to the fifth day of observation. The bumblebee queen in colony A was found dead on the eleventh day of observation. Medians, minimum, and maximum are shown.
Mortality and drifting may have occurred at a rapid rate because we could only record a maximum of 20 honeybees in colony A and 21 in colony B within < 24 h after we introduced the second cohort of 30 honeybees in each nest (Figure 5). That is, approximately 30% of the introduced honeybees disappeared from the bumblebee nests in less than 1 day. During the 10 subsequent days of observations, the daily maximum number of recorded honeybees had another marked decline (~ 50%) in colony A (median, 9; interquartile range (IQR), 1.75) but remained relatively stable in colony B (median, 16; interquartile range (IQR) 5.75). The bumblebee queen in colony A was found dead during this 10-day period on the eleventh day of observation. However, the behaviour of honeybees in this colony remained unaltered relative to both their behaviour prior to losing the queen and the behaviour of honeybees in colony B (Online resources 1 and 2).
The honeybee population in both colonies kept declining over the remaining days of observations (10 days for colony A and 17 days for colony B). For these last days of observations, the daily maximum number of honeybees had a median of 5 honeybees (interquartile range (IQR) 1.75) for colony A and 7 honeybees (interquartile range (IQR) 5.75) for colony B. Despite the declining tendency in the honeybee population during the observation period, there were instances in both colonies that over subsequent days, we recorded an increment in the daily number of honeybees, indicating that their population not merely declined persistently but fluctuated over time.
4 Discussion
Our study provides the first report of mixed-species colonies composed of two differently evolved genera of social bees, honeybees, and bumblebees. In an attempt to explore the evolutionary roots of the primary contact between individuals performing the waggle dance and those following it, we considered the possibility that bumblebees, which use a primitive recruiting system and can use honeybees as a source of foraging information outside the nest (Dawson and Chittka 2012), might develop a readiness, dependant on learning, to establish contact with and attend to nestmate honeybee dancers—comparable to honeybees that learn some elements of the dance communication (Dong et al. 2023; Su et al. 2008). We failed to find support for this idea in our experiments because bumblebees never initiated interactions with or showed attraction towards nestmate honeybees, even in the context of conspicuous waggle dances. Nonetheless, the findings of our study contribute insights about honeybee behaviour that are worth remarking.
Honeybees disappeared at a high rate from the bumblebee nests within 7 days after their introduction. This decrease continued along the rest of the study until the honeybee population stabilised at a modest quantity. Although this finding somewhat matches the 10% daily mortality rate typically expected in honeybee colonies (Seeley 1995), we do not rule out that drifting also caused the loss of honeybees, since related honeybee hives were readily available in the vicinity of our colonies. Honeybees introduced into conspecific hives can drift at a rate of up to 60% within a period equivalent to ours, which intensifies after the death of the host queen (Pfeiffer and Crailsheim 1998). Perhaps in our study, the initial absence of a honeybee queen may have caused honeybees’ drifting to neighbour colonies. Yet, it is intriguing to note that a subset of honeybees remained in both nests for the complete duration of the study, and colony A, which lost its queen in the middle of the observation period, had a more rapid decline in its honeybee population than the queenright colony B. These findings raise the question whether bumblebee queen pheromones might have driven the establishment of some honeybees in the bumblebee nests. Indeed, we found that honeybees consistently interacted with the bumblebee queen throughout the observation period, giving some support to this possibility. Within a honeybee hive, workers interact with their queen and use specific behaviours to disperse her pheromones (see Seeley 1995). Queen’s pheromones in turn signal her presence, integrating workers’ activities in the colony (Pettis et al. 1995). Whether honeybees might have detected the bumblebee queen’s pheromones and responded to them in a similar fashion is a question that merits further examination in light of recent findings on cross-species behavioural effects of the honeybee queen mandibular pheromone on bumblebees (Princen et al. 2019).
Honeybees that remained in the bumblebee nests moved repeatedly between the nests and the outside environment, as shown by their fluctuating daily population. Thus, they established the bumblebee nests as their central place foraging for over a month of observations. Bees, like many other insects, are central place foragers that build nests to provision and protect their offspring, and thus, homing—the ability to return to a spatially restricted nest—is necessary for survival (Collett et al. 2013). In our study, no honeybee offspring to provision for was present in the nests, yet some honeybees displayed homing, resulting from learning, and remembering the location of the nests (Tait et al. 2019; Capaldi and Dyer 1999). Before commencing their foraging careers, honeybees display orientation flights whereby they learn the landscape and their hive features, which enables homing behaviour (Capaldi and Dyer 1999; Degen et al. 2016). Our honeybees did perform orientation flights that, along marking the bumblebee nests with the attracting pheromone from their Nasanov’s glands, may have aided homing behaviour (Guerrero 2009) in the bees that did not drift to neighbouring hives.
Our main indicator of foraging activity in honeybees was the frequency at which they entered and exited the nests. We observed both behaviours practically throughout the study, but their frequency was higher in the second half of the observation period (Figure 1), with bees’ aged between 15 and 26 days. This somewhat aligns with published data for honeybee colonies indicating that bees’ foraging activity begins to rise from ~ 10 days old (Seeley 1982; Seeley and Kolmes 1991). This trend suggests that our honeybees may have developed in their two broad natural behavioural stages, with 20 + days old honeybees performing foraging and younger bees concentrating their activity in the nest (Seeley 1982). In our case, however, future work should elucidate whether young bees can perform any in-nest tasks within the bumblebee nests. Foraging in honeybee colonies is a complex process demanding considerable coordination to distribute labour efficiently among food sources (Seeley 1995). Contrary to bumblebee colonies, where the same workers collect and process food (Goulson 2010), honeybees’ foraging is a more strictly partitioned activity that involves the collection and processing of food resources by separate individuals (Seeley 1995). It cannot be clearly discerned from our observations whether honeybees adhered to their natural partitioned foraging, foraged as individuals, or merely consumed the resources collected by bumblebee nestmates. Our evidence allows us to hypothesise that the former was the case since honeybees deployed waggle and tremble dances, which they naturally use to respectively keep nectar collection and processing in balance. This indicates that despite their reduced population, honeybees in the bumblebee nests might have been able to detect the colonies’ fluctuations in nectar influx and adjust their foraging activities accordingly via dance communication.
Occurrences of the tremble and waggle dance amounted only to a handful of occasions in isolated observation sessions. This may be unsurprising considering both the scarcity of honeybees in the nests and that in natural conditions, the fraction of returning foragers that perform a dance is generally less than 10% (Seeley 1995). Both dance signals operate complementarily to modulate honeybees’ nectar collecting and processing; whereas the waggle dance boosts the nectar collecting rate, the tremble dance stimulates an increase in the processing rate (Seeley 1995). We first observed tremble dances 2 weeks earlier than waggle dances and thereafter occurred irregularly with only two observation sessions in which both dances coincided. This seeming imbalance of both types of dances may be explained by either tremble or waggle dances being performed out of observation sessions, or tremble dances resulting from contexts other than modulating nectar acquisition (e.g. peril at a food source; Lam et al. 2017). In any case, the factors causing honeybees to perform these signals in bumblebee nests demand further study.
Unlike tremble dances, honeybees performed waggle dances during a defined period in the second half of the study with their age ranging between 16 and 37 days . This age is roughly consistent with that of honeybees performing the waggle dance in natural conditions, 12–22 days (Ai et al. 2017). Waggle dances had a relatively low frequency, but their mean duration (12 waggle runs) lies within the range of 1–100 waggle runs reported in honeybee hives (Seeley 1995). Thus, waggle dances in our study largely resembled, in their signal strength, the dances occurring in honeybee hives. Nearly all waggle dances had conspecific followers, but despite the tactile, acoustic and chemosensory conspicuousness of this behaviour, nestmate bumblebees were not attracted to it.
Bees that perform the waggle dance maintain trophallactic contacts (food transfer) with nestmates attracted to the dance, but trophallaxis is not restricted to this context (Farina and Grüter 2009). We observed non-dancing honeybees having trophallactic contacts with both conspecifics and heterospecific bumblebees that do not perform this behaviour naturally. If bumblebees experienced any food rewards during trophallaxis, one would expect that these rewards could be associated with the presence of a honeybee nestmate, as it occurs in foraging contexts (Dawson and Chittka 2012). Then, this association coupled with relevant olfactory stimuli of flowers conveyed by dancers could potentially direct bumblebees’ attention to the waggle dance, which however did not occur. Future work should thus investigate what learning processes and sensory information might underpin the readiness of uninformed individuals to attend distinctive motor displays of knowledgeable others that lead to reward (Chittka 2022).
Whilst honeybees’ trophallactic contacts occurred throughout the observation period, these interactions were more common in bees with an age of 16–27 days, rising in parallel with foraging activity in the second half of the study. Our observation contrasts with reports in honeybee colonies (Seeley and Kolmes 1991) where trophallactic contacts are described to rise in frequency from an earlier age (~ 5 days). This temporal discrepancy might reflect that in our study, initial trophallactic contacts possibly mediated intermittent information transmission between young honeybees (Farina and Grüter 2009), but once they initiated foraging activity, trophallaxis became more prevalent for food transfer. This idea offers support to the possibility that our honeybees may have adhered to their natural foraging behaviour, with bee collectors transferring nectar to bee processors. Trophallaxis is not only a mechanism of transferring and distributing nectar in the colony, but the trophallactic flow also mediates the transmission of important chemosensory information from one bee to another, thus modulating the worker’s activities for the social community to work (Farina and Grüter 2009; Crailsheim 1998). Interestingly, we observed a few instances where honeybees initiated and held trophallactic contacts with bumblebees. These contacts mainly took place before honeybees foraging activity became more intensive. It is thus possible that at the time honeybees engaged in interspecific trophallaxis, they were at a young age, at which they tend to participate in trophallaxis mostly as recipients (Crailsheim 1998). We therefore speculate that interspecific trophallaxis might have primarily served communicational purposes for young honeybees to obtain relevant chemosensory information from their nestmate bumblebee counterparts.
The frequency at which honeybees in our study groomed themselves and nestmate conspecifics remained stable throughout the observation period. This is in accordance with observations in honeybee hives where bees maintain self and social grooming constant over time regardless of their age (Kolmes 1985c; Seeley and Kolmes 1991). The permanence of both self and social grooming over bees’ lifetime is in conjunction with the hygienic and social functions of these behaviours (Foose et al. 2022). We also observed honeybees displaying lateral shakes, sometimes described as the ‘grooming invitation dance’ (Land and Seeley 2004), yet bees performing this behaviour were not groomed by conspecific nestmates. Further, it is noteworthy that most social grooming was directed at heterospecific bumblebees. The diversity of factors causing grooming in honeybees makes it difficult to interpret honeybees’ motivation to groom bumblebees so diligently, but it is possible that interspecific grooming might have been a social mechanism for honeybees to maintain their odour profile through frequently interacting with host bumblebees (see Bagnères and Lorenzi 2010). Honeybees held antennal contact with both conspecifics and heterospecifics, but antennation with the former had a higher frequency. Antennal contacts play an essential role in honeybees’ social communication (Goyret and Farina 2003); our results thus suggest that honeybees might have discriminated between members of their own and different species and accordingly maintained intraspecific communication via antennal contact.
The adaptive use of biologically relevant behaviours that are part of honeybees’ innate repertoire (e.g., homing, foraging and dance communication) enabled their establishment and subsistence in the bumblebee nests. In contrast with honeybee colonies where thousands of individual bees function as the basic unit of a highly integrated social system (Seeley 1995), our findings reveal that a minor portion of this system can integrate into an unfamiliar yet socially organised collective, such as a bumblebee nest, and honeybees can maintain intraspecific social interactions and communication via specific behavioural mechanisms. Future work should investigate whether honeybees embedded in this social environment might operate and respond to environmental challenges either as a ‘subsystem’ in the nest, in conjunction with heterospecific bumblebees, through the interspecific social interactions documented here, or as independent organisms. Our experimental approach has the potential to be a reliable basis for the systematic study of behavioural patterns in honeybees and further develop the ideas exposed in this work, as well as investigating diverse topics of social insects’ biology, such as division of labour and recognition systems. Although our original question regarding the origins of followers’ response to the waggle dance remains unanswered, this work supplies novel insights about the behaviour and cognition of honeybees.
Data availability
The footage and datasets generated and analysed during this study are available from the corresponding author upon reasonable request.
References
Ai H, Kobayashi Y, Matake T, Takahashi S, Hashimoto K, Maeda S, Tsuruta N (2017) Development of honeybee waggle dance and its differences between recruits and scouts. BioRxiv. https://doi.org/10.1101/179408
Bagnères A, Lorenzi MC (2010) Chemical deception/mimicry using cuticular hydrocarbons. In: Blomquist G, Bagnères A (eds) Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, New York, pp 283–324
Bateson M, Martin PR (2021) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
Biesmeijer JC (2003) The occurrence and context of the shaking signal in honey bees (Apis mellifera) exploiting natural food sources. Ethology. https://doi.org/10.1046/j.01791613.2003.00939.x
Capaldi EA, Dyer FC (1999) The role of orientation flights on homing performance in honeybees. J Exp Biol. https://doi.org/10.1242/jeb.202.12.1655
Chittka L (2022) The mind of a bee. Princeton University Press, Princeton
Chittka L, Rossi N (2023) Bees learn to dance. Science. https://doi.org/10.1126/science.adg6020
Collett M, Chittka L, Collett TS (2013) Spatial memory in insect navigation. Curr Biol. https://doi.org/10.1016/j.cub.2013.07.020
Crailsheim K (1998) Trophallactic interactions in the adult honeybee (Apis mellifera L.). Apidologie. https://doi.org/10.1051/apido:19980106
Dawson EH, Chittka L (2012) Conspecific and heterospecific information use in bumblebees. PLoS ONE. https://doi.org/10.1371/journal.pone.0031444
Degen J, Kirbach A, Reiter L, Lehmann K, Norton P et al (2016) Honeybees learn landscape features during exploratory orientation flights. Curr Biol. https://doi.org/10.1016/j.cub.2016.08.013
Dong S, Lin T, Nieh JC, Tan K (2023) Social signal learning of the waggle dance in honey bees. Science. https://doi.org/10.1126/science.ade1702
Dornhaus A, Brockmann A, Chittka L (2003) Bumble bees alert to food with pheromone from tergal gland. J Comp Physiol. https://doi.org/10.1007/s00359-002-0374y
Dornhaus A, Chittka L (1999) Evolutionary origins of bee dances. Nature. https://doi.org/10.1038/43372
Dyer AF (2002) The biology of the dance language. Annu Rev Entomol. https://doi.org/10.1146/annurev.ento.47.091201.145306
Farina WM, Grüter C (2009) Trophallaxis: a mechanism of information transfer. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioural and theoretical approaches. CRC Press, New York, pp 183–197
Foose AM, Westwick RR, Vengarai M, Rittschof CC (2022) The survival consequences of grooming in the honey bee Apis mellifera. Insectes Soc. https://doi.org/10.1007/s00040-022-00868-2
Goulson D (2010) Bumblebees: behaviour, ecology, and conservation. OUP, Oxford
Goyret J, Farina WM (2003) Descriptive study of antennation during trophallactic unloading contacts in honeybees Apis mellifera carnica. Insectes Soc. https://doi.org/10.1007/s00040-003-0678-0
Guerrero A (2009) Inter and intraspecificity of chemical communication. In: Hardege JD (ed) Chemical Ecology 1. Eolss Publishers, Oxford, pp 415–437
Kaspar RE, Cook CN, Breed MD (2018) Experienced individuals influence the thermoregulatory fanning behaviour in honey bee colonies. Anim Behav. https://doi.org/10.1016/j.anbehav.2018.06.004
Kolmes SA (1985a) An information-theory analysis of task specialization among worker honey bees performing hive duties. Anim Behav. https://doi.org/10.1016/S00033472(85)80131-7
Kolmes SA (1985b) An ergonomic study of Apis mellifera (Hymenoptera: Apidae). J Kans Entomol Soc 58:413–421
Kolmes SA (1985c) A quantitative study of the division of labour among worker honey bees. Z Tierpsychol 68:287–302
Krasnec MO, Breed MD (2012) Eusocial evolution and the recognition systems in social insects. In: López-Larrea C (ed) Sensing in nature. Springer, New York, pp 78–92
Lam C, Li Y, Landgraf T, Nieh J (2017) Dancing attraction: followers of honey bee tremble and waggle dances exhibit similar behaviors. Biol Open. https://doi.org/10.1242/bio.025445
Land BB, Seeley TD (2004) The grooming invitation dance of the honey bee. Ethology. https://doi.org/10.1046/j.1439-0310.2003.00947.x
Lindauer M, Kerr WE (1960) Communication between the workers of stingless bees. Bee World. https://doi.org/10.1080/0005772X.1960.11095309
O’Donnell S, Foster RL (2001) Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae). Ethology. https://doi.org/10.1046/j.1439-0310.2001.00668.x
Perez AA, Johnson BR (2019) Task repertoires of hygienic workers reveal a link between specialized necrophoric behaviors in honey bees. Behav Ecol Sociobiol. https://doi.org/10.1007/s00265-019-2731-7
Pettis JS, Winston ML, Slessor KN (1995) Behavior of queen and worker honey bees (Hymenoptera: Apidae) in response to exogenous queen mandibular gland pheromone. Ann Entomol Soc Am. https://doi.org/10.1093/aesa/88.4.580
Pfeiffer KJ, Crailsheim K (1998) Drifting of honeybees. Insectes Soc. https://doi.org/10.1007/s000400050076
Princen SA, Van Oystaeyen A, Petit C, Van Zweden JS, Wenseleers T (2019) Cross activity of honeybee queen mandibular pheromone in bumblebees provides evidence for sensory exploitation. Behav Ecol. https://doi.org/10.1093/beheco/arz191
Riessberger U, Crailsheim K (1997) Short-term effect of different weather conditions upon the behaviour of forager and nurse honey bees (Apis mellifera carnica Pollmann). Apidologie. https://doi.org/10.1051/apido:19970608
Robinson GE (1987) Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. https://doi.org/10.1007/BF00300679
Romero-González JE, Solvi C, Chittka L (2020) Honey bees adjust colour preferences in response to concurrent social information from conspecifics and heterospecifics. Anim Behav. https://doi.org/10.1016/j.anbehav.2020.10.008
Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol. https://doi.org/10.1007/BF00299306
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press, Cambridge
Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees — illusion or reality? Ethology. https://doi.org/10.1111/j.1439-0310.1991.tb00253.x
Slaa EJ, Hughes WOH (2009) Local enhancement, local inhibition, eavesdropping, and the parasitism of social insect communities. In: Jarau S, Hrncir M (eds) Food Exploitation by Social Insects. CRC Press, Boca Raton, pp 147–164
Su S, Cai F, Si A, Zhang S, Tautz J, Chen S (2008) East learns from west: Asiatic honeybees can understand dance language of European honeybees. PLoS ONE. https://doi.org/10.1371/journal.pone.0002365
Tait C, Mattise-Lorenzen A, Lark A, Naug D (2019) Interindividual variation in learning ability in honeybees. Behav Processes. https://doi.org/10.1016/j.beproc.2019.103918
Toth AL, Robinson GE (2005) Worker nutrition and division of labour in honeybees. Anim Behav. https://doi.org/10.1016/j.anbehav.2004.03.017
Van Der Blom J (1993) Individual differentiation in behaviour of honey bee workers (Apis meltifera L.). Insectes Soc. https://doi.org/10.1007/BF01253898
Von Frisch K (1965) Tanzsprache und Orientierung der Bienen. Springer, Berlin
Winston ML, Punnett EN (1982) Factors determining temporal division of labor in honeybees. Can J Zool. https://doi.org/10.1139/z82-372
Yang M-X, Tan K, Radloff SE, Phiancharoen M, Hepburn HR (2010) Comb construction in mixed-species colonies of honeybees, Apis cerana and Apis mellifera. J Exp Biol. https://doi.org/10.1242/jeb.035626
Acknowledgements
We thank Dr. Meredith Johnson for the illustrations in Fig. 3, Dr. James Makinson for technical advice, Dr. Paul Hurd for kindly supplying newly emerged honeybees, and Amelia Kowalewska for help with video recording.
Funding
JER-G was supported by CONACyT and QMUL, CVU 446980, and China Postdoctoral Science Foundation under grant 300148.
Author information
Authors and Affiliations
Contributions
JER-G, CS, LC: study conception and experimental design; JER-G: data collection; JER-G: data analysis; JER-G wrote the paper; all authors were involved in data interpretation and manuscript revisions.
Corresponding author
Ethics declarations
Ethics approval
The research described here aligns with the ASAB/ABS Guidelines for the Use of Animals in Research. No licences or permits were required for this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: James Nieh
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romero-González, J.E., Solvi, C., Peng, F. et al. Behaviour of honeybees integrated into bumblebee nests and the responses of their hosts. Apidologie 55, 50 (2024). https://doi.org/10.1007/s13592-024-01086-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01086-4