Abstract
Background
Salidroside (Sal) is a natural product commonly isolated from Rhodiola rosea L., which has been found to have numerous pharmacological activities (e.g., ameliorating apoptosis and inflammation, and acting as an antioxidant) in various diseases, but its concrete function in rheumatoid arthritis (RA) has not been revealed yet. Here, we aimed to explore the specific role and underlying mechanisms of Sal in RA-fibroblast-like synoviocytes (RA-FLSs).
Methods
Cell counting kit 8 (CCK-8) was used to assess the viability of normal-FLSs and RA-FLSs. Cell apoptosis in RA-FLSs was evaluated by flow cytometry. Western blotting was prepared to examine the levels of apoptosis- and signaling-related proteins. Wound-healing and Transwell assays were conducted to examine RA-FLSs migration and invasion. Enzyme-linked immunosorbent assay (ELISA) was used to assess the effect of Sal on tumor necrosis factor-alpha (TNF-α)-induced inflammation in RA-FLSs. RA animal model was established through complete Freund’s adjuvant (CFA) induction, and the histopathological changes in synovial tissues of the rat model were analyzed by H&E staining.
Results
RA-FLSs were treated with 200 μM Sal for 24 h, and cell viability was significantly suppressed. Sal promoted RA-FLSs apoptosis. The migratory and invasive abilities of RA-FLSs were markedly inhibited by Sal. Sal incubation reduced the levels of inflammatory cytokines interleukin‑8 (IL-8), IL-1β, and IL‑6 in RA-FLSs under the stimulation of TNF‑α. Subsequently, Sal downregulated phosphorylated phosphatidylinositol‑3 kinase (p-PI3K) and protein kinase (p-AKT) expression in RA-FLSs. After the treatment with pathway activator 740Y‑P (20 μM) in RA-FLSs, the promotive effect of Sal on cell apoptosis was reversed, and inhibitory effects of it on cell viability, migration, invasion, and inflammatory response were abolished. Sal inhibited RA development in the CFA-induced rat model.
Conclusion
Sal suppressed cell growth and inflammation in RA-FLSs by inactivating PI3K/AKT-signaling pathways.
Zusammenfassung
Hintergrund
Salidrosid (Sal) ist ein natürliches Produkt, das gewöhnlich aus Rhodiola rosea L. isoliert wird und bei dem zahlreiche pharmakologische Wirkungen (z. B. Verbesserung von Apoptose und Inflammation sowie Wirkung als Antioxidans) bei verschiedenen Krankheiten festgestellt wurden, aber seine konkrete Funktion bei rheumatoider Arthritis (RA) ist noch nicht dargelegt worden. In der vorliegenden Arbeit war es das Ziel, die spezifische Rolle und die zugrunde liegenden Mechanismen von Sal bei mit RA assoziierten fibroblastenartigen Synoviozyten (RA-FLS) zu untersuchen.
Methoden
Zur Prüfung der Lebensfähigkeit von normalen FLS und RA-FLS wurde das Zellzählungssystem CCK‑8 („cell counting kit 8“) eingesetzt. Zellapoptose bei RA-FLS wurde mittels Durchflusszytometrie gemessen. Mit Westernblots wurde der Gehalt an apoptose- und signalwegassoziierten Proteine bestimmt. Zur Untersuchung der RA-FLS-Migration und -Invasion wurden Wundheilungs- und Transwell-Assays durchgeführt. ELISA-Tests wurden zur Bestimmung der Wirkung von Sal auf durch Tumornekrosefaktor-alpha (TNF-α) induzierte Inflammation in RA-FLS verwendet. Ein RA-Tiermodell wurde mithilfe der Induktion durch CFA („complete Freund’s adjuvant“, komplettes Freund-Adjuvans) etabliert, und die histopathologischen Veränderungen im Synovialgewebe des Rattenmodells wurden anhand der Hämatoxylin-Eosin(HE)-Färbung analysiert.
Ergebnisse
RA-FLS wurden mit 200 μM Sal für 24 h behandelt, und die Zelllebensfähigkeit wurde so wesentlich unterdrückt. Sal förderte die RA-FLS-Apoptose. Die Migrations- und Invasionsfähigkeit von RA-FLS wurde durch Sal deutlich inhibiert. Durch Sal-Inkubation reduzierten sich die Werte der inflammatorischen Zytokine Interleukin‑8 (IL-8), IL-1β und IL‑6 in RA-FLS unter der Stimulation von TNF‑α. In der Folge wurde durch Sal die Expression von phosphorylierter Phosphatidylinositol-3-Kinase (p-PI3K) und Proteinkinase (p-AKT) in RA-FLS downreguliert. Nach Behandlung mit dem Signalwegaktivator 740Y‑P (20 μM) in RA-FLS kehrte sich der fördernde Effekt von Sal auf die Zellapoptose um, und seine inhibitorischen Effekte auf die Zelllebensfähigkeit, -migration, -invasion und entzündliche Reaktion wurden aufgehoben. Sal inhibierte also die RA-Entstehung im CFA-induzierten Rattenmodell.
Schlussfolgerung
Sal unterdrückte Zellwachstum und entzündliche Reaktionen in RA-FLS durch Inaktivierung des PI3K/AKT-Signalwegs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a type of autoimmune disease, and RA patients often suffered from the swelling, stiffness, and undesirable musculoskeletal pain, which can impair the life quality of the RA patients [1]. Hyperplasia of synovial tissues is one of the main RA pathological features, and a large number of activated fibroblast-like synoviocytes (FLSs) exist in them [2,3,4]. In addition, tumor-like properties including increased proliferation, decreased apoptosis and prolonged survival can be found in RA-FLSs. More importantly, inflammation can be caused in RA-FLSs by producing pro-inflammatory cytokines [5], playing an essential role in RA development. Thus, it is of great significance to find new targets that can affect cell growth and inflammatory response of RA-FLSs, thus, improving the treatment strategy for RA.
Salidroside (Sal) extracted from Rhodiola rosea L. has been found to possess various pharmacological activities like anti-inflammatory [6], anti-depressive [7], cardioprotective [8], neuroprotective [9], and anti-apoptotic [10] effects. Many studies have discussed the role of Sal in bone-related diseases, particularly in osteoarthritis. For example, Sal alleviates cartilage degeneration via the nuclear factor-kappaB (NF-κB) pathway [11]. Sal protects ATDC5 chondrocyte cells against lipopolysaccharides-induced inflammatory response via regulating miR-145 expression [12]. Moreover, its powerful anti-apoptotic, anti-phlogistic and pro-proliferating effects also have been revealed in osteoarthritis [13]. Furthermore, the protective effect of Sal on cognitive dysfunction induced by arthritis in the blood and brain tissues was shown in a previous study [14]. In addition, Liu et al. revealed the inhibitory effect of Sal on gouty arthritis [15]. However, its concrete role and underling mechanism in RA have not been discussed yet.
It has been widely reported that the inhibition of phosphoinositol-3 kinase/threonine kinase (PI3K/AKT) signaling by molecule drugs is a novel research strategy for RA treatment [16, 17]. PI3K/AKT signaling participates in the growth and apoptosis of RA-FLSs, as well as the secretion of inflammatory factors in the synovium [18]. The regulatory effect of Sal on PI3K/AKT signaling has been widely reported [19, 20]. Thus, it is necessary to understand the relationship between Sal and PI3K/AKT signaling in RA.
Here, we hypothesized that Sal may exerted its pro-proliferating, anti-inflammatory, and anti-apoptotic functions in RA via the PI3K/AKT signaling pathway. A cell model of RA-FLSs and an RA rat model induced by complete Freund’s adjuvant (CFA) were used to verify this hypothesis.
Materials and methods
Cell culture and treatment
Human fibroblast-like synoviocytes (normal-FLSs) and human fibroblast-like synoviocytes: rheumatoid arthritis (RA-FLSs) were purchased from Cell Applications (San Diego, CA, USA). Cells were maintained at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) containing carbon dioxide (5%), foetal bovine serum (FBS) (10%), penicillin (100 U/mL), and streptomycin (100 μg/mL) (all from Gibco, Grand Island, NY, USA).
Salidroside (purity: 99.79%; MedChemExpress, Shanghai, China) was dissolved in 0.1% dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and diluted to the final concentrations. Both normal-FLSs and RA-FLSs were treated with Sal at different concentrations (25, 50, 100, and 200 μM) for the indicated number of hours (24, 48, and 72 h) to confirm the optimal doses and treatment time using cell counting kit-8 (CCK‑8) assay. Meanwhile, the cells in control group were treated with DMSO (0.1%) as vehicle. Finally, RA-FLSs treated with 200 μM Sal for 24 h were used for the in vitro assays. To detect the effect of Sal on inflammation, RA-FLSs were stimulated with 10 ng/mL tumor necrosis factor-alpha (TNF‑α; Sigma) for 12 h. To conduct the rescue assay, RA-FLSs were treated with pathway activator 740Y‑P (20 μM; MedChemExpress) for 24 h following Sal treatment.
CCK-8 assay
Normal-FLSs and RA-FLSs were seeded in 96-well plates (1 × 104 cells/well) for 24 h. After the indicated treatment of Sal or Sal with 740Y‑P, cells were added with CCK‑8 solution (10 μL; Solarbio, Beijing, China). Then, normal-FLSs and RA-FLSs were further incubated for 24, 48, and 72 h, respectively, to determine viability. Absorbance at a wavelength of 450 nm was measured using a microplate reader (REAGEN, Shenzhen, China).
Flow cytometry
After the 200 μM Sal treatment for 24 h, RA-FLSs were harvested with trypsin and resuspended in binding buffer (500 μL) in a centrifuge tube. Subsequently, annexin V‑fluorescein isothiocyanate (FITC) (5 μL) and propidium iodide (PI) (10 μL) (APPLYGEN, Beijing, China) were added and cultured with the RA-FLSs in the dark for 15 min. Finally, the apoptosis in RA-FLSs was analyzed by flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Proteins were extracted from the treated RA-FLSs by lysis buffer (Beyotime Biotechnology, Shanghai, China) and quantified by bicinchoninic acid (BCA) kit (Beyotime Biotechnology). Subsequently, proteins (25 μg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into polyvinylidene difluoride (PVDF membranes [Millipore, Billerica, MA, USA]). Then, the membranes were blocked with 5% non-fat milk (Sigma) at room temperature for 1 h in Tris-buffered saline (Sigma). Primary antibodies of Bcl‑2 (ab182858), Bax (ab32503), cleaved caspase‑3 (ab32042), cleaved caspase‑9 (FNab01296), p‑PI3K (ab278545), PI3K (ab180967), p‑AKT (ab38449), AKT (ab131168), GAPDH (ab8245), and horse radish peroxidase (HRP)-conjugated secondary antibody were obtained from Abcam (Shanghai, China) and FineTest (Wuhan, China). Membranes were treated with the primary antibody at 4 °C overnight, and then incubated with HRP-conjugated secondary antibody for 1 h at room temperature after washing with phosphate buffer saline (PBS; Sigma). Enhanced chemiluminescence kit (ECL, Millipore) was used to visualize the protein blots. The protein band intensity was determined using the ImageJ software.
Wound-healing assay
RA-FLSs were seeded in 12-well plate (5 × 105 cells/well) and cultured with 200 μM Sal for 24 h. Subsequently, a pipette tip was used to make the wounds, and the width of the wounds were observed under a microscope. Then, photographs of each group of cells were taken immediately at 0 h and at 24 h, and the migratory ability of RA-FLSs was analyzed.
Transwell assay
To examine cell migration, RA-FLSs (5 × 104 cells per well) were resuspended in serum-free medium and cultured into the uncoated upper 24-well Transwell chamber with 8 μm pore size (Corning Inc., Corning, NY, USA). Transwell chamber precoated with Matrigel (40 μL; BD Biosciences) was used for the invasion assay. Then the DMEM containing FBS (10%) as a chemoattractant was added to the lower chamber. After 24 h of incubation at 37 °C, RA-FLSs that had migrated into the lower chambers were fixed in methanol (Sigma) for 15 min and stained with crystal violet (0.1%; Sigma) at room temperature for 30 min. The degree of cell migration and invasion was determined by counting under a microscope (Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The levels of inflammatory cytokines (IL‑8, IL-1β, and IL-6) in the supernatants of treated RA-FLSs under the stimulation of TNF-α were evaluated using corresponding ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Complete Freund’s adjuvant (CFA) animal model
Sprague–Dawley (SD) rats (male; n = 24; 220–250 g, Shanghai Experimental Animal Center, Shanghai, China) were housed in their cages (n = 5 in each cage) under standard laboratory conditions with free access to food and water. After 1 week, rats were randomly divided into three groups (n = 8 in each group): normal group, CFA group, and CFA + Sal group. Rats in CFA and CFA + Sal groups were immunized with 200 μg stable emulsion (2 g/ml bovine type II collagen solution emulsified with an equal volume of complete Freund’s adjuvant; Sigma). In addition, rats were given 100 μg booster injection on day 21 to establish the RA models. Rats in the control group were given normal saline only. In addition, rats in CFA + Sal group were treated with Sal (20 mg/kg and day) orally via intragastric tube for 14 days after the establishment of the RA model. At the end, all the rats were euthanized using 10% chloral hydrate (Sigma). The Institutional Animal Care and Use Committee of Qinghai University Affiliated Hospital approved all the animal procedures.
Hematoxylin and eosin staining
The knee joints were removed from the rats on the last day of the experiments. The removed knee joints were fixed in paraformaldehyde (4%), embedded in paraffin, and cut into slices (5 μm), which were then stained with hematoxylin and eosin (H&E; YEASEN, Shanghai, China) to determine pathological changes. Histopathological assessment was conducted based on a previous study [21], and the score (0–5) for inflammation, synovial hyperplasia, and pannus was then determined.
Statistical analysis
SPSS 17.0 software (IBM, Armonk, NY, USA) was used to analyze all data, which are presented as mean ± standard deviation (SD). To compare the difference between two groups, Student’s t test was utilized. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to compare the significant differences between multiple groups. The nonparametric Kruskal–Wallis rank-sum test was used to analyze the nonnormally distributed data, such as the histopathological score (0–5). The number of samples (n) and biological replicates (N) are shown in figure legends. The differences were considered statistically significant at P < 0.05.
Results
Sal inhibits cell proliferation and promotes cell apoptosis in RA-FLSs
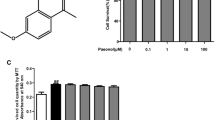
Normal-FLSs and RA-FLSs were all stimulated with different doses of Sal (25, 50, 100, and 200 μM) for 24, 48, or 72 h to evaluate cell viability using CCK‑8 assay. We found that 200 μM Sal treatment reduced cell viability of RA-FLSs most significantly at all of the analyzed time points (24, 48, and 72 h). More importantly, the viability of normal-FLSs had no significant change after the treatment of Sal at any concentrations (25, 50, 100, and 200 μM) for 24 h, indicating no cytotoxic effect. We thus confirmed that 200 μM Sal with 24 h of treatment time can be selected for the subsequent experiments (Fig. 1a). Flow cytometry revealed that both early and late apoptosis rates in the Sal group were markedly enhanced compared to the control in RA-FLSs (Fig. 1b,c). Consistently, the protein level of Bcl‑2 (anti-apoptotic protein) was downregulated and the protein levels of Bax, cleaved caspase‑3, and cleaved caspase‑9 (pro-apoptotic proteins) were upregulated in the RA-FLSs treated with Sal, suggesting that Sal promoted RA-FLSs apoptosis (Fig. 1d).
Salidroside (Sal) inhibits cell proliferation and promotes cell apoptosis in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). a Cell viability was assessed in normal-FLSs and RA-FLSs after the Sal treatments at different concentrations (25, 50, 100, and 200 μM) using cell counting kit-8 (CCK‑8) assay. b,c Flow cytometry revealed early apoptosis rate and late apoptosis rate in RA-FLSs treated with Sal (200 μM). c,d Western blotting revealed the levels of apoptosis-related proteins in RA-FLSs treated with Sal (200 μM). All data are presented as mean ± standard deviation (SD) (N = 3). *p < 0.05, **p < 0.01, ***p < 0.001
Sal inhibits cell migration, invasion, and inflammation in RA-FLSs
We then further detected the effects of Sal on RA-FLSs migration and invasion. As shown by the wound-healing assay, the migratory ability of RA-FLSs was suppressed by Sal treatment compared to the control group (Fig. 2a). Consistently, the Transwell assay showed decreased migrated cells in the Sal group compared to the control (Fig. 2b). Subsequently, the effects of Sal on TNF-α-induced inflammation were assessed using ELISA in RA-FLSs. As expected, TNF‑α stimulation led to increased levels of IL‑8, IL-1β, and IL‑6 in the supernatant of the cells. In contrast, Sal reduced the levels of these inflammatory cytokines significantly, suggesting the anti-inflammatory action of Sal during RA (Fig. 2d).
Salidroside (Sal) inhibits cell migration, invasion, and inflammation in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). a The wound-healing assay was used to analyze the changes in cell migration. b,c Transwell assay was used for assessing cell migration and invasion in RA-FLSs treated with Sal (200 μM). d Enzyme-linked immunosorbent assay (ELISA) showed the levels of inflammatory cytokines in RA-FLSs stimulated with TNF‑α (10 ng/mL) and treated with Sal (200 μM). All data were represented as mean ± standard deviation (SD) (N = 3). ***p < 0.001 vs control group; ###p < 0.001 vs TNF‑α group
Sal inactivates PI3K/AKT signaling pathway in RA-FLSs
Evidence has confirmed the participation of the PI3K/AKT signaling pathway in regulating cell apoptosis and inflammatory process, which has been confirmed as a new target of RA treatment [22]. Thus, we used western blotting to assess the effect of Sal on this signaling pathway. Surprisingly, Sal significantly downregulated the levels of p‑PI3K and p‑AKT in RA-FLSs. When the cells were further treated with pathway activator 740Y‑P, the levels of p‑PI3K and p‑AKT were almost restored to the control levels (Fig. 3a). CCK‑8 revealed that Sal suppressed the RA-FLSs viability, and 740Y‑P promoted it compared with the Sal group (Fig. 3b). Flow cytometry indicated that the increased early and late apoptosis rates induced by Sal in RA-FLSs was obviously inhibited by 740Y‑P (Fig. 3c,d). Consistent with that, 740Y‑P also enhanced the level of Bcl‑2 and restrained the levels of Bax, cleaved caspase‑3, and cleaved caspase‑9 compared with the Sal group, suggesting that Sal inhibited cell proliferation and enhanced cell apoptosis by inactivating the PI3K/AKT signaling pathway in RA-FLSs (Fig. 3e,f).
Salidroside (Sal) inactivates phosphoinositol-3 kinase/threonine kinase (PI3K/AKT) signaling pathway in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLs). a Western blotting was used to examine the levels of signaling-related proteins in RA-FLSs treated with Sal (200 μM) and signaling activator 740Y‑P (20 μM). b Cell counting kit-8 (CCK‑8) showed the changes in cell viability. c,d Flow cytometry revealed cell apoptosis rate. e,f Western blotting presented the expression of apoptosis-related proteins in RA-FLSs treated with Sal (200 μM) and 740Y‑P (20 μM). All data were represented as mean ± standard deviation (SD) (N = 3). **p < 0.01, ***p < 0.001 vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs Sal group
Sal inhibits cell migration, invasion, and inflammation in RA-FLSs via the PI3K/AKT signaling pathway
To confirm whether the PI3K/AKT signaling pathway influenced the inhibitory effect of Sal on RA-FLSs migration and invasion, RA-FLSs were further treated with Sal and pathway activator 740Y‑P was further investigated to conduct the subsequent experiments. As expected, the reduction in cell migration and invasion after Sal treatment was significantly increased by 740Y‑P in RA-FLSs, as shown by Transwell assays (Fig. 4a,b). In TNF-α-stimulated RA-FLS, Sal suppressed the levels of inflammatory cytokines IL‑8, IL-1β, and IL‑6. However, the inhibition on these inflammatory cytokines was then abolished by 740Y‑P, indicating that Sal had an anti-inflammatory role during RA via the inactivation of the PI3K/AKT signaling pathway (Fig. 4c).
Salidroside (Sal) inhibits cell migration, invasion, and inflammation in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs) via the phosphoinositol-3 kinase/threonine kinase (PI3K/AKT) signaling pathway. a,b Transwell assay revealed the changes in cell migration and invasion in RA-FLSs treated with Sal (200 μM) and 740Y‑P (20 μM). c Enzyme-linked immunosorbent assay (ELISA) showed the levels of inflammatory markers in RA-FLSs stimulated with TNF‑α (10 ng/mL) and treated with Sal (200 μM) and 740Y‑P (20 μM). All data were represented as mean ± standard deviation (SD) (N = 3). **p < 0.01, ***p < 0.001 vs control group; ##p < 0.01, ###p < 0.001 vs Sal or TNF‑α group; &&&p < 0.001 vs TNF-α+ Sal group
Sal alleviates RA development in CFA rat model
To further investigate the role of Sal in vivo, rats were randomly divided into three groups (n = 8 in each group): normal group, CFA group, and CFA + Sal group. According to the results of H&E staining, smooth and clear tissues without inflammatory cell infiltration were presented in normal control group. Inversely, we observed obvious hyperplasia of synovial tissues, increased inflammatory cell infiltration, damaged articular cartilage, and narrowed articular cavity in the CFA group, while the Sal-treated rat model presented significantly improved histopathological changes (Fig. 5a). In addition, the histopathological scores for inflammation, synovial hyperplasia, and pannus in the rat model was evaluated. Apparently, the scores in CFA + Sal group were obviously lower than those in the CFA group, suggesting that Sal had a mitigating effect on RA development in rat model (Fig. 5b).
Salidroside (Sal) alleviates rheumatoid arthritis (RA) development in Complete Freund’s adjuvant (CFA) rat model. a Hemotoxylin and eosin (H&E) staining of synovial tissues, and b histopathological score in normal, CFA, and CFA + Sal groups. All data were represented as mean ± standard deviation (SD; n = 8 in each group). **p < 0.01, ***p < 0.001 vs normal group; ##p < 0.01 vs CFA group
Discussion
In recent years, many bioactive molecules from traditional Chinese medicine have been revealed to exert potent anti-RA effects through various mechanisms. For example, piperlongumine alleviates RA by inhibiting the proliferation, migration and invasion of RA-FLSs [23]. Cinnamomi ramulus is also one of the anti-RA traditional Chinese medicine formulas that has anti-proliferative and migratory effects on RA-FLSs [24]. Eugenol existing in a variety of plants suppresses RA progression by inhibiting TNF-α-induced cell growth and inflammation of RA-FLSs, and promoting RA-FLSs apoptosis [25]. In this study, we for the first time confirmed the anti-RA potential of Sal through the inactivation of the PI3K/AKT signaling pathway.
Normal-FLSs are responsible for keeping the stability and lubrication of the articular cavity by secreting collagen, hyaluronic acid, and fibronectin, thus, playing a crucial role in RA development. In contrast, RA-FLSs present an active proliferative state and impaired apoptosis [26]. Here, Sal significantly suppressed the RA-FLSs viability and promoted the cell apoptosis. More importantly, the protein level of Bcl‑2 (anti-apoptotic protein) was downregulated and the protein levels of Bax, cleaved caspase‑3, and cleaved caspase‑9 (pro-apoptotic proteins) were upregulated in the RA-FLSs treated with Sal, suggesting the anti-RA potential of Sal.
Emerging evidence has suggested that inhibiting RA-FLSs migration and invasion is a critical therapeutic target for treating RA [27]. Sal has been reported to inhibit the cell proliferation, migration, and invasion activity of various cell types, like RF/6A cells and BV2 microglia [28, 29]. Here, we found that Sal markedly inhibited the migratory and invasive capacities of RA-FLSs. RA is well-known as an inflammatory immune disease [30]. The activated RA-FLS can cause synovial proliferation, inflammation, and joint damage by producing pro-inflammatory cytokines [31]. The anti-inflammatory action of Sal also has been widely demonstrated [32, 33]. Consistently, we found that Sal prominently suppressed the levels of inflammatory cytokines IL‑8, IL-1β, and IL‑6 in RA-FLSs under the stimulation of TNF‑α, indicating that Sal exerted anti-inflammatory effects during RA.
PI3K/AKT is an important signaling pathway that can regulate various cellular processes, and recent studies highlighted its close relationship with RA development [34, 35]. Sal has been reported to induce the inactivation of PI3K/AKT signaling, which play a beneficial role in many diseases, including cerebral ischemia-reperfusion injury [19], osteoarthritis [13], myocardial injury [36, 37], and atherosclerosis [38]. In our study, Sal treatment repressed the phosphorylation of PI3K and AKT in RA-FLSs. Rescue assays further revealed that the signaling activator 740Y‑P significantly abolished the inhibitory effect of Sal on cell proliferation, migration, invasion, and TNF-α-induced inflammation in RA-FLSs and reversed its promotive effect on RA-FLSs apoptosis, indicating that Sal alleviated RA development by inactivating the PI3K/AKT pathway.
To further prove the anti-RA potential of Sal, the RA rat model was established through CFA induction to conduct in vivo assays. Previous studies have reported many joint pathological changes including swelling, synovial inflammation, and joint damage in the CFA-induced animal model [39]. Similarly, we observed, obvious hyperplasia of synovial tissues, increased inflammatory cell infiltration, damaged articular cartilage, and narrowed articular cavity in CFA group compared with the normal rats. Particularly, Sal significantly inhibited synovial hyperplasia, reduced inflammatory cell infiltration, and alleviated cartilage damage relative to the CFA group, which is further evidenced by prominently reduced histopathological score for inflammation, synovial hyperplasia, and pannus in Sal-treated rats.
However, some limitations still exist in this study. For example, the underlying mechanism of Sal has not been investigated in vivo. In the future, a more representative in vivo tissue samples will be prepared, and more complete in vivo experiments will be designed to further detect the role of Sal in RA.
Conclusion
Sal had an inhibitory effect on cell growth and inflammation in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs) by inactivating the phosphoinositol-3 kinase/threonine kinase (PI3K/AKT) signaling pathways and mitigated inflammatory symptoms and destruction of synovial in the Complete Freund’s adjuvant (CFA)-induced RA rat model. The results of our study provide a new idea for investigating RA development, and Sal may become a novel target to treat RA.
References
Sparks JA (2019) Rheumatoid Arthritis. Ann Intern Med 170(1):Itc1
Bottini N, Firestein GS (2013) Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 9(1):24–33
Chen Z et al (2019) Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 15(1):9–17
Bartok B, Firestein GS (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233(1):233–255
Korb-Pap A et al (2016) Stable activation of fibroblasts in rheumatic arthritis-causes and consequences. Rheumatology 55(suppl 2):ii64–ii67
Wang J et al (2015) The effect of synthetic salidroside on cytokines and airway inflammation of asthma induced by diisocyanate (TDI) in mice by regulating GATA3/T-bet. Inflammation 38(2):697–704
Zhu L et al (2015) Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci Lett 606:1–6
Zhu L et al (2015) Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-κB/AP1 pathway. Inflammation 38(4):1589–1598
Zhu L et al (2021) Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother Res 35(10):5767–5780
Gui D et al (2017) Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway. BMC Pulm Med 17(1):191
Gao H et al (2020) Salidroside alleviates cartilage degeneration through NF-κB pathway in osteoarthritis rats. Drug Des Devel Ther 14:1445–1454
Liu M et al (2019) Salidroside protects ATDC5 cells against lipopolysaccharide-induced injury through up-regulation of microRNA-145 in osteoarthritis. Int J Immunopharmacol 67:441–448
Wu M et al (2019) Salidroside suppresses IL-1β-induced apoptosis in chondrocytes via phosphatidylinositol 3‑Kinases (PI3K)/Akt signaling inhibition. Med Sci Monit 25:5833–5840
Zhu L et al (2016) Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology 103:134–142
Liu Y et al (2019) Frontline science: reprogramming COX‑2, 5‑LOX, and CYP4A-mediated arachidonic acid metabolism in macrophages by salidroside alleviates gouty arthritis. J Leukoc Biol 105(1):11–24
Li X, Wang YJI (2020) Cinnamaldehyde attenuates the progression of rheumatoid arthritis through down-regulation of PI3K/AKT signaling pathway. Inflammation 43(5):1729–1741
Yu Z et al (2018) Foxc1 promotes the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis via PI3K/AKT signalling pathway. Tissue Cell 53:15–22
Yang B et al (2019) miR-124a inhibits the proliferation and inflammation in rheumatoid arthritis fibroblast-like synoviocytes via targeting PIK3/NF-κB pathway. Cell Biochem Funct 37(4):208–215
Wei Y et al (2017) Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral Ischemia in rats. Inflammation 40(4):1297–1309
Xu MC et al (2013) Salidroside attenuates myocardial ischemia-reperfusion injury via PI3K/Akt signaling pathway. J Asian Nat Prod Res 15(3):244–252
Liu R et al (2016) The suppressive effects of the petroleum ether fraction from atractylodes lancea (Thunb.) DC. On a collagen-induced arthritis model. Phytother Res 30(10):1672–1679
Song B et al (2019) BMP9 inhibits the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via the PI3K/AKT signaling pathway. Int Immunopharmacol 74:105685
Xu S et al (2018) Piperlongumine inhibits the proliferation, migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm Res 67(3):233–243
Liu J et al (2020) Anti-proliferation and anti-migration effects of an aqueous extract of Cinnamomi ramulus on MH7A rheumatoid arthritis-derived fibroblast-like synoviocytes through induction of apoptosis, cell arrest and suppression of matrix metalloproteinase. Pharm Biol 58(1):863–877
Wang M et al (2022) Eugenol suppresses the proliferation and invasion of TNF-α-induced fibroblast-like synoviocytes via regulating NF-κB and COX‑2. Biochem Biophys Res Commun 612:63–69
Mo BY et al (2018) Long non-coding RNA GAPLINC promotes tumor-like biologic behaviors of fibroblast-like synoviocytes as microRNA sponging in rheumatoid arthritis patients. Front Immunol 9:702
Huang M et al (2017) Indirubin inhibits the migration, invasion, and activation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Inflamm Res 66(5):433–440
Yang H, Yang Q, Zheng L (2022) Inhibition of hypoxia-inducible factor‑1 by salidroside in an in vitro model of choroidal neovascularization. Cutan Ocul Toxicol 41(3):203–209
Hu H et al (2014) Salidroside reduces cell mobility via NF- κ B and MAPK signaling in LPS-induced BV2 microglial cells. Evid Based Complement Alternat Med p:383821
Wu J et al (2019) Kirenol inhibits the function and inflammation of fibroblast-like synoviocytes in rheumatoid arthritis in vitro and in vivo. Front Immunol 10:1304
Sivalingam SP et al (2007) In vivo pro- and anti-inflammatory cytokines in normal and patients with rheumatoid arthritis. Ann Acad Med Singap 36(2):96–99
Hu R et al (2020) Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J Pharmacol 867:172797
Luan X et al (2022) Salidroside mitigates airway inflammation in asthmatic mice via the AMPK/Akt/GSK3β signaling pathway. Int Arch Allergy Immunol 183(3):326–336
Smith MD, Walker JG (2004) Apoptosis a relevant therapeutic target in rheumatoid arthritis? Rheumatology 43(4):405–407
Malemud CJ (2015) The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem 7(9):1137–1147
He H et al (2015) Salidroside mitigates sepsis-induced myocarditis in rats by regulating IGF-1/PI3K/Akt/GSK-3β signaling. Inflammation 38(6):2178–2184
Chen L et al (2017) Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med 21(12):3178–3189
Xing S et al (2015) Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol 72:141–152
Gul A et al (2017) N‑(2-Hydroxyphenyl)acetamide: a novel suppressor of RANK/RANKL pathway in collagen-induced arthritis model in rats. Inflammation 40(4):1177–1190
Acknowledgements
We appreciate all participants who contributed to the study
Author information
Authors and Affiliations
Contributions
Y. Qin was the main designer of this study. Y. Qin and J. Su performed the experiments and analyzed the data. Y. Qin and J. Su drafted the manuscript. Y. Qin and J. Su read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y. Qin and J. Su declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The Institutional Animal Care and Use Committee of Qinghai University Affiliated Hospital approved all the animal procedures.
Additional information
Redaktion
Ulf Müller, Ladner, Bad Nauheim
Uwe Lange, Bad Nauheim
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Qin, Y., Su, J. Salidroside suppresses cell growth and inflammatory response of fibroblast-like synoviocytes via inhibition of phosphoinositol-3 kinase/threonine kinase signaling in rheumatoid arthritis. Z Rheumatol 83 (Suppl 1), 78–87 (2024). https://doi.org/10.1007/s00393-023-01431-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-023-01431-5
Keywords
- Synovial tissue hyperplasia
- Herbal medicine
- Complete Freund’s adjuvant
- TNF-α
- Fibroblast-like synoviocyte migration