Abstract
Campos de altitude are high-altitude grasslands found in the mountain ranges of southeastern and southern Brazil, which are characterized by high species richness and endemism. Because of the difficulty in delimiting this vegetation type, measuring biodiversity patterns is challenging. Here, we compared the application of two methods using spatial data to estimate angiosperm diversity in campos de altitude: (1) filtering occurrence data by elevation, canopy height, location and keywords and; (2) the same as the first, however, adding a filter of “campos de altitude” in the vegetation type of the Flora e Funga do Brasil database. Also, we discuss conservation status, plant collections, endemism, vegetation data and similarity among 14 sites harboring campos de altitude. Our two resulting lists indicated between 1087 and 2398 angiosperm species and infraspecific taxa in campos de altitude, mostly belonging to Asteraceae and Poaceae and endemic to Brazil. Extrapolations of species richness suggest a potential number of up to 4000 species. Of the taxa assessed for conservation status, 53–65% are threatened or near threatened. The flora of campos de altitude is more similar on closely located mountains rather than on mountains with similar geological characteristics and origin. We provide an editable list online destined to seek help from taxonomists to generate a more accurate species list, to support advances in knowledge on this unique tropical montane ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Montane floras have a high degree of endemism and diversity (Chen et al. 2009; Merckx et al. 2015). As on islands, the isolation of flora on mountaintops leads to similar patterns of dispersal, speciation and extinction (Merckx et al. 2015; Rahbek et al. 2019). Depending on the context and on the organism, mountains can function as bridges or barriers for species flow (Perrigo et al. 2020). Covering around 25% of the planet’s terrestrial area, some estimates suggest that mountain regions harbor more than 85% of the terrestrial species; this pattern is especially observed in the Neotropics, such in the Andes and the mountains of the Atlantic Forest (Rahbek et al. 2019).
Campos de altitude (Fig. 1) are montane ecosystems consisting of unique high-altitude grasslands that occur in the highest and coldest regions of Atlantic Forest in southeastern and southern Brazil (Eiten 1983; Veloso et al. 1991; Safford 1999a, b). While being surrounded by a dense forest matrix and under the influence of similar climatic conditions, campos de altitude differ by having shallow, acidic, and extremely nutrient-poor soils (Safford 1999a, b), favoring their grasslands condition. Typically, these grasslands occur at or above ca.1800–2000 m a.s.l., especially in Caparaó, Serra dos Órgãos, and Itatiaia national parks (Safford 1999a, b). However, the proximity to the ocean and latitude strongly influence this elevational threshold; in some cases, this formation starts at ca.1500 m a.s.l. (Vasconcelos 2011) or even at ca.1300 m in southernmost Brazil (Paraná and Santa Catarina states) (Martinelli and Orleans e Bragança 1996; Struminski 1996; Mocochinski and Scheer 2008; ICMBIO 2021). Elevation and soil type are known to influence the phylogenetic relationships and distribution of species (Neri et al. 2017; Gastauer et al. 2020). Thus, delimiting campos de altitude based solely on elevation is complex, since these grasslands usually form mosaics with the adjacent cloud forests, with different micro-climates and biotas.
Campos de altitude and their flora. a Landscape view of Parque Estadual dos Três Picos (PETP); b Grassland in Parque Nacional do Itatiaia (PNIT); c Vriesea itatiaiae Wawra in Parque Nacional do Itatiaia; d Zygopetalum maculatum (Kunth) Garay in Parque Nacional da Serra dos Órgãos; e Barbacenia irwiniana L.B.S.m. in Parque Nacional da Serra dos Órgãos. Photos: Igor M. Kessous
In Brazil, campos de altitude are not the only type of natural mountaintop grasslands, a common misconception in the literature and databases. Campos de altitude occur only in the Atlantic Forest usually on igneous or metamorphic rocks, in the mountaintops of the Serra do Mar and Serra da Mantiqueira (Safford 1999a). Also, the Serra Geral, at the southernmost part of the Serra do Mar and extending into the interior of Brazil and Paraguay (Frank et al. 2009), harbors grasslands that are recognized as campos de altitude (ICMBIO 2021). Despite originating from the same tectonic event in the Paleocene (Almeida and Carneiro 1998), the Serra do Mar and the Serra da Mantiqueira exhibit significant structural, geographical, and biological differences, making them distinct “vegetation islands”. The Serra da Bocaina is located between these two mountain ranges and is also considered part of the Serra do Mar. Recent climatic events have favored the emergence of a unique biota in the region (Behling et al. 2007).
Other open montane vegetation types occur in Brazil, mainly in the Cerrado (campos rupestres); and in the Amazon (tepuis). In contrast with campos de altitude, these vegetation types occur on sandstone or quarzitic rocks (Alves and Kolbek 2010; Silveira et al. 2020). Many plant collections from these other grasslands include the term “campo de altitude” on their labels; however, these plant communities are biogeographically different. The flora of the campos rupestres is more closely related to that of the tepuis, while campos de altitude are more related to the Andes (Safford 1999b, 2007; Vasconcelos 2011; Guedes et al. 2020). Because of their similarities to Andean Páramos, campos de altitude came to be known as Brazilian Páramos, although with a greater influence of seasonality (Safford 1999a, 2007). Nevertheless, the term campos de altitude was already widely used in the literature and in the national biogeographic classification and is therefore more commonly used today to refer to this vegetation.
The flora of the campos de altitude includes ancient plant lineages (Silveira et al. 2020) and has high species richness and endemism (Safford 1999a; Neri et al. 2017). Advances in taxonomy and associated technology over the past 20 years have dramatically increased the number of known species in Brazil. Previous work focusing on the diversity of campos de altitude suggested that they contain fewer than 1000 vascular plant species (Safford 2007), encompassing 100 endemics (Alves and Kolbek 2010). However, in these studies, the scope of the concept of campos de altitude was not always clear, and most of them included only those high-altitude grasslands typical of the northern Serra do Mar and Serra da Mantiqueira. With the recent expansion of open-source data repositories, the use of spatial data has enabled us to reach a better understanding of species distribution patterns, habitats, and conservation.
Here, we compared two methods using spatial data to estimate the diversity of angiosperms in campos de altitude. We provide two lists of species and infraspecific taxa, the first based on sensu lato searching (filtering occurrence data by elevation, canopy height, location and keywords) and the other based on sensu stricto searching (the same as the first, however adding a filter of “campos de altitude” in the vegetation type of the Flora e Funga do Brasil). Furthermore, we aimed to provide an editable list of angiosperm species of the campos de altitude, and estimate the extrapolated species richness, under the hypotheses that (1) angiosperm taxa occurring in campos de altitude are underestimated in previous inventories; (2) campos de altitude of the Serra da Mantiqueira and Serra do Mar are internally more similar than to each other; and (3) the application of “filtering” methods to spatial data and habitat information can be used to estimate the biodiversity in campos de altitude. Based on the information collected, we estimated the number of taxa per family, threatened taxa, life forms, habitat, and endemism, and provide explanations for species distribution patterns.
Methods
To construct lists of angiosperm taxa (specific and infraspecific) occurring in campos de altitude and surroundings, we downloaded from Global Biodiversity Information Facility (GBIF) 9,101,129 records (GBIF.org -09 February 2022- GBIF Occurrence Download https://doi.org/10.15468/dl.e9c9cf) of all preserved specimens of Magnoliopsida (Eudicots + Basal angiosperms) and Liliopsida (Monocots) from all of Brazil. We excluded ambiguities and all records not identified at least to species level.
Backbone list
To obtain a backbone list (BBL), we divided the search into two steps: first, we obtained a list of taxa with georeferenced data (decimalLatitude and decimalLongitude) further filtered by elevation and canopy height; second, we obtained a list of taxa filtered by keywords to also encompass the occurrences without geographical coordinates.
In the first step, we removed duplicates and cleaned biased records using the R (R Core Team 2020) package CoordinateCleaner (Zizka et al. 2019), flagging and removing “capitals”, “centroids”, “equal”, “gbif”, “institutions”, “zeros” and “countries”. We then downloaded two raster files containing elevation information (Earth Resources Observation and Science—EROS (2018), with 30 m resolution: https://www.usgs.gov/centers/eros/science/usgs-eros-archive-digital-elevation-shuttle-radar-topography-mission-srtm?qt-science_center_objects=0#qt-science_center_objects ) and canopy height, with 1 km resolution: and extracted the information for each point using the R package raster 3.5–29 (Hijmans et al. 2022). There are no official maps specifically containing the sites of campos de altitude (Vasconcelos 2016), thus we specified elevation filters according to the literature for each site where they occur (Table 1, Fig. 2) or when lacking information, using Google Earth®. In addition, we applied a < 5 m filter for canopy height, since campos de altitude are covered predominantly by grasses, shrubs, and forbs, with only a few scattered small trees.
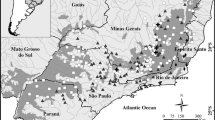
Map with the campos de altitude analyzed here. Map color references the elevation raster. Acronyms are explained in Table 1
We then downloaded the map of the Brazilian protected areas (MMA, http://mapas.mma.gov.br/i3geo/datadownload.htm ), rasterized using the functions shapefile and rasterize of the R package raster (Hijmans et al. 2022), and extracted the information from each coordinate point to determine the distribution of each taxon in the different campos de altitude. We excluded the records corresponding to other Brazilian mountaintop vegetation types, such as the grasslands of central and northern Brazil.
For the keyword search, we used the raw list downloaded from GBIF, including records with and without coordinate data, and performed the search in the locality column: “campo de altitude” and “campos de altitude”, ignoring capitals. Again, we filtered the search output according to the elevation obtained by the GBIF database, keeping only the records according to Table 1, or those that had no elevation information. We removed all records not corresponding to campos de altitude and duplicates.
Final lists
To evaluate incorrect occurrences and obtain data on vegetation type, conservation status, endemism, life form, family, and habitat, we merged our BBL data with previously published data (Moreira et al. 2020; Carrijo et al. 2018), and unpublished lists obtained by our research group (in PNIT and PNCP), and extracted the information from Flora e Funga do Brasil (http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/). For this, we performed the function get.taxa of the R package flora 0.3.5 (Carvalho 2021). The Flora e Funga do Brasil database is based on information from about 1000 taxonomists who collaborated in the description of the Brazilian Flora for more than a decade (Brazil Flora Group 2021). For the synonymized taxa not recognized in this R package, we performed searches for the accepted name of each separately, using the platforms World Flora Online (http://www.worldfloraonline.org/) and Tropicos (https://www.tropicos.org/), and resubmitted them for analysis in the package flora. We excluded all taxa considered to not occur in Brazil. The final output, covering all taxa and localities, is referred to here as the sensu lato list (SLL), representing species and infraspecific taxa that occur in campos de altitude and possibly in surrounding vegetation types. Because of methodological issues of resolution of the rasters and in order to include border species, we considered the use of “campos de altitude and surroundings” to refer to SLL. Preliminary sensu lato lists were made for all localities (Table 1) separately and then merged.
To obtain our sensu stricto list (SSL), we applied a “campos de altitude” filter to the vegetation type column in our preliminary sensu lato lists, representing only those taxa that occur in campos de altitude according to the Flora e Funga do Brasil. Thus, we were able to compare the results and analyses between the two datasets. The resulting lists were compared by the non-parametric Mann–Whitney U test (function wilcox.test) performed in R to verify differences in the number of taxa. Here, we considered only campos de altitude located at or above at least 1300 m and with more than 50 taxa in SLL (except by the outgroup), after a detailed literature search for each specific case. We did not include sites that are doubtful as to their vegetation or “high altitude rupestrian complexes” (see Benites 2002; Garcia 2003; Garcia and Pirani 2005; Vasconcelos 2011). An editable SSL-based angiosperm taxa list can be found in https://doi.org/10.5281/zenodo.8114353.
Cluster, network, dissimilarity analyses, conservation status, and collections
We fully coded the presence and absence of each taxon by site and performed clustering analyses for both datasets, using the R package BinMat (van Steenderen 2022). For the hierarchical cluster analyses, we chose the Jaccard distance method, applying the clustering method of WPGMA (Weighted Pair Group Method with Arithmetic Mean), since we considered that there is an unequal number of taxa in the sites/clusters. We performed the function upgma and applied 10,000 bootstrap replications to calculate the p-value of each cluster. Reliability was measured by approximately unbiased (AU) probability values and bootstrap (BP). We then performed the dissimilarity analyses using the Jaccard method, and obtained the number of taxa for each site, using the functions vegdist and specnumber, respectively, of the R package vegan 2.6.2 (Oksanen et al. 2022).
We performed a network analysis using the eLasso (least absolute shrinkage and selection operator) method (van Borkulo et al. 2014) for SLL and SSL, using the R package IsingFit 0.3.1 (van Borkulo et al. 2016). This procedure allowed us to use binary data, using the Extended Bayesian Information Criterion (EBIC; Chen and Chen 2008), which helps to decrease false-positive rates (van Borkulo et al. 2014). We applied the AND-rule and chose the higher gamma value (1.0) to avoid unreliable connections, especially false positives (van Borkulo et al. 2014, 2016). To statistically compare the output networks resulting from SLL and SSL, we submitted our binary datasets to a direct comparison test (network comparison test), using the NCT function of the R package NetworkComparisonTest 2.2.1 (van Borkulo et al. 2019), with the same parameters as for the network construction.
Conservation threat status (CR: Critically Endangered; VU: Vulnerable; and EN: Endangered) of the taxa were obtained from the latest Official National List of Endangered Plant Species (MMA 2022). Information for near threatened (NT) and least concern (LC) taxa was obtained by use of the package flora 0.3.5 (Carvalho 2021). Life form, habitat, endemism, and the corresponding families (according to the Flora e Funga do Brasil), in addition to the conservation status, were plotted using the R package ggplot2 (Wickham 2016). We included only accurate and unambiguous information about habitat and life form. After obtaining the final lists, we estimated the mean number of collections per taxon (Table 2). We plotted the graphical networks using the package qgraph 1.9.3 (Epskamp et al. 2022).
We estimated species-accumulation curves (SACs) through the method of “rarefaction” and the extrapolated species richness for the GBIF species dataset (based on the SLL vouchers), as well as for each site separately using the R package vegan 2.6.2 (Oksanen et al. 2022). For the extrapolated species richness analyses, we used multiple estimators, as suggested by Brose et al. (2003): Chao, Jacknife (first and second order, Jack1 and Jack2 respectively) and Bootstrap (Boot). All codes and list of vouchers are available on GitHub (https://github.com/kessous/campos_de_altitude).
Results
Sensu lato list
Our sensu lato list (SLL) resulted in 2398 species and infraspecific taxa, 651 genera, and 131 families (Online Appendix 1). The estimated number of angiosperm taxa in each campos de altitude (and surroundings) is given in Table 2. Asteraceae is the best represented family, encompassing 345 taxa, followed by Poaceae (191 taxa) and Orchidaceae (162 taxa) (Fig. 3a). Most of the taxa are herbs (997 taxa; shrubs: 311; trees: 184; Fig. 3a), terrestrial (1792 taxa; epiphytes: 108; rupicolous: 61; Fig. 3a), and endemic to Brazil (1407 taxa; non-endemic: 926, Fig. 3a). A total of 224 taxa of angiosperms are threatened (CR: 44; VU: 45; EN: 135; Fig. 3a) and 47 taxa are near threatened. In general, our results indicated a larger number of taxa in sites with more collections and higher mean numbers of collections per taxon (Table 2). Of the four most diverse sites, PNSB had the fewest collections per taxon (2.4), while PNIT and PNSJ had the most collections per taxon (4.0 and 5.3, respectively). PEDS, PESP, PECJ, APAP, and PIMA had fewer than two collections per taxon.
Number of taxa by: 1- life form; 2- endemism; 3- family; 4- conservation status; 5- habit. a sensu lato list (SLL); b sensu stricto list (SSL). Data obtained from the flora package, according to the Flora e Funga do Brasil (2022) and MMA 2022. IUCN categories: LC least concern, NT near threatened, EN endangered, CR critically endangered, VU vulnerable. Values of each line are explained on the side of the pink bar
Sensu stricto list
The sensu stricto list (SSL) list resulted in 1087 species and infraspecific taxa, 347 genera, and 89 families (Online Appendix 2). This list showed similar taxonomic patterns to the SLL. The Asteraceae was also the best represented family, with 225 taxa, followed by Poaceae (112 taxa) and Fabaceae (58 taxa) (Fig. 3b). Also, most of the taxa are herbs (539 taxa; shrubs: 131 taxa; subshrubs:108 taxa; Fig. 3b), terrestrial (809 taxa; rupicolous: 37 taxa; epiphyte: 15 taxa; Fig. 3b), and endemic to Brazil (686 taxa; non-endemic: 384 taxa, Fig. 3b). According to the SSL, 133 taxa of angiosperms (12%) are threatened (CR: 33 taxa; VU: 19 taxa; EN: 81 taxa; Fig. 3b), and 20 taxa are near threatened according to the IUCN criteria. Of the total number of angiosperm taxa, about 223 (20% in the SSL) are endemic to the campos de altitude vegetation. The number of taxa did not present significant differences between the two lists (W = 157, p = 0.07).
Species accumulation curves and extrapolation
The analysis of SACs and extrapolated species richness demonstrate a continued increase in the number of species within campos de altitude, suggesting that the number of species can reach up to 4052 species. (Fig. 4; Table 2). Across the entire dataset, the observed richness accounted for mean 68.5% (58–83%) of the extrapolated richness (Chao: 64%, Jack1: 69%, Jack2: 58%; Boot: 83%). The completeness means varied across the different sites. PNIT and PNCP had the highest means (76.2% and 75%, respectively), while PESB and PIMA exhibited the lowest means (35.5% and 47.4%, respectively). Similar patterns were observed in the separate datasets, with the minimum percentage typically around 45–50%, often associated with Jack2, and the highest percentage occurring in Boot, ranging from 75 to 85% (refer to Table 2 for precise values).
Species accumulation curves (SACs) per years. a SAC of the entire GBIF species dataset. The blue line represents the SAC estimated by the “rarefaction” method. The boxplots curve represents the SAC estimated by the “random” method; b SAC of each site separately using the GBIF species dataset. Confidence index is represented by the shadows. Acronyms are explained in Table 1
Hierarchical relationship and dissimilarity
The hierarchical cluster dendrograms based on the SSL and SLL data showed very similar topologies and branch support (Fig. 5; acronyms explained in Table 1). Three main clusters were formed: 1– Northern Serra do Mar (PNSO, PEDS, APAP), with PETP nested together only in SSL; 2– Serra da Mantiqueira and Serra da Bocaina; and 3– Southern Serra do Mar + Serra Geral. Importantly, the Southern and Northern Serra do Mar did not cluster together in any of the analyses; however, the Northern Serra do Mar emerged together with the Serra da Mantiqueira in SSL, and partially in SLL, excluding PETP. Internally to the Southern Serra do Mar + Serra Geral, the localities in Paraná state (PEPP and ARAC) clustered with strong support in both analyses. In the Northern Serra do Mar communities, PNSO and PEDS clustered together in both analyses. In the Serra da Mantiqueira, we observed a strongly supported cluster of PNSB, PNIT, and PNCP, with PNCP and PNIT clustering together. The relationship among PDMI, PESP, PECJ, and PESP was inconclusive, with PECJ + (PESP + PDMI) in SLL and PESP + (PECJ + PDMI) in SSL. The dissimilarity analyses (Table 3) showed congruent results to the cluster analyses, although with some differences. However, analyzing the lowest dissimilarity between each site, we observed several of the same pairs in both datasets (Table 3).
Hierarchical clusters dendrogram generated by the R package BinMat. a data from the sensu lato list (SLL); b data from the sensu stricto list (SSL). The number above the branches refers to the bootstrap value (BP) and the number below the branches refers to the approximately unbiased probability values (AU). Colors refer to mountain ranges: reddish-brown-Serra da Mantiqueira; green-Serra do Mar; blue- Serra Geral. Acronyms are explained in Table 1
Network analyses
The network analyses showed similar results regarding positive and negative connections in the datasets, although with more connections (p < 0.05) in SLL than in SSL (Fig. 6). The analysis based on the SLL dataset (Fig. 6a) showed a large number of positive connections. PNSJ (Serra Geral) showed only negative connections, with PNIT, APAP, and PEDS (p < 0.05). SSL network also showed a negative connection between PNSJ and PNIT, in addition to positive connections in three groups: Southern Serra do Mar, Northern Serra do Mar, and Serra da Mantiqueira + PNSB, with the latter two positively connected. The Network Comparison Test showed mostly non-significant differences in comparing the network structures of the SLL and SSL datasets edge weight (per edge: Online Appendix 3; maximum difference in edge weights: p = 1) and global strength (p = 0.77).
Graphical networks among campos de altitude communities generated by the R packages qgraph and IsingFit. Each line weight of the edges indicates positive (green) and negative (red) connection strength. Alpha transparency refers to the p-values, which are transparent where p > 0.05 and less transparent toward 0. Only connections with p < 0.05 are shown. a data from the sensu lato list (SLL), b data from the sensu stricto list (SSL). Acronyms are explained in Table 1
Discussion
Output lists
Few studies have aimed to estimate the number of plant species in the campos de altitude, and most of them have focused on different collection methods, elevation gradients, or specific regions or groups (Pereira et al. 2006; Ribeiro et al. 2007; Mocochinski and Scheer 2008; Pompeu et al. 2014; Campos-Cordeiro and Neri 2019; Campos et al. 2020). Considering the sites analyzed here, we estimate that the number of angiosperms in campos de altitude is between our SLL (2398) and SSL (1087). The former number is higher since it probably contains species of other vegetation types. On the other hand, the latter may have discarded some records, particularly of grassland plants that occur bordering forests. Further advances in developing reliable species lists of the campos de altitude will depend on collection efforts and record validation by specialists on different families. Therefore, an online and editable list is available at https://doi.org/10.5281/zenodo.8114353.
Previous studies suggested that fewer than 1100 plant taxa (Fundação Florestal 2015), including fewer than 1000 vascular plants (Safford 2007) occur in campos de altitude. We demonstrate that it is not possible to estimate accurately the number of angiosperms in campos de altitude solely through the Flora e Funga do Brasil information on habitat occurrence. Our analyses confirmed that number of taxa were underestimated in previous inventories. According to Flora e Funga do Brasil database (searched in August 2022), an estimated 2353 species (and 179 infraspecific taxa) of angiosperms are associated to campos de altitude. However, this number includes certain taxa that do not occur in campos de altitude but in other Brazilian ecosystems, such as the Amazon (tepuis), Cerrado (campos rupestres), and Pampas (campos sulinos). Although some authors have used the term campos de altitude for Amazonian montane grasslands (e.g., Martinelli 2007), we here considered the more recognized use in literature for campos de altitude, it means, only those from the Atlantic Forest. Applying an “Atlantic Forest” filter, this number reduces to 2170 species (and 163 infraspecific taxa), although still including more than 1000 taxa that occur in Rio Grande do Sul state, outside the usual defined geographic range of the campos de altitude (after Martinelli and Orleans e Bragança 1996; Safford 1999a), and not included in the present study.
Family and functional diversity
Grasslands are regions of utmost importance for human survival, representing more than a third of the Earth’s land cover (Buisson et al. 2022). Despite the term “grasslands”, not only grasses, but especially forbs and shrubs are present in these environments (Dixon et al. 2014). Brazil ranks seventh among countries in total grassland area (ca. 17% of its entire land area; White et al. 2000). Here, we found slightly divergent results between the two datasets, with SLL including more tree and epiphyte families than SSL, as expected considering the two methods. On the other hand, Asteraceae and Poaceae were consistently the most diverse families in the campos de altitude (Fig. 3), as observed in previous studies of this vegetation (Safford 1999a; Fundação Florestal 2015; Alves et al. 2016; Flora e Funga do Brasil) and of the alpine flora as a whole in the Americas (Figueroa et al. 2022).
High-altitude environments around the world are occupied by herb- and shrub-rich communities, usually without tall trees (White et al. 2000; Alves and Kolbek 2010; Blair et al. 2014; Dixon et al. 2014; Le Stradic et al. 2015; Mucina 2018; Rada et al. 2019). Here, we found that the number of herbaceous plants is dramatically higher (ca. 3–4 times) than the number of shrubs, the second most common life form in both datasets, which can be explained by the difficult environmental conditions for a tropical vegetation. The campos de altitude environment is subject to wide variations in daily and seasonal temperature, solar radiation, and precipitation (Safford 1999a, b). In the Andean Páramos, grasses and forbs are estimated to be more resistant to water deficiency and low-temperature injury than woody plants (Rada et al. 2019), which could explain the predominance of these life forms in campos de altitude.
The difference in frequency of life forms between our two datasets (i.e., the larger number of tree-like life forms in SLL) reflects that SLL captured cloud forest species, while SSL was more closely restricted to species of campos de altitude. Orchidaceae and Bromeliaceae are represented in campos de altitude (Safford 1999a); however, these families showed a considerable increase in SLL, with a high number of epiphytic species. We also observed an increase in the number of Myrtaceae and Rubiaceae in SLL, which was clearly induced by the surrounding cloud forests that harbors many species of these families (Pompeu et al. 2014).
Conservation, endemism and biogeography
About 59–63% of the angiosperm taxa occurring in campos de altitude are endemic to Brazil, which is similar to the proportion for the Atlantic Forest as a whole (ca. 63%; Flora e Funga do Brasil). Also, our analysis showed that ca. 20% of the taxa (in SSL) occur only in campos de altitude, in general agreement with previous estimates based on more restricted sampling (ca. 21%; Safford 1999a). While their environmental conditions have led to high endemism, campos de altitude are hypothesized to be gateways for plant groups into the Brazilian Shield, especially regarding the Andes-Atlantic Forest pathway, as in the case of certain Bromeliaceae (Givnish et al. 2011) and liverworts (Santos and Costa 2010), and through the co-occurrence of several other groups (Martinelli and Bragança 1996; Safford 1999a). In addition to the Andean similarity, campos de altitude share taxa with several other disjunct phytogeographic elements (temperate, holarctic, austral-antarctic; see Safford 1999a) and with other South American mountain regions (Safford 2007; Alves et al. 2007).
Montane ecosystems are rich in endemic species, which makes them a priority for conservation (Chen et al. 2009; Merckx et al. 2015; Assis and Mattos 2016; Rahbek et al. 2019). Most localities harboring campos de altitude are included in protected areas (see Table 1), and are even covered by a broader national law (the Brazilian “Lei da Mata Atlântica”). However, over time, campos de altitude are subject to many kinds of human pressure (Martinelli 2007; Ribeiro and Freitas 2010) and are especially vulnerable to climate change (Neri et al. 2017). Only ca. 20% of the taxa in both lists have been assessed for their conservation (SLL: 513; SSL: 231), of which a high proportion presented a threatened or near threatened status (SLL: 53%; SSL: 65%). One of the primary factors affecting endemic and endangered campos de altitude species is human-caused fires during the dry season. Conversely, natural fires during the wet season are more limited and may even be beneficial for some species (Safford 1999a, 2001; Aximoff and Rodrigues 2011). These results demonstrate the urgency of acting to protect the campos de altitude, through specific national plans for this vegetation and a greater effort to assess the risks to this environment and its species.
Similarity among campos de altitude communities
Similarity analyses focused on different South American mountain ranges suggest the proximity of the campos de altitude flora to floras in other high-elevation regions in South America, especially the Andean region (Safford 2007), although the internal relationship between them still lacks a clear resolution. Our results refuted our expectation of a higher similarity among floras of the same geological formations.
In contrast to our expectation, Southern Serra do Mar sites are more similar to the Serra Geral (PNSJ) than to those in the Northern Serra do Mar (APAP, PNSO, PEDS, and PETP). One explanation for the pattern observed is the greater distance of the Southern Serra do Mar from the Serra da Mantiqueira + Serra da Bocaina (PEPP to PECJ: ca. 400 km) compared to the Northern Serra do Mar (PNSO to PNIT: ca.150 km), facilitating the flow of species between them. Proximity also seemed to influence the clustering, and networking Northern Serra do Mar.
The Serra da Bocaina, clustered with the sites of the Serra da Mantiqueira, also explained by their greater proximity (40 km from PNIT, although set apart by the lowland Paraiba do Sul river valley). On the other hand, proximity does not explain the relationship of the PNIT + PNCP cluster, which are ca. 350 km apart. One explanation for the massive presence of shared taxa in PNIT and PNCP probably relates to the recent Last Glacial Maximum, which led to the vegetation of the campos de altitude extending to lower elevations, which reached about 700 m in the Caparaó surroundings, forming a “corridor”. These regions were subsequently isolated as the Holocene advanced, as supported by palynological and zoological data (Safford 1999a, b; Gonçalves et al. 2007; Meireles and Shepherd 2015).
Some previous analyses have attempted to investigate the similarity of the campos de altitude to other mountain ecosystems and surrounding physiognomies (Safford 2007; Meireles and Shepherd 2015; Mendonça 2017). Meireles and Shepherd (2015) found that cloud forests at high altitudes are similar to each other, which seemed to be reflected in our locality networks, as SLL showed many more connections than SSL. On the other hand, Mendonça (2017) suggested that the flora of the campos de altitude of the Serra da Mantiqueira is similar to the floras of the campos rupestres of south-central Minas Gerais and the campos sulinos (here also considered as campos de altitude) of the Serra do Mar of Paraná, in contrast to our results. Also, Safford (2007) suggested that the campos de altitude (sensu Safford 2007) are floristically similar to the Aparados da Serra (near PNSJ). Here, PNSJ clustered, with low support, with PEPP + ARAC, and is the only one site located in the Serra Geral, at the southern limit of the Serra do Mar.
Extrapolation of species richness and methodological considerations
The lack of collections can lead to premature conclusions in spatial analyses (Farooq et al. 2021). As expected, our species accumulation curves (SACs) show an increasing trend in the number of species in most sites and in the total dataset. The areas differ greatly in size and accessibility, which affects sampling sufficiency and collection efforts. Taxonomic, geographic, and temporal biases (according to Meineke and Daru 2021) are often observed in these datasets.
The SACs and extrapolated richness have demonstrated that the expected number of species can vary depending on the specific site. However, it is important to note that our SAC analyses did not account for the inclusion of imputed species from recent reviews of two significant sites (PNIT and PNCP, as detailed in the methods section), which could have positively influenced the comprehensiveness of our species lists.
Considering the entire dataset, we found that at least 58% of the species were accounted for in the true richness, with variations of up to 83% depending on the estimation method used. It is crucial to acknowledge that while SACs can provide an estimate of expected species numbers, they are not always considered reliable sources (Longino et al. 2002; Brose et al. 2003).
Moreover, it is noteworthy that the campos de altitude represent only approximately 350 km2 of the Atlantic Rainforest (Safford 1999a), which covers a vast area of about 200,000 km2. At first glance, it might seem unlikely that this relatively small region encompasses a quarter of the native species found within this domain. The Flora and Funga do Brasil estimates the Atlantic Rainforest to host approximately 16,000 angiosperm species. Nevertheless, it is plausible that the count of angiosperms in the Atlantic Forest is underestimated. Furthermore, due to the notable distinctiveness of the campos de altitude habitat in comparison to the forest matrix, it is conceivable that they contribute a substantial portion, potentially a quarter, of the species within this domain.
It is important to mention that the shape of the SAC can be influenced by the spatial distribution of species, which can vary depending on the habitat and environmental conditions (Brose et al. 2003). Hence, it is crucial to exercise caution when interpreting the results obtained from SACs and to consider other factors that may impact estimates of species richness. By employing multiple methodologies, we can obtain a more robust estimate of true species richness and enhance the reliability of our conclusions.
Although SACs extrapolate the number of species in our dataset, our statistical analyses were based on a random subset of all occurring species. In our study, the sites with the largest number of collections were mostly those with the most species, being influenced by several aspects and influencing the analyses performed. PNCP, PNSJ, and PNIT have the largest extent of campos de altitude and have the most collections; however, the sampling bias goes beyond these aspects, especially regarding accessibility. This factor becomes clear when sites near large cities, universities, and research institutions or with easy access are better sampled (Meineke and Daru 2021). Some of the sites are only accessible by long trails, sometimes requiring more than a day of walking (e.g., PDMI), while others can be reached by car (e.g., PNIT). Our results evidenced a disproportionate collecting effort among the campos de altitude, making it highly necessary for further collections to focus on these less collected sites, especially PEDS, PESP, PECJ, APAP, and PIMA (mean collections per taxon < 2). On the other hand, even the most intensely collected sites did not reach saturation in the number of species.
Methodological bias is always present in statistical analyses. Here we tried to standardize biases and errors, since all data were submitted to the same method, without further external intervention. However, the collection bias is present in spatial data databases such as GBIF (Beck et al. 2014), a frequent problem in analyses of large-scale occurrence data. GBIF data come from different collections, which have different collection methods and focus, and therefore some groups and sites are better sampled than others. Also, this can be problematic when performing SACs, as there may not have clear sampling events. On the other hand, by using automated methods to clean the raw matrix, we eliminated some of these biases, although it was then necessary to deal with the information loss (according to Zizka et al. 2020), one of the main issues of filter methods. In traditional checklist methods, some taxonomic groups are better studied than others, and often taxonomic revisions are performed by researchers who are not specialists in all the families involved, resulting in disproportionate effort in the accuracy of species identification. Therefore, to reduce the bias of information loss, and assist to reach the total coverage of species, we here proposed the creation of an editable list to seek an accurate number and the identity of taxa that occur in campos de altitude.
Conclusions
According to the two methods we tested, between 1087 and 2398 angiosperm taxa are present in the Brazilian campos de altitude, an increase over previous estimates. However, according to our extrapolation analyses, this number could potentially exceed 4000. Geographical proximity is a better predictor of floristic similarity than presence in the same mountain range. Also, we found that a relatively few taxa in campos de altitude have conservation assessments, and of these, ca. 53–65% are threatened or near threatened. In campos de altitude, many collections have focused on only a few sites, and this should be changed for a more comprehensive knowledge of this unique tropical ecosystem. New collections in under-sampled sites should be carried out, as only then will we be able to determine the true diversity of these sites.
Overall, our SLL and SSL showed similar patterns, indicating that our “filtering” method based on location, elevation and canopy height may be suitable for estimating biodiversity in campos de altitude and in other mountain environments. The estimated numbers and analyses are mostly proportionally similar between the two datasets. The differences between the SLL and SSL networks and number of taxa were statistically not significant.
Herewith, we make available an online and editable list to seek contributions from taxonomists to eventually estimate a precise number of angiosperm species, to serve as a baseline for phytogeographic and conservation analyses. Finally, we suggest that further conservation and biogeographic studies explore the real state-of-the-art of campos de altitude species conservation status and drivers of their great diversity.
Data availability
The online version contains supplementary material available along with this article and at https://github.com/kessous/campos_de_altitude.
References
Almeida FFM, Carneiro CDR (1998) Origem e evolução da Serra do Mar. Braz J Geol 28:135–150
Alves RJV, Kolbek J (2010) Can campo rupestre vegetation be floristically delimited based on vascular plant genera? Plant Ecol 207:67–79. https://doi.org/10.1007/s11258-009-9654-8
Alves RJV, Cardin L, Kropf MS (2007) Angiosperm disjunction “Campos rupestres –restingas”: a re-evaluation. Acta Bot Bras 21:675–685. https://doi.org/10.1590/S0102-33062007000300014
Alves RG, Zaú AS, Oliveira RD (2016) Flora dos campos de altitude em quatro áreas do Maciço do Itatiaia, nos estados do Rio de Janeiro e Minas Gerais, Brasil. Pesquisas Botânica 69:109–140
Assis MV, de Mattos EA (2016) Vulnerabilidade da vegetação de campos de altitude às mudanças climáticas. Oecologia Aust 20:24–36. https://doi.org/10.4257/oeco.2016.2002.03
Aximoff I, Rodrigues RDC (2011) Histórico dos incêndios florestais no Parque Nacional do Itatiaia. Cienc Florest 21:83–92. https://doi.org/10.5902/198050982750
Beck J, Böller M, Erhardt A, Schwanghart W (2014) Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol Inform 19:10–15. https://doi.org/10.1016/j.ecoinf.2013.11.002
Behling H, Dupont L, Safford HD, Wefer G (2007) Late Quaternary vegetation and climate dynamics in the Serra da Bocaina, southeastern Brazil. Quat Int 161:22–31. https://doi.org/10.1016/j.quaint.2006.10.021
Benites VM (2002) Caracterização dos solos e das substâncias húmicas em complexos rupestres de altitude. PhD Thesis, Universidade Federal de Viçosa
Blair J, Nippert J, Briggs J (2014) Grassland ecology. In: Monson RK (ed) Ecology and the environment. Springer, New York, pp 389–423
Brazil Flora Group (2021) Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon 71:178–198. https://doi.org/10.1002/tax.12640
Brose U, Martinez ND, Williams RJ (2003) Estimating species richness: sensitivity to sample coverage and insensitivity to spatial patterns. Ecology 84:2364–2377. https://doi.org/10.1890/02-0558
Buisson E, Archibald S, Fidelis A, Suding KN (2022) Ancient grasslands guide ambitious goals in grassland restoration. Science 377:594–598. https://doi.org/10.1126/science.abo4605
Campos PV, Villa PM, Schaefer CEGR, Nunes JA, Porembski S, Neri AV (2020) Community composition, beta diversity and structure of high altitude grasslands along an altitudinal gradient in southeastern Brazil. Rev Biol Trop 68:977–986. https://doi.org/10.15517/RBT.V68I3.37704
Campos-Cordeiro AA, Neri AV (2019) Spatial patterns along an elevation gradient in high altitude grasslands. Brazil. Nord J Bot 37:e02065. https://doi.org/10.1111/njb.02065
Carrijo TT, Alves-Araújo AG, Amorim AMA, Barbosa DEF, Barcelos LB, Baumgratz JF, Bueno VR, Coelho RLG, Costa DP, Couto DR, Delgado CN, Dutra VF, Flores TB, Furtado SG, Giacomin LL, Goldenberg R, Gomes M, Gonzaga DR, Guimarães EF, Heiden G, Kameyama C, Labiak PHE, Lírio EJ, Lohmann LG, Matos, FB, Moraes PLR, Meireles LD, Menini-Neto L, Monteiro D, Moreira MM, Morim MP, Mota MCA, Oliveira, JRPM, Pastore JFB, Pederneiras LC, Pereira LC, Rapini A, Salimena FRG, Silva AV, Silva-Neto SJ, Sobral MEG, Souza MC, Sylvestre LS, Trovó M, Viana PL, Forzza RC (2018) Lista de espécies de plantas terrestres do Parque Nacional do Itatiaia. In: Catálogo de Plantas das Unidades de Conservação do Brasil. Jardim Botânico do Rio de Janeiro. Disponível em: https://catalogo-ucs-brasil.jbrj.gov.br. Accessed 21 June 2022
Carvalho G (2021) Package ‘flora’. https://cran.r-project.org/web/packages/flora/flora.pdf. Accessed 5 May 2022
Chen J, Chen Z (2008) Extended bayesian information criteria for model selection with large model spaces. Biometrika 95:759–771. https://doi.org/10.1093/biomet/asn034
Chen IC, Shiu HJ, Benedick S, Holloway JD, Chey VK, Barlow HS, Hill JK, Thomas CD (2009) Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc Natl Acad Sci USA 106:1479–1483. https://doi.org/10.1073/pnas.0809320106
Cronemberger C, Viveiros-de-Castro EB (2007) Ciência e conservação na Serra dos Órgãos. IBAMA, Brasília
Dixon AP, Faber-Langendoen D, Josse C, Morrison J, Loucks CJ (2014) Distribution mapping of world grassland types. J Biogeogr 41:2003–2019. https://doi.org/10.1111/jbi.12381
Eiten G (1983) Classificação da vegetação do Brasil. CNPq/Coordenacao Editorial, Brasília
Epskamp S, Costantini G, Haslbeck J, Isvoranu A, Cramer AO, Waldorp LJ, Schmittmann VD., Borsboom D (2022). Package ‘qgraph’. https://cran.r-project.org/web/packages/qgraph/qgraph.pdf. Accessed 15 May 2022
Farooq H, Azevedo JA, Soares A, Antonelli A, Faurby S (2021) Mapping Africa’s biodiversity: more of the same is just not good enough. Syst Biol 70:623–633. https://doi.org/10.1093/sysbio/syaa090
Figueroa HF, Marx HE, de Souza Cortez MB, Grady CJ, Engle-Wrye NJ, Beach J, Stewart A, Folk RA, Soltis DE, Soltis PS, Smith SA (2022) Contrasting patterns of phylogenetic diversity and alpine specialization across the alpine flora of the American mountain range system. Alp Bot 132:107–122. https://doi.org/10.1007/s00035-021-00261-y
Flora e Funga do Brasil (2022) Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/. Accessed 24 June 2022
Frank HT, Gomes ME, Formoso ML (2009) Review of the areal extent and the volume of the Serra Geral formation, Paraná Basin, South America. Pesqui Em Geocienc 36:49–57. https://doi.org/10.22456/1807-9806.17874
Freitas L (2002) Biologia da polinização em campos de altitude no Parque Nacional da Serra da Bocaina. PhD Thesis, Universidade de Campinas
Fundação Florestal (2015) Plano de Manejo do Parque Estadual de Campos do Jordão. São Paulo. https://smastr16.blob.core.windows.net/fundacaoflorestal/2017/02/Diagn%C3%B3stico-e-Planejamento.pdf. Accessed 20 May 2022
Garcia RJF (2003) Estudo florístico dos campos alto-montanos e matas nebulares do Parque Estadual da Serra do Mar-Núcleo Curucutu, São Paulo, SP, Brasil. PhD Thesis, Universidade de São Paulo
Garcia RJF, Pirani JR (2005) Análise florística, ecológica e fitogeográfica do Núcleo Curucutu, Parque Estadual da Serra do Mar (São Paulo, SP), com ênfase nos campos junto à crista da Serra do Mar. Hoehnea 32:1–48
Gastauer M, Thiele J, Porembski S, Neri AV (2020) How do altitude and soil properties influence the taxonomic and phylogenetic structure and diversity of Brazilian paramo vegetation? J Mt Sci 17:1045–1057. https://doi.org/10.1007/s11629-019-5403-1
Givnish TJ, Barfuss MH, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Winter K (2011) Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Bot 98:872–895. https://doi.org/10.3732/ajb.1000059
Gonçalves PR, Myers P, Vilela JF, de Oliveira JA (2007) Systematics of species of the genus Akodon (Rodentia: Sigmodontinae) in southeastern Brazil and implications for the biogeography of the campos de altitude. Misc publ—Mus Zool, Univ Mich 197
Guedes TB, Azevedo JA, Bacon CD, Provete DB, Antonelli A (2020) Diversity, endemism, and evolutionary history of montane biotas outside the Andean region. In: Rull V, Carnaval AC (eds) Neotropical diversification: patterns and processes. Springer, Cham, pp 299–328
Hijmans RJ, Van Etten J, Cheng J, Mattiuzzi M, Sumner M, Greenberg JA, Lamigueiro OP, Bevan A, Racine EB, Shortridge A, Hijmans MRJ (2022) Package ‘raster’. https://cran.r-project.org/web/packages/raster/raster.pdf. Accessed 15 Aug 2022
IBAMA (2007) Plano de Manejo da APA de Petrópolis. Resumo Executivo. https://petropolis.rj.gov.br/sma/phocadownload/Documentos/Protecao_Ambiental/resumo_executivo_apa.pdf. Accessed 12 March 2022
ICMBIO (2021) Plano de uso público do Parque Nacional de São Joaquim. https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/mata-atlantica/lista-de-ucs/parna-de-sao-joaquim/arquivos/plano_uso_publico_sao_joaquim.pdf. Accessed 11 March 2022
Le Stradic S, Silveira FA, Buisson E, Cazelles K, Carvalho V, Fernandes GW (2015) Diversity of germination strategies and seed dormancy in herbaceous species of campo rupestre grasslands. Austral Ecol 40:537–546. https://doi.org/10.1111/aec.12221
Longino JT, Coddington J, Colwell RK (2002) The ant fauna of a tropical rain forest: estimating species richness three different ways. Ecology 83:689–702. https://doi.org/10.1890/0012-9658(2002)083[0689:TAFOAT]2.0.CO;2
Martinelli G (2007) Mountain biodiversity in Brazil. Braz J Bot 30:587–597. https://doi.org/10.1590/S0100-84042007000400005
Martinelli G, Orleans e Bragança J (1996) Campos de Altitude. Index, Rio de Janeiro
Meineke EK, Daru BH (2021) Bias assessments to expand research harnessing biological collections. Trends Ecol Evol 36:1071–1082. https://doi.org/10.1016/j.tree.2021.08.003
Meireles LD, Shepherd GJ (2015) Structure and floristic similarities of upper montane forests in Serra Fina mountain range, southeastern Brazil. Acta Bot Brasilica 29:58–72. https://doi.org/10.1590/0102-33062014abb3509
Mendonça JGF (2017) Campos de altitude do Parque Estadual da Serra do Papagaio, Minas Gerais, Brasil: Composição florística, fitogeografia e estrutura da vegetação. Master’s Dissertation, Universidade Federal de Juiz de Fora
Merckx VS, Hendriks KP, Beentjes KK, Mennes CB, Becking LE, Peijnenburg KT, Afendy A, Arumugam N, de Boer H, Biun A, Buang MM (2015) Evolution of endemism on a young tropical mountain. Nature 524:347–350. https://doi.org/10.1038/nature14949
MMA (2022) Portaria MMA Nº 148, de 7 de Junho de 2022. https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Portaria/2020/P_mma_148_2022_altera_anexos_P_mma_443_444_445_2014_atualiza_especies_ameacadas_extincao.pdf. Accessed 02 Aug 2022
Mocochinski AY, Scheer MB (2008) Campos de altitude na serra do mar paranaense: aspectos florísticos. Floresta 38:625–640. https://doi.org/10.5380/rf.v38i4.13158
Moreira MM, Carrijo TT, Alves-Araújo AG, Rapini A, Salino A, Firmino AD, Chagas AP, Versiane AF, Amorim AM, da Silva AV, Tuler AC (2020) A list of land plants of Parque Nacional do Caparaó, Brazil, highlights the presence of sampling gaps within this protected area. Biodivers Data J 8:e59664. https://doi.org/10.3897/BDJ.8.e59664
Mucina L (2018) Vegetation of Brazilian campos rupestres on siliceous substrates and their global analogues. Flora 238:11–23. https://doi.org/10.1016/j.flora.2017.06.007
Neri AV, Borges GRA, Meira-Neto JAA, Magnago LFS, Trotter IM, Schaefer CEG, Porembski S (2017) Soil and altitude drive diversity and functioning of Brazilian Páramos (campo de altitude). J Plant Ecol 10:771–779. https://doi.org/10.1093/jpe/rtw088
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H, Oksanen MJ (2022) Package ‘vegan’. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 15 Aug 2022
Pereira IM, Oliveira-Filho ATD, Botelho SA, Carvalho WAC, Fontes MAL, Schiavini I, Silva AFD (2006) Composição florística do compartimento arbóreo de cinco remanescentes florestais do maciço do Itatiaia, Minas Gerais e Rio de Janeiro. Rodriguésia 57:103–126. https://doi.org/10.1590/2175-7860200657108
Perrigo A, Hoorn C, Antonelli A (2020) Why mountains matter for biodiversity. J Biogeogr 47:315–325. https://doi.org/10.1111/jbi.13731
Pompeu PV, Fontes MAL, Santos RMD, Garcia PO, Batista TA, Carvalho WAC, Oliveira Filho ATD (2014) Floristic composition and structure of an upper montane cloud forest in the Serra da Mantiqueira mountain range of Brazil. Acta Bot Brasilica 28:456–464. https://doi.org/10.1590/0102-33062014abb3239
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/
Rada F, Azócar A, García-Núñez C (2019) Plant functional diversity in tropical Andean páramos. Plant Ecol Divers 12:539–553. https://doi.org/10.1080/17550874.2019.1674396
Rahbek C, Borregaard MK, Colwell RK, Dalsgaard BO, Holt BG, Morueta-Holme N, Nogues-Bravo D, Whittaker RJ, Fjeldså J (2019) Humboldt’s enigma: what causes global patterns of mountain biodiversity? Science 365:1108–1113. https://doi.org/10.1126/science.aax014
Ribeiro KT, Freitas L (2010) Potential impacts of changes to Brazilian forest code in campos rupestres and campos de altitude. Biota Neotrop 10:239–246. https://doi.org/10.1590/S1676-06032010000400029
Ribeiro KT, Medina BMO, Scarano FR (2007) Species composition and biogeographic relations of the rock outcrop flora on the high plateau of Itatiaia, SE-Brazil. Braz J Bot 30:623–639. https://doi.org/10.1590/S0100-84042007000400008
Rodrigues MT, Cassimiro J, Pavan D, Curcio FF, Verdade VK, Pellegrino KCM (2009) A new genus of microteiid lizard from the Caparaó Mountains, southeastern Brazil, with a discussion of relationships among Gymnophthalminae (Squamata). Am Mus Novit. https://doi.org/10.1206/622.1
Safford HDF (1999a) Brazilian Páramos I. An introduction to the physical environment and vegetation of the campos de altitude. J Biogeogr 26:693–712. https://doi.org/10.1046/j.1365-2699.1999.00313.x
Safford HDF (1999b) Brazilian Páramos II. Macro-and mesoclimate of the campos de altitude and affinities with high mountain climates of the tropical Andes and Costa Rica. J Biogeogr 26:713–737. https://doi.org/10.1046/j.1365-2699.1999.00312.x
Safford HDF (2001) Brazilian Páramos. III. Patterns and rates of postfire regeneration in the Campos de Altitude. Biotropica 33:282–302. https://doi.org/10.1111/j.1744-7429.2001.tb00179.x
Safford HDF (2007) Brazilian Páramos IV. phytogeography of the campos de altitude. J Biogeogr 34:1701–1722. https://doi.org/10.1111/j.1365-2699.2007.01732.x
Santos ND, Costa DP (2010) Altitudinal zonation of liverworts in the Atlantic Forest, Southeastern Brazil. Bryologist 113:631–645. https://doi.org/10.1639/0007-2745-113.3.631
Silva RR, Cervi AC, dos Santos ÉP (2010) Flórula do Morro dos Perdidos, Serra de Araçatuba, Paraná, Brasil: Ericaceae. Acta Biol Par 39:87–97. https://doi.org/10.5380/abpr.v39i0.20256
Silveira FA, Dayrell RL, Fiorini CF, Negreiros D, Borba EL (2020) Diversification in ancient and nutrient-poor Neotropical ecosystems: how geological and climatic buffering shaped plant diversity in some of the world’s neglected hotspots. In: Rull V, Carnaval AC (eds) Neotropical diversification: patterns and processes. Springer, Cham, pp 329–368
Struminski E (1996) Parque Estadual do Pico Marumbi, caracterização ambiental e delimitação das áreas de risco. Master’s Dissertation, Universidade Federal do Paraná
van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, Waldorp LJ (2014) A new method for constructing networks from binary data. Sci Rep 4:5918. https://doi.org/10.1038/srep05918
van Borkulo CD, Epskamp S, van Borkulo MC (2016) Package ‘IsingFit’. https://cran.r-project.org/web/packages/IsingFit/IsingFit.pdf. Accessed 30 June 2022
van Borkulo CD, Boschloo L, Borsboom D, Penninx BWJH, Waldorp LJ, Schoevers RA (2019) Package ‘NetworkComparisonTest’. https://cran.r-project.org/web/packages/NetworkComparisonTest/NetworkComparisonTest.pdf. Accessed 7 July 2022
van Steenderen C (2022) Package ‘BinMat’. https://cran.r-project.org/web/packages/BinMat/BinMat.pdf. Accessed 10 July 2022
Vasconcelos MFD (2011) O que são campos rupestres e campos de altitude nos topos de montanha do Leste do Brasil? Braz J Bot 34:241–246. https://doi.org/10.1590/S0100-84042011000200012
Vasconcelos VV (2016) Mapa de estratificação de altitude para vegetação no Brasil. Geosul 31(62):7–18. https://doi.org/10.5007/2177-5230.2016v31n62p7
Veloso HP, Rangel-Filho ALR, Lima JCA (1991) Classificação da vegetação brasileira, adaptada a um sistema universal. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro
White RP, Murray S, Rohweder M, Prince SD, Thompson KM (2000) Grassland ecosystems. World Resources Institute, Washington, DC
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Zizka A, Silvestro D, Andermann T, Azevedo J, Duarte Ritter C, Edler D, Farooq H, Herdean A, Ariza M, Scharn R, Svantesson S (2019) CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol Evol 10:744–751. https://doi.org/10.1111/2041-210X.13152
Zizka A, Carvalho FA, Calvente A, Baez-Lizarazo MR, Cabral A, Coelho JFR, Colli-Silva M, Fantinati MR, Fernandes MF, Ferreira-Araújo T, Moreira FGL (2020) No one-size-fits-all solution to clean GBIF. PeerJ 8:e9916. https://doi.org/10.7717/peerj.9916
Acknowledgements
We thank the members of the Floral Biology Lab—JBRJ for their valuable discussions on the project and Janet Reid for the English revision. We gratefully acknowledge the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro—FAPERJ (204.326/2021; E-26/204.327/2021) for the postdoctoral fellowship and research grant to IMK; Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (PQ:304794/2018-0) and FAPERJ (CNE: E-26/201.000/2022) for the research grant to LF.
Funding
This work was funded by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro, FAPERJ (204.326/2021; E-26/204.327/2021; E-26/201.000/2022).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the design and conception of the study. IMK analyzed the data and wrote the paper, with comments from LF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Both authors have approved the submitted manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
35_2023_298_MOESM3_ESM.csv
Supplementary file3 Online Appendix 3. Comma-separated differences in p-values between SLL and SSL edges from the permutation test (CSV 2 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kessous, I.M., Freitas, L. Implementing spatial analyses to measure angiosperm biodiversity from the high-altitude grasslands of the Atlantic forest. Alp Botany 133, 163–178 (2023). https://doi.org/10.1007/s00035-023-00298-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-023-00298-1