Abstract
Objective and design
Among combustion-derived air pollutants, little is known about jet kerosene characteristics and effects.
Materials and methods
Particles yielded by experimental kerosene combustion in a jet engine were characterized with electron microscopy and X-ray energy dispersive spectroscopy. Immature human monocyte-derived dendritic cells were exposed for 18 h to 10, 25 or 100 μg/mL jet exhaust particles and/or Escherichia coli-derived endotoxin. Antigen-presenting and costimulation molecules (HLA DR, CD40, CD80, CD86, CD11c), tumor necrosis factor-α and interleukin-10 production were measured.
Results
The primary particles of jet exhaust are spherical (9.9 nm), carbonaceous and exert an adjuvant effect on human monocyte-derived dendritic cell maturation in vitro. Concomitant particle and endotoxin stimulation induced a high cytokine production with low antigen-presenting molecules; particle contact prior to endotoxin contact led to an opposite phenotype. Finally, low cytokine production and high costimulation molecules were present when particle adjunction followed endotoxin contact.
Conclusions
Jet exhaust particles act as adjuvants to endotoxin-induced dendritic cell maturation, suggesting possible implications for human health and a role for the time pattern of infectious and pollutant interplay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effect of air pollution on the prevalence and severity of pulmonary and cardiovascular diseases, including asthma and cancer, is now well recognized, but its pathophysiology remains unclear [1, 2]. Recent studies demonstrated that current diesel exhaust levels in urban areas induce small-airway effects resulting in a decline in lung function [3, 4]. Both initiation and exacerbation of allergic asthma may be related to air pollution levels [5, 6]. Aerosols, defined as atmospheric particles in suspension, are mostly of natural origin: sea salt, volcanic or desert dust. Particles less than 10 μm in diameter enter lower airways and affect local homeostasy and immune responses. In urban areas, 15% of the respirable particulate matter that is 10 μm or less (PM10) originates from diesel vehicle exhaust, and this proportion reaches 45% when particles smaller than 0.1 μm are considered [7]. Dendritic cells (DC) play a crucial role in sampling airway particles and locally initiating either an immune response or tolerance [8, 9]. Diesel exhaust particles (DEP), including the fine (<2.5 μm, PM2.5) and ultrafine (<0.1 μm, PM0.1) fractions, induce maturation of DC through multiple pathways including granulocyte-monocyte colony-stimulating factor (GM–CSF) [10]. In a murine model, inhalation of DEP in the presence of lipopolysaccharide (LPS) increases lung production of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) [11]. The biological fraction of inhaled particulate matter has been reported to play an important role in airway phagocyte activity [1, 6]. Unlike DEP, which are now being studied in vitro and in vivo, the residue of air traffic combustion and pollution has received little attention. Yet, aircraft-derived pollution is remarkable by its geographical dispersion (it is not confined to urban areas) and also by direct injection into the troposphere and low stratosphere. Jet exhaust particles (JEP), therefore, persist for a long time in the atmosphere and can exert their effects on climate and health for an extended period (Intergovernmental Panel on Climate Change, 2007). Little is known about the interaction of JEP with the respiratory system or about their ability to adsorb pollens, bacteria or fungi and convey these to airway phagocytes.
We therefore collected JEP emitted from aircraft engines that are largely used within the world fleet. Physico-chemical properties of the particles were assessed before testing their biological effects on monocyte-derived DC in vitro, with and without DC maturation by LPS. JEP alter DC maturation, as reflected by costimulatory molecules acquisition and cytokine production. Moreover, the DC maturation pattern differs according to the time pattern of JEP–LPS adjunction. These results suggest JEP involvement in the onset and maintenance of lung inflammation and allergic responses.
Materials and methods
Subjects
Blood (20 mL) was taken by veinpuncture from 27 healthy donors (20 men and 7 women, mean age 53 ± 15 years, 25–80). All subjects had been informed about the nature and purpose of the study and had provided written consent before enrolment. The study had been approved by the local ethics committee.
Particles
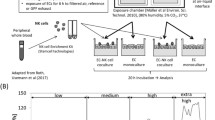
JEP are carbonaceous compounds resulting from kerosene combustion in aircraft turbofan engines. JEP sampling was made on a civil aero-engine bench during take-off/landing cycles. Particles were collected by direct impaction on polycarbonate membranes (Nucleopore®, Isopore), silicon windows (UQG Ltd, Cambridge), and electron microscope grids (Holey carbon film, Oxford Instruments) that were located in the exhaust flow axis at 27 m behind a CFM56 commercial aircraft engine. Physico-chemical analyses were performed by scanning electron microscopy, transmission electron microscopy, and X-ray energy dispersive spectroscopy (XREDS). For cell culture experiments, JEP were solubilized in dimethylsulfoxide at a final concentration of 50 mg/mL and stored at +4°C.
Cell culture reagents
Cell culture reagents were from Invitrogen unless otherwise specified. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamin, 100 U/mL penicillin and 100 μg/mL streptomycin. Recombinant human GM–CSF was purchased from RnDSystems and recombinant human interleukin-4 (IL-4) from AbCys. Low endotoxin specifications were given for all cell culture reagents. E. coli O55:B5 LPS was from Sigma–Aldrich.
Dendritic cells
DC were issued from in vitro differentiation of circulating monocytes. Briefly, fresh heparinized blood was used for leukocyte separation (MSL, Eurobio), then peripheral blood mononuclear cells were allowed to adhere for 1.5 h to cell culture plates (Dutscher). Non-adherent cells were removed and monocytes were cultured for 7 days. Recombinant human IL-4 (300 U/mL) and GM–CSF (100 U/mL) were added at the onset of cultures and every other day. On the sixth day, non-adherent immature DC were harvested, counted, transferred to 24-well plates (Nunc) and used for maturation experiments as follows. Standard DC maturation was induced with 1 μg/mL endotoxin. JEP were added to DC cultures (a) on day 6, or (b) on day 6 in conjunction with LPS, or (c) on day 6 after LPS adjunction on day 5, or (d) on day 5 followed by endotoxin adjunction on day 6. Thus, JEP effects were tested on immature DC (settings a and d), on mature DC (c) and on DC undergoing the maturation process (b). Three JEP concentrations were tested: 100, 25, and 10 μg/mL, following preliminary dose–response experiments on monocytic THP-1 cells, then on human monocyte-derived DC, showing no toxic effects with JEP concentrations of 100 μg/mL or less.
On day 7, DC supernatants were harvested and stored at −80°C for cytokine determination, DC viability was assessed by trypan blue exclusion, then DC were labeled for flow cytometry.
Cytokine assays
Cultures of DC were stimulated as indicated above. On the seventh day, supernatants were harvested and stored at −80°C for further cytokine determination. Levels of TNF-α and IL-10 in DC supernatants were measured using commercial quantitative non-competitive sandwich ELISAs (Quantikine, RnDSystems). For each patient, cytokine production by mature DC was expressed as fold induction of the corresponding immature DC cytokine production. For DC exposed to both JEP and LPS, cytokine production was expressed as fold induction of LPS-matured DC.
Flow cytometry
Fluorescently labeled mouse anti-human antibodies (HLA DR, CD40, CD80, CD86) and isotype-matched control antibodies were purchased from Beckman Coulter. CD11c-FITC and its isotype-matched control antibody were from DakoCytomation. After harvesting, DC were washed, stained with test or control antibodies for 30 min in the dark at room temperature, rinsed and resuspended in phosphate-buffered saline containing 1% formaldehyde. Flow cytometry experiments were performed with an Epics XL (Beckman Coulter).
For each maturation condition of each donor, i.e. immature DC or DC + JEP or DC + JEP + LPS, the fluorescence measurement of the isotypic control was subtracted from measurements of all surface molecule densities, in order to avoid possible artifacts due to particle aggregates or particle autofluorescence.
The percentage of positive cells and the median fluorescence intensity for each surface molecule were then collected.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Data sets were compared and tested for significance using ANOVA, then Student’s t test. Statistical significance was accepted for p < 0.05.
Results
Physical and chemical characterization of JEPs
The particulate matter emitted by the aircraft engine consisted of small aggregates (Fig. 1a), with a mean gyration diameter of 89 ± 4 nm. These aggregates were made of primary particles (Fig. 1b) with geometries very similar to spheres and a mean diameter of 9.9 ± 1.7 nm. Size distributions of both aggregates and primary particles followed a log-normal law. The complex geometry of JEP aggregates can be described by their fractal dimension, which was found to be 1.92 ± 0.05.
Jet exhaust particle characterization: aggregates were collected on a porous membrane (a, scanning electron micrograph); primary particles were spherical and consisted of concentric layers (b, transmission electron micrograph); turbostratic structure was demonstrated through electron diffraction pattern of primary particles (c)
In addition to their spherical morphology, primary JEP exhibited an onion-like structure of concentric graphene layers with a lateral extension of about 2–3 nm (Fig. 1b). The electron diffraction patterns of these particles showed the specular reflection and a set of diffuse rings, typical of turbostratic structures, corresponding in this case to the (002), (10), and (11) reflections (Fig. 1c). XREDS analyses demonstrated the predominance of carbon atoms (98 ± 3%), with a few oxygen atoms (1.5 ± 0.4%) and traces of sulfur (0.12 ± 0.05%, not shown).
When compared to DEP [12, 13], JEP proved similar in terms of size range, elemental composition, turbostratic structure, and fractal dimension values. Yet, primary JEP diameter was smaller, ranging from one-third to half the average DEP diameter (Table 1).
High combustion temperatures together with immediate collection of JEP and further sterile handling avoided LPS contamination of JEP samples. The absence of LPS contamination of JEP was also checked with the measure of CD83 expression on immature and JEP-exposed DC. Indeed, CD83 upregulation is a sensitive detector of LPS stimulation, even when minimal amounts of LPS are involved.
Modulation of DC cytokine production
JEP concentrations of 100, 25, and 10 μg/mL were chosen, according to previous studies on ambient particulate matter [14] and to preliminary experiments in our laboratory that had confirmed the absence of toxic effects of 100 μg/mL JEP or less on monocytic and monocyte-derived DC cell cultures (not shown). For each JEP concentration, kinetics of the JEP-DC interaction was studied.
Immature DC produced very low levels of TNF-α and IL-10, and adjunction of JEP alone resulted in a nonsignificant elevation of both cytokines (Fig. 2a). As expected, LPS maturation induced an approximate 500-fold increase in TNF-α production and a 30-fold increase in IL-10 production (Fig. 2a, p < 10−7).
Cytokine production by JEP-exposed DC compared to LPS-matured DC and immature DC. JEP at 10, 25 or 100 μg/mL induced a slight, nonsignificant increase in cytokine production as compared with immature dendritic cells. Data for 25 μg/mL JEP are represented here (a); concomitant JEP and LPS adjunction upregulated TNF-α and IL-10 production in a dose-dependent fashion, while JEP adjunction preceding or following LPS exerted opposite effects (b, c)
TNF-α production was further increased in DC simultaneously exposed to LPS and JEP, with higher JEP concentrations being more efficient (Fig. 2b). Thus, 100 μg/mL JEP induced a twofold increase in TNF-α production as compared with standard LPS-matured DC (p = 3 × 10−5).
On the contrary, JEP adjunction before or after LPS maturation downregulated TNF-α production. Maximal effect was seen with 25 μg/mL JEP adjunction following LPS maturation, inducing a threefold decrease in TNF-α production of DC (Fig. 2b, p < 10−5).
IL-10 production showed a similar pattern of induction: simultaneous treatment of DC with LPS and JEP resulted in an elevated IL-10 production, while other experimental designs led to decreased levels of IL-10 (Fig. 2c). The lowest IL-10 production was noted with LPS maturation following the adjunction of 100 μg/mL JEP, resulting in a threefold decrease of IL-10 production (p < 10−5).
Thus, DC maturation was altered following the time pattern of JEP adjunction: simultaneous JEP and LPS maturation upregulated both cytokine production, while JEP adjunction before or after LPS maturation downregulated it. Changes in JEP concentration further influenced DC maturation.
Effect of JEP on immature DC phenotype
The absence of JEP toxicity was checked through trypan blue exclusion from DC. The myeloid DC differentiation molecule CD11c, which is not expressed by circulating monocytes, was expressed by 96–100% of cells after a 6-day culture and remained stable thereafter. Immature DC displayed weak expression of HLA DR and CD40. Low levels of CD80 were expressed by 44% of immature DC, while CD86 was detected on 57% of these cells (Table 2).
At any of the concentrations tested, JEP alone did not induce DC maturation in an efficient manner, as reflected by weak, nonsignificant increases in the percentage and/or median fluorescence intensity of HLA DR, CD80 and CD86-expressing DC (Fig. 3). JEP adjunction for 24 or 48 h prior to analysis yielded similar results. As expected, LPS-induced maturation led to significant increases in surface densities of HLA DR and CD40 and to the recruitment of virtually all cells to CD80 and CD86-expressing DC (Table 2 and Fig. 3, p < 10−3).
Modulation of LPS-induced DC costimulatory pattern
JEP adjunction altered DC maturation, mainly through changes in HLA DR and CD86 median fluorescence intensity.
Concomitant JEP and LPS maturation resulted in an impaired acquisition of HLA DR expression: half the median fluorescence intensity was measured at the surface of this DC group as compared with standard LPS maturation (Fig. 4a, p = 0.001).
JEP time and dose-dependent modulation of LPS maturation. HLA DR surface intensity was downregulated by concomitant JEP and LPS adjunction and upregulated by JEP exposure prior to or following LPS maturation (a). CD86 intensity was higher when JEP exposure had been preceded by LPS, but lower in the other experimental settings (c). Significant differences (p < 0.05) from LPS-matured DC are indicated by asterisks
On the contrary, HLA DR acquisition was stimulated by JEP contact prior to LPS maturation, with a maximum of 39% increase in the median fluorescence intensity with 25 μg/mL JEP (p = 0.03). Finally, JEP adjunction after the onset of LPS maturation was not associated with significant changes in HLA DR median fluorescence, although a slight increase was noted with 25 μg/mL JEP.
CD86 paralleled HLA DR in DC exposed concomitantly to 10 or 25 μg/mL JEP plus LPS, showing a slight decrease (Fig. 4b).
CD86 expression also decreased with JEP adjunction at any concentration prior to LPS.
Finally, JEP adjunction after the onset of LPS maturation was associated with an increase in CD86 median intensity. A concentration of 10 μg/mL JEP yielded a 62% increase in CD86 expression.
Taken together, JEP modulation of surface molecule acquisition or upregulation involved mainly HLA DR and CD86 and showed less dose-dependent changes than did cytokine production.
Experimental patterns of DC maturation
Taken together, markers of DC maturation showed distinct patterns, depending on LPS versus JEP experimental scheme. Based on HLA DR and CD86 surface intensity and TNF-α and IL-10 production, three patterns of DC maturation were identified (Figs. 2, 4, 5). DC maturation under concomitant LPS and JEP (25 or 100 μg/mL) stimulation presented with abundant TNF-α and IL-10 production and weak HLA DR expression, while CD86 remained stable. Low TNF-α and IL-10, high HLA DR and low CD86 were induced by JEP contact prior to LPS adjunction. Finally, LPS prior to JEP treatment yielded similarly low cytokine output, but stimulated HLA DR and CD86 expression. These results show differential DC activation and thus suggest that the time pattern of JEP adjunction may induce distinct immune responses downstream.
Discussion
Air traffic has been growing by 5% annually since 1990 [15] and the extended persistence of its waste in the atmosphere prompted us to study the effect of aircraft exhausts on human health. We collected JEP at the ground level during experimental take-off/landing cycles of a civil aircraft engine, established their physico-chemical properties and described alterations they induce in human monocyte-derived DC maturation in vitro.
Primary JEP were spherical, with a mean diameter of 9.9 ± 1.7 nm. The complex geometry of JEP aggregates could be described by their fractal dimension (D f = 1.92 ± 0.05), although they did not completely fulfil requirements for fractals. However, the term has been commonly used for many years to characterize fractal-like particle geometry [16, 17]. JEP displayed physical, chemical, and geometrical properties close to diesel exhaust [12, 13]. Nevertheless, fractal structures of JEP were less compact. This result indicates larger surfaces for small molecule adsorption and, therefore, efficient shuttling of allergens and/or carcinogens to lungs [7, 14]. Moreover, a slightly smaller size of aircraft-derived elementary particles suggested higher deposition rate in tissues.
Final concentrations of 100 μg/mL JEP were active but not toxic in our hands both on THP-1 cells and on human monocyte-derived DC, consistent with studies on ambient particulate matter [18]. We chose human monocyte-derived, GM–CSF and IL-4 driven DC as a model, because these cells are closely related to myeloid, monocyte-derived DC that replace lung resident DC in vivo in acute conditions [19]. We showed that JEP alone did not induce DC maturation. Instead, JEP exert an adjuvant activity in DC maturation. The ability of particulate matter to induce DC maturation is a controversial issue. Lack of direct particle effect on DC maturation was reported in earlier studies with ambient particulate matter or diesel exhaust particles, but also in a more recent paper using standardized, spark generated elemental carbon-ultrafine particles in a murine model of allergy and inhaled ultrafine particles interaction [20]. Some authors, therefore, concluded that DEP displayed little, if any, biological activity in murine and human models in the absence of a biological stimulus, i.e. microbial components or allergenic challenge [10, 11, 20]. Nevertheless, in other studies DEP were able to induce DC maturation and an adaptive immune response [21]. This may be due to the experimental design allowing epithelium-DC crosstalk (DEP instillation into mouse airways leads to bronchial epithelial activation and subsequent DC recruitment and maturation). Conversely, there is major variability in DEP chemical and toxicological properties [22]. Finally, bacterial contamination of the particulate matter is not often assessed, despite the extreme sensitivity of DC to these stimuli.
On the contrary, the combined action of JEP and endotoxin exerted potent effects on DC maturation. Cytokine production was more sensitive to higher JEP concentrations than surface molecule expression. Moreover, different experimental time patterns yielded distinct DC responses.
Simultaneous JEP and LPS exposure induced DC to produce high amounts of TNF-α and IL-10, low HLA DR and stable CD86. TNF-α promotes neutrophilic responses and stimulates DC migration to secondary lymph organs, while IL-10 is the major cytokine involved in DC induction of T regulatory responses. Surface density of class II molecules including HLA DR modulate DC effectiveness in antigen presentation to native and memory T cells. Thus, simultaneous JEP and LPS activation, through high TNF-α and IL-10 and low HLA DR should not favor an effector T response, but rather a regulatory one.
In contrast, JEP exposure of immature DC (prior to LPS activation) yielded mature DC with high levels of HLA DR expression, low IL-10 production and low CD86 expression. CD86, a member of the B7 family of costimulation molecules, participates in the regulation of the Th1/Th2 balance. On the other hand, CD86 and HLA DR were both expressed at high levels by DC exposed to LPS prior to JEP, along with a diminished TNF-α and IL-10 production.
Although the amplitude of changes does not exceed a twofold induction, these results confirm and complete recent studies. Indeed, IL-10 expression by antigen-presenting DC is usually considered tolerogenic, but endogenous IL-10 upregulation might contribute to allergic inflammation [23, 24]. Selective upregulation of costimulatory molecules and altered production of IL-10 and TNF-α have been associated with fungal allergen-induced Th2 polarization of DC [23]. Finally, IL-10 was unchanged in bronchoalveolar fluid of sensitized mice exposed to elemental carbon-ultrafine particles inhalation before allergenic challenge [20].
On the other hand, DEP have been shown to synergize with LPS to increase inflammatory cell recruitment to the lung and to upregulate local TNF-α production [11]. In vivo, native but not heat-inactivated ambient PM2.5–10 induce airway monocytes to produce TNF-α mRNA [1]. Highly polluted DEP shift maturation and functionality of murine bone marrow-derived DC toward a Th2-polarized, pro-allergic inflammation [25]. The adjuvant effect of ultrafine particles can also synergize with allergenic proteins [20]. Interestingly, in the paper by Alessandrini et al. [20], early effects of ultrafine particles were analyzed at the transcription factor level, uncovering a discrepancy between slight but significant particle effects per se at the molecular level (lung tissue peroxidation, lung NFκB activation immediately after particle exposure) and the well-accepted lack of a significant effect on bronchoalveolar fluid inflammatory infiltrates, lung functional tests or later lung NFκB activation. Thus, LPS and other biological molecules shuttled by airborne particles play decisive roles in the activation of the lung mononuclear phagocyte system.
CD80 and CD86 are upregulated on myeloid DC in vivo following carbon black particle plus ovalbumin intranasal administration [26]. CD80 and CD86 expression is associated with T helper response decision. CD80 to CD86 ratio might reflect the ability of DC to induce Th1 rather than Th2 immune responses. Although the Th1/Th2 paradigm has evolved into a more complex picture including Treg and Th17 responses [27, 28], its interplay with asthma and allergy has not been fully depicted yet.
The time pattern of particle and infectious stimulation seems to play a critical role. Concomitant exposure to traffic-related particles and house-dust endotoxin had a synergistic effect on persistent wheezing during childhood [29]. In ovalbumin-sensitized mice exposed to diesel exhaust particles prior to influenza virus infection, lung allergic inflammation was evidenced through eosinophil recruitment, Th2-type cytokine production and the absence of IL-10 induction [30].
In conclusion, we show here that JEP are physically, chemically, and biologically related to, but different from, DEP. They act as adjuvants, exerting little functional effects on human immature DC expression of costimulatory molecules and cytokine production, but synergize with LPS to induce distinct maturation patterns depending on the time sequence of JEP and LPS activation. Further investigations should provide insights into the mechanisms of cellular alteration, particularly with respect to the generation of reactive oxygen species and help characterize responses to JEP and DEP in the context of phenotypic, secretory, T cell activation, and dendritic-bronchial epithelial crosstalk.
References
Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy donors. J Allergy Clin Immunol. 2006;117:1396–403.
Andersen ZJ, Loft S, Ketzel M, Stage M, Scheike T, Hermansen MN, et al. Ambient air pollution triggers wheezing symptoms in infants. Thorax. 2008;63:710–6.
Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. New Engl J Med. 2007;357:2338–47.
McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to Diesel traffic in persons with asthma. New Engl J Med. 2007;357:2348–58.
Lippmann M. Health effects of airborne particulate matter. New Engl J Med. 2007;357:2395–6.
Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005;115:221–8.
Highwood EJ, Kinnersley RP. When smoke gets in our eyes: the multiple impacts of atmospheric black carbon on climate, air quality and health. Environ Int. 2006;32:560–6.
Holt PG, Strickland DH, Wickström ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–52.
Vitte J, Bongrand P. Immunotoxicologie. In: Viala A, Botta A, Andrejak M, Aubert C, editors. Toxicologie. Paris: Lavoisier; 2005. p. 59–69.
Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol. 2006;176:7431–7.
Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Fujitani Y, et al. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology. 2007;238:99–110.
Neer A, Köylü ÜÖ. Effect of operating conditions on the size, morphology, and concentration of submicrometer particulates emitted from a diesel engine. Combust Flame. 2006;146:142–54.
Smekens A, Godoi RHM, Berghmans P, Van Grieken R. Characterization of soot emitted by domestic heating, aircraft and cars using diesel or biodiesel. J Atmos Chem. 2005;52:45–62.
Bartra J, Mullol J, del Cuvillo A, Davila I, Ferrer M, Jauregui I, et al. Air pollution and allergens. J Investig Allergol Clin Immunol. 2007;17S(2):3–8.
Akerman J. Sustainable air transport–on track in 2050. Transp Res Part D. 2005;10:111–26.
Brasil AM, Farias TL, Carvalho MG. A recipe for image characterization of fractal-like aggregates. J Aerosol Sci. 1999;30:1379–89.
Köylü ÜÖ, Faeth GM, Farias TL, Carvalho MG. Fractal and projected structure properties of soot aggregates. Combust Flame. 1995;100:621–33.
Williams MA, Porter M, Horton M, Guo J, Roman J, Williams D, et al. Ambient particulate matter directs nonclassic dendritic cell activation and a mixed TH1/TH2-like cytokine response by naïve CD4+ T cells. J Allergy Clin Immunol. 2007;119:488–97.
Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204.
Alessandrini F, Beck-Speier I, Krappmann D, Weichenmeier I, Takenaka S, Karg E, et al. Role of oxidative stress in ultrafine particle-induced exacerbation of allergic lung inflammation. Am J Resp Crit Care Med. 2009;179:984–91.
Provoost S, Maes T, Willart MAM, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 2010;184:426–32.
Singh PD, DeMarini M, Dick CA, Tabor DG, Ryan JV, Linak WP, et al. Sample characterization of automobile and forklift diesel exhaust particles and comparative pulmonary toxicity in mice. Environ Health Perspect. 2004;112:820–5.
Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–9.
Prasse A, Germann M, Pechkovsky DV, Markert A, Verres T, Stahl M, et al. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J Allergy Clin Immunol. 2007;119:464–71.
Braun A, Bewersdorff M, Lintelmann J, Matuschek G, Jakob T, Göttlicher M, et al. Differential impact of diesel particle composition on pro-allergic dendritic function. Toxicol Sci. 2010;113:85–94.
de Haar C, Kool M, Hassing I, Bol M, Lambrecht BN. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol. 2008;121:1246–54.
Larché M. Regulatory T cells in allergy and asthma. Chest. 2007;132:1007–14.
Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67.
Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age three. Am J Resp Crit Care Med. 2009;180:1068–75.
Jaspers I, Sheridan PA, Zhang W, Brighton LE, Chason KD, Hua X, et al. Exacerbation of allergic inflammation in mice exposed to diesel exhaust particles prior to viral infection. Part Fibre Toxicol. 2009;6:22.
Acknowledgments
We thank Dr. Patrick Sudour and Dr. Nathalie Charvin for helpful discussions and assistance with formal issues and Ms. Elisabeth Wostrowski and Ms. Dolores Migneret for technical assistance.
This work was supported by Assistance Publique Hôpitaux de Marseille, Institut National de la Santé Et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Université de la Méditerranée.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Ferry, D., Rolland, C., Delhaye, D. et al. Jet exhaust particles alter human dendritic cell maturation. Inflamm. Res. 60, 255–263 (2011). https://doi.org/10.1007/s00011-010-0262-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0262-9