Abstract
Objectives
To evaluate the inhibition of indirubin in FLSs migration, invasion, activation, and proliferation in RA FLSs.
Methods
The levels of IL-6 and IL-8 in cultural supernatants were measured by ELISA. RA FLS migration and invasion in vitro were measured by the Boyden chamber method and the scratch assay. Signal transduction protein expression was measured by western blot. FLS proliferation was detected by Edu incorporation. F-actin was measured by immunofluorescence staining.
Results
We found that indirubin reduced migration, invasion, inflammation, and proliferation in RA FLSs. In addition, we demonstrated that indirubin inhibited lamellipodium formation during cell migration. To gain insight into molecular mechanisms, we evaluated the effect of indirubin on PAK1 and MAPK activation. Our results indicated that indirubin inhibited the activity of PAK1 and MAPK.
Conclusions
Our observations suggest that indirubin may be protective against joint destruction in RA by regulating synoviocyte migration, invasion, activation, and proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a common chronic joint inflammatory disease with abnormal synovial hyperplasia and progressive destruction of cartilage and bone [1]. Fibroblast-like synoviocytes (FLSs) in the synovial intimal lining are considered to be key effector cells in the pathogenesis of RA [2]. They can produce a variety of pro-inflammatory cytokines, chemokines, and inflammatory enzymes, which provides an inflammatory microenvironment [3, 4]. Increasing evidence indicates that stable activated RA FLSs exhibit characteristics of malignant cells [5, 6] and play a critical role in the development of pannus by migrating and invading into cartilage and bone [7–9]. Therefore, inflammatory mediators and migration and invasion of FLSs are crucial for the pathogenesis of RA, and the inhibition of migration and invasion of FLSs may be a therapeutic strategy for the destructive progress of RA.
Indirubin is the active component of Danggui Longhui Wan, a traditional Chinese medicine formulation, used against chronic myelocytic leukemia [6]. Natural indirubin and its multiple synthetic derivatives inhibit cyclin-dependent kinases (CDKs) and glycogen synthase kinase-3β (GSK-3β) [10]. Several studies have confirmed that indirubin have anti-tumor activity in several human cancer cells [10–15] and have anti-inflammatory activity. Indirubin suppresses lipopolysaccharide(LPS)-induced pro-inflammatory cytokine expression in macrophages [16] and inhibits inflammatory response in TNF-α-stimulated human umbilical vein endothelial cells [17]. Indirubin also inhibits inflammatory reactions in delayed-type hypersensitivity in a mouse model [7]. However the effects of indirubin on RA FLS have not been addressed so far. Therefore, in this study, we determined whether indirubin exerts suppressive effects against RA FLS motility and activation. We then showed that indirubin inhibits the migration, invasion, activation, and proliferation of RA FLS.
Materials and methods
Patients and isolation of FLS
Synovial tissue specimens were obtained from RA patients and normal control patients, who are undergoing synovectomy or joint replacement surgery. Briefly, synovial tissues were cut into small pieces and digested with collagenase I (Sigma, St. Louis, USA) in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA) for 4 h at 37 °C, 5% CO2. After centrifugation, the cells were cultured in DMEM/F12 medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator of 5% CO2, 21% O2, and 75% N2. When the cells reached confluence, the cells were trypsinized, and passaged. The cells at passages of 3–7 were used in this study. The study was approved by the Medical Ethical Committee of the First Affiliated Hospital, Sun Yat-sen University, China and performed according to the recommendations of the Declaration of Helsinki.
Cell viability assay
Indirubin was obtained from Selleck Chemicals. RA FLSs were pretreated with indirubin at different concentrations (1–25 μM) for 48 h. The culture supernatants were removed, and the adherent cells were incubated for 4 h with a solution of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) salt. MTT formazan was dissolved in dimethyl sulfoxide (DMSO), and the absorbance was read at 540 nm with microplate reader.
In vitro migration and invasion assay of FLS
Chemotaxis assay of FLS was determined by the transwell (Corning, New York, USA) migration assay. The bottom chambers were filled with DMEM/F12 medium containing 10% FBS which was used as a chemoattractant. The top chambers were seeded with 200 μL DMEM/F12 medium (without FBS) and FLS with indirubin for 24 h. After 12 h, the non-migrating cells were removed from the upper surface of the filter by a cotton swab. The migrated cells on the bottom side of the membrane were fixed in methanol and stained with 0.1% Crystal Violet. Images were taken using a ZEISS digital microscope and the stained cells were counted as the mean number of cells per 8 random fields for each assay. The assays were replicated three times.
For the in vitro invasion assay, similar experiments were performed using inserts coated with a Matrigel basement membrane matrix (BD Biosciences, Oxford, UK). The bottom chambers were filled with DMEM/F12 medium containing 15% FBS which was used as a chemoattractant. The top chambers were seeded with 200 μL DMEM/F12 medium (without FBS) and FLS with indirubin for 24 h. After 24 h, the non-invaded cells were removed from the upper surface of the filter by a cotton swab. The invaded cells on the bottom side of the membrane were fixed in methanol and stained with 0.1% Crystal Violet.
Wounding migration
RA FLSs treated with indirubin for 24 h, plated to confluence on 60-mm culture dishes, were wounded with pipette tips and then treated with or without 10% FBS. After 24 h of incubation, migration was quantified by counting the cells that had moved beyond a reference line.
Proliferation assays
5-Ethynyl-2′-deoxyuridine (EdU) is a thymidine analogue that is incorporated into replicating DNA when cells are dividing and is used to label proliferating cells. RA FLSs were trypsinized, counted, and seeded onto 96-well plates. RA FLSs were grown to 80% confluence and were pretreated with or without indirubin for 24 h. Cell proliferation was then measured using a Cell-Light EdU DNA Cell Proliferation Kit according to the manufacturer’s instructions. Each assay was replicated three times.
Confocal laser scanning fluorescence microscopy
RA FLSs were seeded on sterilized glass coverslips in 35-mm dishes. When became approximately 80% confluent, the cells were stimulated with indirubin for 24 h and then were stimulated with TNF-α (10 ng/mL) for 12 h. Then they were fixed with paraformaldehyde and permeated with 0.1% Triton X-100 in PBS. For detection of F-actin, the cells were incubated with phalloidin overnight. The cells were then incubated with DAPI and the coverslips were mounted on glass slides with antifade mounting media and examined using a confocal fluorescence microscopy (Zeiss LSM710).
ELISA
RA FLSs were treated with indirubin for 24 h and then were stimulated with TNF-α (10 ng/mL) for 24 h. Cell culture supernatants were collected for the measurement of IL-6 and IL-8 using cytokine-specific ELISA kits according to the instructions of the manufacturer (R&D systems, Minneapolis, MO, USA).
RNA isolation and quantitative polymerase chain reaction
After the designated treatments with indirubin for 24 h and then treated with TNF-α (10 ng/mL) for 12 h, total RNAs were extracted using TRIzol (Sigma) and were reverse transcribed to cDNA using miScript Reverse Transcription Kit. Quantitative real-time polymerase chain reaction (PCR) analysis for the expression of IL-6 and IL-8 was performed on cDNA using QuantiTect SYBR Green RT-PCR Kit on StepOnePlusTM Real-Time PCR System (Applied Biosystems). Relative mRNA expression was normalized to the expression of GAPDH.
Western blot analysis
For western blotting, RA FLSs were pretreated with indirubin for 24 h and then were stimulated with or without TNF-α (10 ng/mL) for 10 min. Cells were lysed with cell lysis buffer (CST) for 15 min on ice, and lysates were centrifuged at 12,000 rpm for 15 min at 4 °C. Supernatants were incubated with 2× laemmli sample buffer (Sigma) at 100 °C for 5 min. The equal amounts of samples were then separated with SDS-PAGE gel and transferred on NC membranes and immunoblotted with the indicated antibodies: anti-JNK (Cell signaling), anti-phospho-JNK (Cell signaling), anti-p38 (Cell signaling), anti-phospho-p38 (Cell signaling), anti-ERK (Cell signaling), anti-phosphor-ERK (Cell signaling), anti-phosphor-PAK1 (Abcam), and anti-PAK1 (Abcam).
Statistical analysis
The software of SPSS 13.0 was utilized for statistical analysis. Data were present as means ± standard deviation (SD). Student’s t test or the Mann–Whitney U test was used to detect differences between experimental groups. P values less than 0.05 were considered statistically significant.
Results
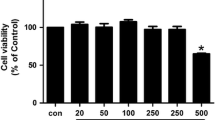
The effect of indirubin on RA FLS viability
The cytotoxic effect of indirubin was evaluated. RA FLSs were cultured until 90% confluent and cytotoxicity was assessed by MTT assay. We observed no significant change in cell viability when cells were treated with different concentrations of indirubin (1–25 μM) for 48 h (Fig. 1).
Indirubin suppresses the migration and invasion of RA FLS
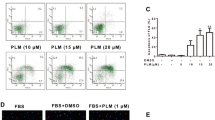
To evaluate the inhibition role of indirubin in RA FLS migration and invasion, we examined the effect of indirubin on FBS-induced migration and invasion in RA FLS. As shown in Fig. 2S, migration and invasion were increased in RA FLS compared with that in normal healthy FLS. As shown in Fig. 2a, c, indirubin treatment markedly inhibited FBS-induced migration and invasion of RA FLS. RA FLS migration was also evaluated in a wound healing assay in the presence or absence of indirubin. Cell movement into the wounded area was detected with light microscopy. As expected, the number of cells that migrated increased in response to FBS. However, indirubin treatment suppressed migration in response to FBS (Fig. 2b). These results reveal an important role of indirubin in inhibiting RA FLS migration and invasion. We also found that anti-IL-6 (tocilizumab) and anti-IL-8 antibodies inhibited RA FLS migration and invasion.
Effect of indirubin on migration and invasion of RA FLS. a Migration was performed in a Boyden chamber and chemotaxis was quantified by counting the migrated cells. Photo images of migration of RA FLS treated with indirubin. b Effect of indirubin on the wounding migration of RA FLS induced by FBS (original magnification ×100). Cells migrating beyond the reference line were photographed and counted. c Invasion was performed in a Matrigel basement membrane matrix chamber and chemotaxis was quantified by counting the invaded cells. Photo images of invasion of RA FLS treated with indirubin. All values represent mean ± SD. *P < 0.05 vs. control
Indirubin suppresses cytoskeleton alterations of RA FLS
Dynamic reorganization of the actin cytoskeleton is critical for RA FLS migration. Therefore, we investigated whether indirubin inhibits the reorganization of the actin cytoskeleton in TNF-α-induced RA FLS. As shown in Fig. 3a, treatment with indirubin affected the number or intensity of F-actin stress fibers in TNF-α-treated RA FLS. Using wound healing assay simulates the cell migration process upon indirubin treatment. After the addition of indirubin, the cells were inhibited to migrate into the created cell-free area. As shown in Fig. 3b, in the presence of indirubin at the concentration of 10 μM, the cell pseudopodia extending was inhibited. These results suggest that indirubin controls RA FLS migration through regulating the reorganization of cytoskeleton.
Effect of indirubin on cytoskeleton alterations of RA FLS. a Effect of indirubin on the alterations of actin cytoskeleton in TNF-α-treated RA FLS. F-actin (red) and nucleus (blue) were stained with phalloidin and DAPI, respectively. b RA FLSs were grown on glass coverslips for 48 h, and then scratched with the narrow end of a sterile pipet tip and were treated with indirubin, and cell migration into cell-free area was assessed after 18 h. F-actin (red) and nucleus (blue) were stained with phalloidin and DAPI, respectively, arrows indicate protruding pseudopodia. Representative images from three independent experiments. (color figure online)
Indirubin suppresses pro-inflammatory mediators in RA FLSs
To evaluate the inhibition role of indirubin in pro-inflammatory mediator expression, we examined the effect of indirubin on TNF-α-induced IL-6 and IL-8 gene expression and secretion of IL-6 and IL-8 in RA FLS. As shown in Fig. 2S, pro-inflammatory cytokines were increased in RA FLS compared with that in normal healthy FLS. Indirubin treatment suppressed TNF-α-induced IL-6 and IL-8 mRNA expression and the secretion of IL-6 and IL-8 in RA FLS, respectively (Fig. 4a, b).
Effects of indirubin on pro-inflammatory cytokine expression and production of pro-inflammatory cytokines in RA FLSs. RA FLSs were pretreated with DMSO, as the control, or various concentrations of indirubin for 24 h and then stimulated with or without TNF-α (10 ng/mL) for 12 (for IL-6 and IL-8 mRNA) or 24 (for IL-6 and IL-8 secretion) hours. a Effect of indirubin on IL-6 and IL-8 expression. mRNA expression of IL-6 and IL-8 was determined by quantitative real-time PCR. Data were normalized to GAPDH. The levels of the IL-6 and IL-8 in cultured cell supernatants were measured by ELISA. All values represent mean ± SD. *P < 0.05 vs. control, # P < 0.05 vs. TNF-α
Indirubin suppresses proliferation in RA FLSs
Previous studies indicate that indirubin suppresses tumor cell proliferation. Therefore, we investigated the effect of indirubin on TNF-α-induced RA FLSs proliferation, which was measured by EdU incorporation. As shown in Fig. 2S, proliferation was increased in RA FLS compared with that in normal healthy FLS. We showed that indirubin treatment reduced TNF-α-induced proliferation of FLSs (Fig. 5).
Effect of indirubin on proliferation in RA FLSs. The cells were pretreated with DMSO or various concentrations of indirubin and then stimulated with TNF-α (10 ng/mL) for 24 h. EdU incorporation was used to assess proliferation of cells. Representative images from 3 independent experiments. All values represent mean ± SD. * P< 0.05 vs. control, # P < 0.05 vs. TNF-α
Indirubin regulates MAPK signal in RA FLS
Since several MAPKs have been implicated in regulating cell migration, proliferation, and inflammation, we next asked whether indirubin had any effect on the activation of MAPKs (p38, JNK, and ERK) in response to TNF-α. As shown in Fig. 6, the phosphorylation of p38, JNK, and ERK was robustly induced after treatment with TNF-α. Indirubin treatment suppressed phosphorylation of p38, JNK, and ERK after TNF-α treatment.
Effect of indirubin on the activation of MAPK signal in RA FLS. Western blot analysis (n = 3) of phosphorylated p38 or JNK or ERK in RA FLS pretreated with indirubin for 24 h followed by 10 ng/mL TNF-α for 10 min. Densitometry was performed and fold change of protein expression is shown below the corresponding band. All values represent mean ± SD. * P< 0.05 vs. control, # P < 0.05 vs. TNF-α
Indirubin regulates TNF-α-induced activation of PAK1 in RA FLS
PAK1, a potential mediator of Rac1/Cdc42 signaling pathway, is involved in regulating RA FLS migration, proliferation, and inflammation. PAKs also regulate some signal kinases, including mitogen-activated protein kinase (MAPK) family members. Therefore, we investigated the role of indirubin in regulating PAK1 activation in response to TNF-α. It was observed that indirubin treatment inhibited phosphorylation of PAK1 in TNF-α-treated RA FLS (Fig. 7).
Effect of indirubin on TNF-α-induced activation of PAK1 in RA FLS. Western blot analysis of phosphorylated PAK1 in RA FLS treated with DMSO or indirubin for 24 h in the presence or absence of TNF-α (10 ng/mL). Densitometry was performed and fold change of protein expression is shown. All values represent mean ± SD. * P< 0.05 vs. control, # P < 0.05 vs. TNF-α
Discussion
The synovial lining undergoes dramatic changes in RA, leading to the formation of hyperplastic, invasive tissue that invades and destroys joint structures. Indirubin has been reported to have beneficial effects on anti-tumor activities and inflammatory diseases [7, 10–13, 17]. In the study, we demonstrate that indirubin prevents migration, invasion, actin cytoskeletal reorganization, proliferation, and inflammation of RA FLS. It is also shown that indirubin suppresses TNF-α-induced RA FLS p38, JNK, and ERK MAPKs activation and PAK1 activation. In light of these findings, these studies identify indirubin as a novel drug of RA FLS activation, migration, and proliferation.
Migration of FLS to cartilage and bone has been considered as a critical step in the aggravation of RA. RA FLS can destruct cartilage or activate osteoclasts to enhance bone erosion and destruction when they arrive in the cartilage or bone [18–20]. To migrate, cells undergo dynamic rearrangements of their actin cytoskeleton to form protrusive structures and generate the intracellular forces required for cell translocation. Actin-based cell motility relies on actin as well as numerous actin-interacting proteins and a wide variety of signaling molecules such as kinases and phosphatases that drive the dynamics of the actin system and govern its spatial organization [21]. Inflammatory mediators including IL-6 and IL-8 exhibited abundant production in RA synovium and high concentration in the synovial and serum of RA, and have been demonstrated to play critical roles in pathogenesis of RA [22]. In response to pro-inflammatory cytokines, FLSs produce chemokines, which further enhance inflammation, hyperplasia, and cartilage destruction [23]. In this work, we demonstrated that indirubin inhibited migration, proliferation, and inflammation of RA FLS.
Mitogen-activated protein kinases, including JNK, ERK, and p38, are involved in the regulation of migration, proliferation, and inflammation [24–26]. In this study, TNF-α stimulation of FLSs resulted in the phosphorylation of MAPKs. Furthermore, indirubin inhibited the phosphorylation of p38, JNK, and ERK. These findings may suggest that indirubin inhibits the MAPK signaling pathways to reduce RA FLS migration, proliferation, and inflammation.
PAK1 also plays an important role in cell migration, proliferation, and inflammation [27–29]. A recent report shows that PAK1 has an important role in regulating migration and invasion of RA FLS [28]. This prompts us to investigate whether indirubin inhibits PAK1 activation. Our studies showed that indirubin decreased PAK1 activity in RA FLS. Taken together, our study suggests that indirubin suppresses TNF-α-induced RA FLS migration, proliferation, and inflammation through the inhibition of MAPK pathway, and suppression of PAK1 activation.
In summary, we have identified that indirubin suppresses RA FLS migration, proliferation, and inflammation through the inhibition of MAPK and PAK1 pathway. Taking these data together, indirubin may serve as an effective therapeutic drug for RA.
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33.
Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55.
Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr Opin Pharmacol. 2013;13:413–9.
Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–900.
Roivainen A, Jalava J, Pirila L, Yli-Jama T, Tiusanen H, Toivanen P. H-ras oncogene point mutations in arthritic synovium. Arthritis Rheum. 1997;40:1636–43.
Muller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102–10.
Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–15.
Pap T, Meinecke I, Muller-Ladner U, Gay S. Are fibroblasts involved in joint destruction? Ann Rheum Dis 2005;64 Suppl 4:iv52–4.
Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–7.
Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 2001;20:3786–97.
Knockaert M, Blondel M, Bach S, Leost M, Elbi C, Hager GL, et al. Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the antiproliferative effects of indirubins. Oncogene. 2004;23:4400–12.
Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005;102:5998–6003.
Ribas J, Bettayeb K, Ferandin Y, Knockaert M, Garrofe-Ochoa X, Totzke F, et al. 7-Bromoindirubin-3′-oxime induces caspase-independent cell death. Oncogene. 2006;25:6304–18.
Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 2006;281:23425–35.
Kim EJ, Park WH, Ahn SG, Yoon JH, Kim SW, Kim SA. 5′-nitro-indirubinoxime inhibits inflammatory response in TNF-alpha stimulated human umbilical vein endothelial cells. Atherosclerosis. 2010;211:77–83.
Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000;410:93–100.
Volin MV, Huynh N, Klosowska K, Chong KK, Woods JM. Fractalkine is a novel chemoattractant for rheumatoid arthritis fibroblast-like synoviocyte signaling through MAP kinases and Akt. Arthritis Rheum. 2007;56:2512–22.
Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8.
McGarry T, Veale DJ, Gao W, Orr C, Fearon U, Connolly M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res Ther. 2015;17:153.
Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–28.
Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–8.
Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26.
Chang YW, Zhao YF, Cao YL, Gu W, Pang J, Zhan HS. Bufalin exerts inhibitory effects on IL-1beta-mediated proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2014;37:1552–9.
Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–28.
Lao M, Shi M, Zou Y, Huang M, Ye Y, Qiu Q, et al. Protein Inhibitor of Activated STAT3 Regulates Migration, Invasion, and Activation of Fibroblast-like Synoviocytes in Rheumatoid Arthritis. J Immunol. 2016;196:596–606.
Fu D, Yang Y, Xiao Y, Lin H, Ye Y, Zhan Z, et al. Role of p21-activated kinase 1 in regulating the migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Rheumatology (Oxford). 2012;51:1170–80.
Dammann K, Khare V, Lang M, Claudel T, Harpain F, Granofszky N, et al. PAK1 modulates a PPARgamma/NF-kappaB cascade in intestinal inflammation. Biochim Biophys Acta. 2015;1853:2349–60.
Yang G, Zhang X, Shi J. MiR-98 inhibits cell proliferation and invasion of non-small cell carcinoma lung cancer by targeting PAK1. Int J Clin Exp Med. 2015;8:20135–45.
Acknowledgements
The authors would like to thank Jinjin Fan for her technical assistance.
Funding
This work is supported by grants from Guangdong Project of Science and Technology (Grant Number 2012B031800375).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of Interest has been declared by authors.
Additional information
Responsible Editor: Liwu Li.
Mingcheng Huang, Lihui Wang, and Shan Zeng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, M., Wang, L., Zeng, S. et al. Indirubin inhibits the migration, invasion, and activation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Inflamm. Res. 66, 433–440 (2017). https://doi.org/10.1007/s00011-017-1027-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1027-5