Abstract
Epidemiologic studies have linked diesel exhaust (DE) to cardiovascular and respiratory morbidity and mortality, as well as lung cancer. DE composition is known to vary with many factors, although it is unclear how this influences toxicity. We generated eight DE atmospheres by applying a 2 × 2 × 2 factorial design and altering three parameters in a controlled exposure facility: (1) engine load (27 vs 82 %), (2) particle aging (residence time ~5 s vs ~5 min prior to particle collection), and (3) oxidation (with or without ozonation during dilution). Selected exposure concentrations of both diesel exhaust particles (DEPs) and DE gases, DEP oxidative reactivity via DTT activity, and in vitro DEP toxicity in murine endothelial cells were measured for each DE atmosphere. Cell toxicity was assessed via measurement of cell proliferation (colony formation assay), cell viability (MTT assay), and wound healing (scratch assay). Differences in DE composition were observed as a function of engine load. The mean 1-nitropyrene concentration was 15 times higher and oxidative reactivity was two times higher for low engine load versus high load. There were no substantial differences in measured toxicity among the three DE exposure parameters. These results indicate that alteration of applied engine load shifts the composition and can modify the biological reactivity of DE. While engine conditions did not affect the selected in vitro toxicity measures, the change in oxidative reactivity suggests that toxicological studies with DE need to take into account engine conditions in characterizing biological effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s vehicle fleet has been rapidly growing in concert with rising populations and improving economies (Health Effects Institute 2010). Diesel vehicle emission controls such as the use of reduced sulfur content in diesel fuel and catalyzed particle traps have been found to reduce several components of the complex mixture of diesel exhaust (DE) while increasing others (Kittelson et al. 2008; McDonald et al. 2004b). However, implementation of new technology will take time, and emissions from older engines are likely to dominate exposure for several years (Zhang et al. 2009). Correspondingly, there is a global trend of increasing airborne concentrations of markers of traffic-related air pollution (TRAP) (World Health Organization 2006). TRAP, which is attributed to both gasoline exhaust (GE) and DE, has been associated with numerous adverse health effects, including cardiovascular disease and inflammation of the respiratory system (Health Effects Institute 2010). The International Agency for Research on Cancer also recently classified DE as a human carcinogen (Benbrahim-Tallaa et al. 2012). The prevalence of vehicles with diesel engines relative to vehicles with gasoline engines has been increasing for the past 20 years and continues to rise in several countries (Zhang et al. 2009).
Application of numerous approaches to investigate health effects resulting from DE exposure—including epidemiology, animal toxicology, and controlled human exposure studies—is particularly useful due to the complex composition of DE. Controlled exposures are ideal for study of the mechanism of health effects caused by exposure because the concentration and duration of the exposure are well defined and it is possible to reduce the effects of confounding co-exposures (Ghio et al. 2012).
One challenge of selecting and designing a system for generation of DE exposures is that the DE composition is known to vary by many parameters including engine technology, fuel type, operating conditions, and ambient environment (Hesterberg et al. 2009; Lloyd and Cackette 2001). The relative toxicity resulting from exposures to these different DE compositions is not well understood. Study of DE is further complicated by the fact that there has not been standardization in the reported characterization of the DE among existing exposure facilities (Birger et al. 2011; Fujitani et al. 2009; Laumbach et al. 2009; McDonald et al. 2004a, b; Rudell et al. 1994; Sawant et al. 2008; Sobus et al. 2008).
In the natural environment, engines operate at various loads depending on the grade of the road, vehicle type, carrying weight, and other conditions that place a draw on the engine. Controlled exposure facilities generally apply one of two options for mimicking the effect of engine load: a static load bank or a variable load dynamometer; alternatively, some facilities have traditionally run engines with no load. Investigations of generated DE to date have applied a wide range of engine loads as a standard operating condition ranging from 40 to 100 % (Birger et al. 2011; Laumbach et al. 2009; McDonald et al. 2004a; Rudell et al. 1994; Sobus et al. 2008). Our research group has usually applied 75 % load with our previous Cummins generator (Gould et al. 2008) and either 75 or 82 % load with our current Yanmar generator.
Photochemical aging of particles is not generally present in controlled exposure facilities, unless it is added to the diesel exhaust generation process by design. In the University of Washington (UW) facility, diluted diesel exhaust particulate (DEP) is typically aged for approximately 5 min. This is similar to other facilities, where residence times of DE prior to exposure range from 3 to 5 min (Birger et al. 2011; Fujitani et al. 2009; Sawant et al. 2008). Oxidation naturally occurs from the interaction of sunlight with DE in the environment, which leads to formation of secondary organic aerosols. The effect of exposing DE to ultraviolet (UV) light has previously been investigated with the use of smog chambers or UV lamps (Ebersviller et al. 2012b; Lichtveld et al. 2012; Robinson et al. 2007; Weitkamp et al. 2007). However, DE in the University of Washington (UW) facility, as in most other exposure facilities, is not UV-irradiated (Birger et al. 2011; Fujitani et al. 2009; Laumbach et al. 2009; McDonald et al. 2004a; Rudell et al. 1994; Sawant et al. 2008; Sobus et al. 2008). Ozone addition, which we applied in the current investigation, is a simplified way to represent the oxidation of near-road DE emissions.
The goal of this work was to evaluate the effects of altering operating condition (engine load), time since emission (residence time), and presence of ozone on DE composition, diesel exhaust particulate (DEP) reactivity, and DEP in vitro toxicity in an exposure facility. We selected these parameters to reflect compositions of DE in the natural environment. In vitro toxicity was assessed in murine endothelial cells with three relatively high-throughput cellular assays.
Methods
Study design
We investigated three parameters of DE generation: engine load, residence time, and ozonation of exhaust. For each parameter, we tested two contrasting conditions. Thus, we studied eight DE atmospheres in a 2 × 2 × 2 factorial design selected to maximize our statistical power to characterize each parameter. We collected measurements of physicochemical properties of DE composition (both particulate and gas components), DEP reactivity, and DEP in vitro toxicity.
We generated a single DE atmosphere on each day of sampling. Each of the eight unique atmospheres was run at least once for each type of measurement; for some measurements, atmospheres were run on multiple days. We averaged observational replicates from the day that the DE atmosphere was generated prior to analysis for all components and toxicity measurements. Summary statistics by condition along with the number of experimental replicates (samples collected on different days) and observational replicates (samples collected side by side or analyzed as laboratory replicates from the same day during DE generation) are shown in Table 1.
Generation of diesel exhaust atmospheres
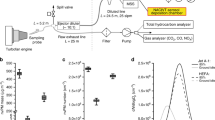
DE was generated within the UW facility using a two-step dilution process with dynamic control of fine particulate matter (PM2.5) mass concentration as previously described (Gould et al. 2008) (with DE parameters in Fig. 1). In 2010, the facility’s engine was replaced with a 5.5-kW, single-cylinder generator (Yanmar model YDG5500EV-6EI) (Table 2). We used ultra-low sulfur diesel fuel with a maximum sulfur content of 15 ppm, the present highway grade diesel fuel obtained from local fuel distributors. The generator lubricating oil was Chevron DELO 400LE, SAE 15 W-40.
We selected the engine load by setting a load bank (Simplex Model Swift-E FT, Springfield, IL) to 1.5 kW (~27 % generator output, “low load”) or 4.5 kW (~82 % generator output, “high load”). The ozonated condition was achieved by applying a series of adjustable UV generators (UV Pro 1100AT and UV Pro 4000, Crystal Air Marketing Inc., Langley, BC, Canada) to radiate the dilution air added to the exhaust, where the ozonated condition had a target excess ozone concentration of 100 ppb, measured by amount of excess nitrogen dioxide (NO2) relative to nitric oxide (NO) via direct reading measurement throughout the sample collection period. (Since oxides of nitrogen (NOx) levels are also altered by engine load, this was a more consistent approach for controlling the degree of oxidation between the DE atmospheres.) The non-ozonated condition was obtained with DE generated without the use of the UV lamps. Differences in the DE residence time since emission were tested by sampling at different points in the exhaust train. “Fresh” exhaust was collected in the baffles of the exposure facility ~5 s after emission, while “aged” exhaust was collected downstream of the baffles within the exposure room ~5 min after emission (Table 2).
Our target concentration of PM2.5 during each of the 8 DE atmospheres was 200 μg/m3. During high engine load DE generation, we applied our automatic dynamic feedback system. The PM level was significantly reduced during low engine load conditions beyond the capability of the automated system, so we manually maintained the PM levels as close to 200 μg/m3 as possible during the low load conditions by partially closing a slide gate in our second-stage dilution air supply.
Sample collection and physicochemical characterization of particles and gases
Various components of diesel exhaust were collected and analyzed, as summarized in Table 3. Particle samples analyzed for PM2.5 and metal concentrations were collected on 37-mm-diameter polytetrafluoroethylene (PTFE, “Teflon”) filters (Teflo with PMP support ring #R2PJ037, Pall Corporation, Port Washington, NY) within open-faced Harvard Impactors with a PM2.5 median cut-size. PM2.5 mass was measured in a humidity- and temperature-controlled room by gravimetric analysis before and after sample collection with a micro-balance (UMT-2 microbalance, Mettler Toledo, Columbus, OH). Elements were analyzed on the Teflon filters via X-ray fluorescence (Cooper Environmental Services, Portland, OR). Among other components measured, comparisons of elements were limited to the six metals that were the most abundant across the average of the eight atmospheres: copper (Cu), zinc (Zn), calcium (Ca), magnesium (Mg), tin (Sn), and iron (Fe). (See Supplemental Material for mass fraction of other metals.)
Samples analyzed for elemental carbon (EC) and organic carbon (OC) were collected on 37-mm-diameter pre-fired quartz filters (Sunset Laboratory Inc., Tigard, OR) within Harvard Personal Environmental Monitors (HPEM) with a PM2.5 median cut-size, and a 1.5-cm2 punch was analyzed using the IMPROVE-A thermal optical reflectance method (Sunset Laboratories, Tigard, OR). A second quartz filter placed downstream of the primary filter within the same HPEM housing served as a dynamic blank for OC results. By absorbing organic gases, this dynamic blank filter used for selected samples allowed the vapor phase artifact present in the primary quartz filter samples to be quantified and thereby separated from the contribution of only particulate matter OC. The dynamic blank OC concentration was found to vary with the OC concentration of the sample filter, so a derived correction factor was determined from pooling the dynamic blanks and paired samples using simple linear regression. We dropped two OC/EC samples from the analysis because of unexplained disparities between the observational replicates.
Dithiothreitol (DTT) and 1-nitropyrene (1-NP) samples were collected on 37-mm-diameter Teflon membrane filters (Zefluor #P5PJ037, Pall Corporation, Port Washington, NY) within open-faced Harvard Impactors in which the impactor stage was removed. For DTT analysis, we adapted the procedure of Li et al. (2003b) and Kumagai et al. (2002) for use with a microplate reader. Briefly, DTT reactivity was analyzed by extracting particles from filters in methanol. The extract was then incubated with DTT and portions of the extract were removed at specific time points and allowed to react with 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) to form 3-thio-6-nitrobenzoate (TNB). The absorbance due to TNB at 412 nm was measured in a microplate reader (SpectraMAX plus, Molecular Devices Corporation, Sunnyvale, CA). The rate of consumption of DTT was calculated from the plot of absorbance versus time with a correction for atmospheric oxidation of DTT based on a reagent blank time series. 1-NP was analyzed by extraction of particles in dichloromethane, followed by evaporation and resuspension in a mixture of ethanol and sodium acetate buffer and analyzed via 2D-HPLC-MS/MS (Agilent 1100 HPLC, Agilent 6410 Triple Quad Mass Spectrometer, Santa Clara, CA), as previously described (Miller-Schulze et al. 2010).
The particle-bound fraction of polycyclic aromatic hydrocarbons (PAHs) was measured via direct reading of induced electrical charge (Ecochem PAS 2000, League City, TX).
The mass median aerodynamic diameter (MMAD) was measured via a 10-stage cascade impactor (MOUDI Model 110-NR, MSP, Shoreview, MN; stage cut-sizes 0.055–18 μm). The estimation of MMAD was restricted to mass collected on stages with cut-size ≤0.55 μm to limit the analysis to a single mode representative of DEP. The impactor flow was operated at 30 l/min, and samples were collected on Zefluor substrates weighed before and after sampling via gravimetric analysis. The count median diameter (CMD) was measured with a P-Trak Ultrafine Particle Counter (Model 8525; TSI, Shoreview, MN) in combination with size-selective diffusion screens (cut-sizes 0.02–0.155 μm).
At least 20 % blanks and 20 % duplicates were collected during each day of DE generation for analysis of all particle samples on filters dedicated to DE composition analysis. Except for OC and EC, which were corrected as detailed above, analyte concentrations of samples were blank-corrected when deemed appropriate, which was generally when the blank concentrations were ≥10 % of the sample concentrations.
In the gas phase, NOx and NO2 were measured continuously by chemiluminescence (Thermo Model 42C, Thermo Fisher Scientific, Waltham, MA). Carbon dioxide (CO2) was measured by non-dispersive infrared detection (Telaire Model 1050, Telaire Systems). Carbon monoxide (CO) was measured by an electrochemical sensor (Langan Model T15n, Langan Products, San Francisco, CA).
We collected samples between April 2010 and May 2012. To allow for comparison across the varying environments, both gas and particle concentrations were normalized to PM2.5 concentration.
Particle preparation, exposure of cells, and cell cultures
Particle samples for in vitro toxicity testing were collected on 3 μm pore size 8” × 10” PTFE-coated fiber filter sheets (Zeflour #P5PI001, Pall Corporation, Port Washington, NY) using a hi-vol sample collector designed for total suspended particulate matter (Method 40 CFR 50, Appendix B, with a flow rate of 1.0 to 1.1 m3/min). Particles were extracted from filters by suspending in methanol, sonicating/shaking, and evaporating to dryness. The extracted particles were resuspended in phosphate-buffered saline (PBS) with <0.1 % dimethyl sulfoxide. A media control was prepared by applying the vehicle (PBS and dimethyl sulfoxide) to cells without extracted particles. A blank filter was processed using the same techniques to be used as a filter control.
SVEC4-10 murine endothelial cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum plus antibiotics (100 μg/ml streptomycin, 100 IU/ml penicillin) at 37 °C in a humidified atmosphere containing 5 % CO2/95 % air. Cells were seeded at an assay appropriate starting density and grown for an additional 18–24 h prior to treatment. Dependent on assay type, suspensions of DEP were then administered at concentrations of 0, 5, 10, 25, or 100 μg/ml for periods of up to 24 h.
Quantification of cell viability, cell proliferation, and cell migration/repair
The MTT assay, a surrogate of cell viability, assesses metabolic reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Sigma-Aldrich, St. Louis, MO) to formazan crystals. SVEC4-10 cells were plated and treated with DEP in 24-well microtiter plates. Subsequent to DEP treatment, medium was removed and cells were washed with PBS. MTT solution was added to the wells and then removed after 1-h incubation. Precipitated formazan was dissolved with 100 μl DMSO, and samples were centrifuged at 10,000×g to remove any residual DEP from the formazan suspension. Absorbance at 405 nm was read in a SpectraMax spectrophotometer (McConnachie et al. 2013).
For the colony formation assay, a measure of cell proliferation, 50 SVEC4-10 cells/well were introduced into a six-well tissue culture plate. After attachment, DEP was introduced at a concentration of 5 μg/ml. Seven days later, cell colonies were fixed and stained with 1 % Crystal Violet in 20 % methanol. Gray-scale images of the plates were then obtained using a BioRad Gel-Doc system (BioRad, Hercules, CA), and the change in relative optical density per well was used as an index of total colonies formed (Franken et al. 2006).
Wound healing was measured to indicate cell migration and repair by scratching a confluent monolayer of SVEC4-10 cells with a P200 pipette tip. Phase contrast images of the “wound” were obtained using an Olympus IMT2 inverted microscope (Olympus, Center Valley, PA). Time-dependent image analysis was utilized to assess cell migration into the “wound” induced into the monolayer (Liang et al. 2007). Results were calculated as the change in “wound” area over time.
Toxicity was assessed with a dose of 5 μg DEP/ml for all three cellular assays, and additional doses for the MTT (25 and 100 μg DEP/ml) and scratch assays (10 μg DEP/ml). All toxicity effects are measured as the percentage difference in response of DEP dose compared to filter blank.
Statistical analysis
All data were analyzed in Stata (StataCorp v 11.0, College Station, TX) using simple linear regression of the mean of replicate samples collected on a single day. (In Table 1 “Experimental Observations” represent the number of days of sampling, with a unique DE atmosphere each day). For estimates of the effect of each of the three DE generation factors on either the exposure composition or toxicity, a Bonferroni correction factor was applied to the multivariate analysis, leading to a statistical significance level of p = 0.0167. To estimate the dose–response for the MTT assay, we applied a linear mixed model that accounted for the repeat MTT measures on individual filters, where filters correspond to unique conditions.
Results
Gas and particle characteristics of DE atmospheres
The average daily PM2.5 concentration in the exposure facility was 233 μg/m3 (range 97 to 376 μg/m3) across all tested conditions. Different sampling durations were applied based on the ideal PM loading for each component, varying between 1.5 and 6 h. The average daily temperature and relative humidity in the exposure facility during sample collection was 24 °C (range of daily averages, 20–27 °C) and 36 % (range, 0–11 %), respectively. Within a day of collection, the temperature increased by an average of 4 °C and the relative humidity increased by an average of 3 % over a 6-h period.
Particulate-matter-normalized measurements were higher for low engine load versus high engine load by >15-fold for 1-NP (not statistically significant), approximately 2-fold for DTT (p = 0.010), approximately 2-fold for CO (p < 0.001), and approximately 1.5-fold for OC (p < 0.001) (Table 1 and Fig. 2). The mass fraction of particle-bound PAHs was approximately 2-fold higher for high engine load (p = 0.013).
Mass fraction of exposure components (1-NP, OC, and PAH) and reaction rate of reactivity (DTT) by engine load. Data points represent all experimental replicates; some DE atmospheres were repeated. (See Table 1 for number of experimental replicates.) Black point estimates and black error bars to the right of data points represent mean and standard error by load
With the addition of ozone, NO2 was approximately 4-fold higher (p = 0.002), whereas NO was depleted by a similar magnitude (4-fold difference, p < 0.001), as would be expected due to titration. No exposure components differed statistically significantly by residence time.
The particle mass distribution of DEP, measured as the MMAD, was not found to vary by the three parameters. Although MMAD of DEP was estimated using a restricted particle size distribution, particles were collected with a median cut-size of up to 18 μm in the MOUDI cascade impactor. In review of the full spectrum of particles collected in the MOUDI, during generation of DE from a typical condition, 97 % of particles by mass were less than a median 1.8-μm-diameter cut-size, and 48 % of particles by mass were less than a median cut-size of 0.1 μm.
In vitro cytotoxicity
All cytotoxicity results were normalized with a corresponding cell culture medium-only control. Cell viability averaged across the DEP from the eight atmospheres was 84 % (SD 13) for a 5 μg DEP/ml dose versus 99 % in filter control samples, 55 % (SD 13) for 25 μg DEP/ml versus 95 % in filter controls, and 34 % (SD 10) for 100 μg DEP/ml versus 85 % in filter controls. We observed a statistically significant linear decline in viability across the three doses (p < 0.001, Fig. 3). The average half maximal effective concentration (EC50) for a response in viability of the average of the eight DE atmospheres obtained from doses of 5, 25, and 100 μg/ml is 13.6 μg DEP/ml (SD 7.7). Average cell proliferation across the eight atmospheres was lower with a 5 μg DEP/ml dose than in filter control samples (75 % (SD 9) vs 102 %, respectively). Average wound healing was lower across the eight atmospheres compared to filter controls for both 5 μg/ml DEP (88 % (SD 4) vs 100 %) and 10 μg/ml DEP (90 % (SD 10) vs 100 %).
We report the relative toxicity by each of the DE generation parameters (engine load, residence time, and ozonation) in Table 1. There were no statistically significant differences in the in vitro toxicity of DE when varied by these parameters.
Discussion
In this study of DE generated in a controlled exposure facility, we found that altering engine load had the greatest effect on exposure characteristics and oxidative reactivity. Ozonation of the exhaust stream and modification of the residence time prior to particle collection had less impact.
Diesel exhaust atmospheres
The two-step design in our controlled facility allows for rapidly quenching particle conditions in the first dilution and adjustment of particle concentration to levels that are more ideal for study in the second step. With the application of these design features, we were able to compare the exposure components, reactivity, and toxicity of the DE generation parameters by adjusting for PM2.5 concentration.
Operating engines at low load generally results in less complete combustion—as demonstrated by the increase in the ratio of OC to EC with decreasing engine load that we and others have found (McDonald et al. 2011; Shi et al. 2000). The efficiency of engine operation also tends to alter emissions of PAHs, as the engine temperature is the main determinant of PAH emissions, and engine load is proportional to engine temperature (Scheepers and Bos 1992). In accordance, we measured more particle-bound PAH with high engine load. However, it is worth noting that our measurement of particle-bound PAH with the Ecochem aerosol monitor relies on selective photoionization of PAHs and cannot be compared directly with standard mass-spectrometry-based approaches.
In contrast with particle-bound PAHs, 1-NP decreased with increased load, which has similarly been reported by Schuetzle and Perez (1983). While many nitro-PAHs are formed following emissions in the atmosphere, the majority of 1-NP (>80 %) is formed in the exhaust stream (Scheepers and Bos 1992). Our finding of a >15-fold higher concentration of 1-NP in low versus high load conditions was not statistically significant; this may be attributed to increased variability caused by an interaction with one or both of the other DE generation parameters (residence time or ozonation), although we were unable to test for interactions in this small data set. 1-NP is particularly important among the components of DE because it is one of the largest contributors to mutagenicity, measured by the Ames test (Sjogren et al. 1996). At the same time, 1-NP does not appear to be related to cell proliferation, migration, or viability, since we observed no significant effects by engine load for these cytotoxicity measurements.
We found no general trends in metal content by the DE generation parameters, although we did observe a significant elevation of Mg with ozone addition. We do not have a hypothesis for this finding, and chance is a very plausible explanation. In contrast, Sharma et al. (2005) reported a modest decrease in several metals (Fe, Mg, Ca, Cr, Ni, Pb, Zn, and Ba) with increasing load on a larger four-cylinder truck engine, which they attribute to the increase of the engine’s thermal efficiency (brake specific fuel consumption) at higher engine loads. However, our small engine is designed to produce a relatively constant electrical power output over a range of engine loads, and therefore, their results may not be directly comparable to ours.
As expected, DE in the UW facility has a high content of particles in the ultrafine size range. A measure of mass distribution (MMAD) was not found to vary substantially by load, in agreement with findings by McDonald et al. (2011).
The OC-to-EC ratio (0.10) in our facility with the Yanmar generator, under high load, in the aged location and without ozone addition (a typical operating condition) is lower than a previous estimate from our facility with the Cummins engine (0.34), and that reported by McDonald et al. with a Yanmar generator (0.41) (Supplemental Material). This difference in the OC-to-EC ratio may be attributed to use of different engine models, differences in the applied engine loads, an update of the analytical method for carbon (IMPROVE-A vs NIOSH method), and the use of dynamic blanks to correct OC in the present study (correction reduces OC concentration by ~50 %) (Gould et al. 2008; McDonald et al. 2004a).
Our surrogates of two types of photochemical aging of particles, the time since emission and the presence or absence of ozonation, were simplified ways to represent the processes in the natural environment. Neither time since emission nor ozone addition was found to induce a significant change in the components of DEP.
Oxidative reactivity
Oxidative reactivity represents an acellular approach providing information bridging toxicity and composition measurements, as it indicates the ability of particulate matter to produce reactive oxygen species (2008). Oxidative stress is thought to initiate a cascade of steps that lead to inflammation, which is hypothesized to play a role in more severe health outcomes such as cardiovascular morbidity, asthma, and diabetes (Brook et al. 2010; Li et al. 2003a; Lugrin et al. 2013; Moller et al. 2008; Yin et al. 2013). While the relationship to human health effects is not currently understood, DTT reactivity is a sensitive measurement that is relatively easy to obtain Ayres et al. (2008).
Our average DTT measurement for the eight conditions (0.64 ng/min/μg (SD 0.32), or 145 ng/min/m3 (SD 57)) is about 10 times higher than the average measurement our research group obtained from six urban areas in the USA as part of the National Particle Component Toxicity Initiative (NPACT, 11.04 ng/min/m3 (SD 3.62)), which applied a similar analysis method, other than the use of quartz filters (Vedal et al. 2013). Our DTT measurements are about five times less than those measured from urban ambient samples from California (3.2–5.5 ng/min/μg) (Ntziachristos et al. 2007) and DE generated in another controlled exposure facility (2.7–3.5 ng/min/μg) (Cheung et al. 2009). These differences may be partially explained by the collection of particles in a liquid impinger in both of these latter investigations rather than the use of particles extracted from filters in our study. Differences in extraction of particles and the applied analytical method may result in large differences in measured DTT activity values. Our research group uses methanol extraction, which is a mid-polarity solvent. Other researchers extract particles for DTT measurement with either dichloromethane, a non-polar organic solvent that would result in greater extraction of PAHs such as quinones or “quinone-like” species (Pan et al. 2004; Shinyashiki et al. 2009), or an aqueous solvent that would result in extraction favoring metals (Biswas et al. 2009; Charrier and Anastasio 2012).
To our knowledge, this is the first report of a difference in DTT activity by engine load. Our finding suggests that low engine load produces a more reactive environment, indicative of greater toxicity, than high load.
In vitro screening
To assess the relative toxicity of different diesel exhaust compositions, we measured the in vitro toxicity of diesel exhaust particulate (DEP) toward murine endothelial cells. Endothelial cells play an important role in the vasculature and have been previously investigated as relevant targets in DEP-induced cytotoxicity and oxidative stress (Bai et al. 2001; Hirano et al. 2003; Mattingly and Klinge 2012; Weldy et al. 2011). Murine SVEC4-10 endothelial cells, specifically, have also been used in several investigations of the vasculature and vascular reactivity (Choi et al. 2011; Kang et al. 2011; Wang et al. 2012; Weldy et al. 2011; You et al. 2012).
The MTT, colony formation, and scratch assays are in vitro toxicity assessments that provide insight regarding the gross impact of DE exposure on aspects of cell physiology, including cell viability, proliferation, and wound healing, respectively (Cory 2011; Katz et al. 2008; Peplow and Chatterjee 2013; Sylvester 2011). These toxicity assays were not intended to provide detailed mechanistic information about DE, but rather served as a screening tool to demonstrate which DE parameters from the different DE exposure atmospheres impact toxicity in general.
The MTT assay is generally interpreted as a measure of mitochondrial function and is one of the most common measurements of cell viability. The MTT assay has been applied in several investigations to measure toxicity in endothelial cells resulting from different pollutants (Hirano et al. 2003; Hsu et al. 2009; Liu et al. 2005; Mattingly and Klinge 2012; Napierska et al. 2009; Weldy et al. 2011). Comparison of cell viability attributed to different types of particles is limited by many factors, including differences in protocols and types of endothelial cell lines applied, yet the trend among these studies suggests that particle toxicity measured as cell viability is generally highest for wood smoke, followed by silica and DEP, and lowest for cigarette smoke (Hirano et al. 2003; Hsu et al. 2009; Liu et al. 2005; Napierska et al. 2009; Weldy et al. 2011).
The colony formation assay and the scratch assay are less standard techniques for assessing cytotoxicity but are similarly rapid and straightforward. The colony forming assay is considered a sensitive measure of cytotoxicity, since the formation of a visible colonies over a 7-day period from single, plated cells requires both maintenance of cell viability and replication through multiple cell cycles in the presence of the toxicant. The scratch assay is a surrogate for a wound healing response following the introduction of a scratch across a cell monolayer with a pipette tip. The culture is then monitored over time for how long it takes to “repair” the wound as cells fill in the opened area left by the scratch. This involves both migration of cells from the leading edge of the wound as well as replication of cells.
These three in vitro toxicity assays have been previously used to demonstrate DEP-induced cytoxicity (Danielsen et al. 2008; LaGier et al. 2013; Mattingly and Klinge 2012; Okayama et al. 2006; Poss et al. 2013; Tau et al. 2013), which we also observed. Based on the mean toxicity compared to controls, our results suggest that the order of sensitivity of these assays to a low dose of DEP (5 μg/ml) is highest for the colony formation assay (75 %, SD 9 %), followed by the MTT assay (84 %, SD 13 %), and lowest for the scratch assay (88 %, SD 4 %). Although differences between these assays may be specific to cell type and toxicants, the crystal violet assay (similar to the colony formation assay) was similarly found to be more sensitive than the MTT assay when applied to cancer cells (Martin and Clynes 1993).
Few published studies have investigated the differential effects of DEP with varying composition. Although we found no differences in toxicity by the three DE generation parameters tested, an overall effect of DEP versus controls was observed for all three in vitro toxicity assays studied, suggesting that these are appropriate models. In contrast with our analysis of the effect of DEP on MTT toxicity, which did not result in a meaningful difference by the three DE generation parameters, Liu et al. exposed epithelial cells to gaseous extracts of DE and observed a trend of increasing toxicity in the MTT assay with decreasing engine load (Liu et al. 2009). Perhaps this difference is explained by Liu et al.’s focus on the gaseous fraction of DE versus our focus on particles for toxicity testing.
Limitations
The goal of this study was to generally explore the influence of DE generation parameters (engine load, residence time, and presence of ozone). Applying a factorial design provided greater statistical power to investigate these three parameters for the number of samples tested. We used the statistical analysis as a tool to highlight exposure components, reactivity, and toxicity measures of particular interest for future study. With a small data set, we are not able to also consider other factors, such as measurement error, another controlled factor, or even one of the other measured parameters that have affected our data.
In the natural environment, the effects of residence time and ozonation are generally intertwined, resulting in complex particle growth, particle and gas phase interactions, and formation of secondary organic aerosols. The ozone generators inserted in the DE train for this investigation did not achieve the degree of oxidation that occurs with sunlight or smog chambers, for which an increase in markers of inflammation and cytotoxicity has been reported (Doyle et al. 2007; Ebersviller et al. 2012b; Sexton et al. 2004).
While the introduced parameters were intended to mimic real-world differences in ambient DE in the UW facility’s generated DE, they were not perfect representations. Engine load was likely the best rendition in the facility, as the application of the load bank on the engine is directly comparable to load changes induced by driving vehicles. This may also partially explain why the effects of engine load on composition and reactivity were the most pronounced.
While using a toxicity screening approach with efficient laboratory procedures is arguably appropriate for the purpose of this study, there is considerable concern about measuring toxicity with exposures from extracted particles. DEP extracts exclude gases, which are thought to be an important contributor to DE toxicity, and the process of extraction is thought to remove important components from the surface of particles. These issues likely led to reduced toxicity from exposure to DEP compared to complete DE (Ebersviller et al. 2012a; Lichtveld et al. 2012; Totlandsdal et al. 2012). A smaller overall effect for DEP would likely reduce the ability to detect differences between the eight atmospheres. Our research group is currently working on exposing cell cultures to native DE without extraction. Further, the toxicological impacts of the gas/particle DE mixture in the environment can be more comprehensively evaluated in human and animal inhalation studies such as those typically conducted in our facility. The study described in this paper was designed to inform decision-making for future inhalation studies.
Conclusions
Alteration of engine load, compared to addition of ozone or changes in residence time, resulted in the largest compositional differences: 1-NP, DTT activity, and OC were elevated with low load, while PAHs were elevated with high load. All three high-throughput assays selected to screen for gross in vitro toxicity in murine endothelial cells demonstrated an effect of DEP. However, these effects on cytotoxicity of particle exposures were not modified by alteration of engine load, residence time, and ozonation.
Studies involving controlled DE exposures are generally limited to comparison of effects following exposure to a single mixture (though sometimes at varying concentrations) versus a filtered air exposure. Given that natural DE composition can shift widely with different vehicle parameters and conditions of the environment, future work is needed to identify which components are associated with the numerous toxic effects. Our findings, though limited, suggest that important compositional aspects and biological reactivity may be altered by engine operating characteristics.
Abbreviations
- Ba:
-
Barium
- Ca:
-
Calcium
- CMD:
-
Count median diameter
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- Cr:
-
Chromium
- Cu:
-
Copper
- DE:
-
Diesel exhaust
- DEP:
-
Diesel exhaust particulate
- DTNB:
-
5,5’-Dithiobis-(2-nitrobenzoic acid)
- DTT:
-
Dithiothreitol
- EC:
-
Elemental carbon
- Fe:
-
Iron
- GE:
-
Gasoline exhaust
- HPEM:
-
Harvard personal environmental monitor
- Mg:
-
Magnesium
- MMAD:
-
Mass median aerodynamic diameter
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Ni:
-
Nickel
- 1-NP:
-
1-Nitropryene
- NO:
-
Nitric oxide
- NOx :
-
Oxides of nitrogen
- NO2 :
-
Nitrogen dioxide
- OC:
-
Organic carbon
- PAH:
-
Polycyclic aromatic hydrocarbon
- Pb:
-
Lead
- PBS:
-
Phosphate-buffered saline
- PM2.5 :
-
Fine particulate matter
- PTFE:
-
Polytetrafluoroethylene
- SD:
-
Standard deviation
- Sn:
-
Tin
- TNB:
-
3-Thio-6-nitrobenzoate
- TRAP:
-
Traffic-related air pollution
- UV:
-
Ultraviolet
- UW:
-
University of Washington
References
Ayres JG et al (2008) Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—a workshop report and consensus statement. Inhal Toxicol 20:75–99
Bai Y, Suzuki AK, Sagai M (2001) The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radic Biol Med 30:555–562
Benbrahim-Tallaa L et al (2012) Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol 13:663–664. doi:10.1016/S1470-2045(12)70280-2
Birger N, Gould T, Stewart J, Miller MR, Larson T, Carlsten C (2011) The Air Pollution Exposure Laboratory (APEL) for controlled human exposure to diesel exhaust and other inhalants: characterization and comparison to existing facilities. Inhal Toxicol 23:219–225. doi:10.3109/08958378.2011.562256
Biswas S, Verma V, Schauer JJ, Cassee FR, Cho AK, Sioutas C (2009) Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ Sci Technol 43:3905–3912. doi:10.1021/es9000592
Brook RD et al (2010) Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121:2331–2378
Charrier JG, Anastasio C (2012) On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos Chem Phys Discuss 12:11317–11350. doi:10.5194/acpd-12-11317-2012
Cheung KL et al (2009) Chemical characteristics and oxidative potential of particulate matter emissions from gasoline, diesel, and biodiesel cars. Environ Sci Technol 43:6334–6340
Choi JH et al (2011) 5-(4-Hydroxy-2,3,5-trimethylbenzylidene) thiazolidine-2,4-dione attenuates atherosclerosis possibly by reducing monocyte recruitment to the lesion. Exp Mol Med 43:471–478. doi:10.3858/emm.2011.43.8.053
Cory G (2011) Scratch-wound assay methods in molecular biology (Clifton, NJ) 769:25–30 doi:10.1007/978-1-61779-207-6_2
Danielsen PH, Loft S, Moller P (2008) DNA damage and cytotoxicity in type II lung epithelial (A549) cell cultures after exposure to diesel exhaust and urban street particles Particle and fibre toxicology 5:6-8977-8975-8976 doi:10.1186/1743-8977-5-6
Doyle M, Sexton KG, Jeffries H, Jaspers I (2007) Atmospheric photochemical transformations enhance 1,3-butadiene-induced inflammatory responses in human epithelial cells: the role of ozone and other photochemical degradation products. Chem Biol Interact 166:163–169
Ebersviller S, Lichtveld K, Sexton KG, Zavala J, Lin YH, Jaspers I, Jeffries HE (2012a) Gaseous VOCs rapidly modify particulate matter and its biological effects - Part 1: Simple VOCs and model PM. Atmospheric Chem Phys Discuss: ACPD 12:5065–5105
Ebersviller S, Lichtveld K, Sexton KG, Zavala J, Lin YH, Jaspers I, Jeffries HE (2012b) Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 2: Complex urban VOCs and model PM. Atmos Chem Phys 12:12293–12312
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319
Fujitani Y, Hirano S, Kobayashi S, Tanabe K, Suzuki A, Furuyama A, Kobayashi T (2009) Characterization of dilution conditions for diesel nanoparticle inhalation studies. Inhal Toxicol 21:200–209. doi:10.1080/08958370802339491
Ghio AJ, Sobus JR, Pleil JD, Madden MC (2012) Controlled human exposures to diesel exhaust. Swiss Med Wkly 142:w13597. doi:10.4414/smw.2012.13597
Gould T, Larson T, Stewart J, Kaufman JD, Slater D, McEwen N (2008) A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal Toxicol 20:49–52
Health Effects Institute (2010) Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects vol 17. Boston, MA
Hesterberg TW, Long CM, Bunn WB, Sax SN, Lapin CA, Valberg PA (2009) Non-cancer health effects of diesel exhaust: a critical assessment of recent human and animal toxicological literature. Crit Rev Toxicol 39:195–227. doi:10.1080/10408440802220603
Hirano S, Furuyama A, Koike E, Kobayashi T (2003) Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells. Toxicology 187:161–170. doi:10.1016/S0300-483X(03)00053-2
Hsu C-L, Wu Y-L, Tang G-J, Lee T-S, Kou YR (2009) Ginkgo biloba extract confers protection from cigarette smoke extract-induced apoptosis in human lung endothelial cells: role of heme oxygenase-1. Pulm Pharmacol Ther 22:286–296. doi:10.1016/j.pupt.2009.02.003
Kang K et al (2011) Vascular tube formation and angiogenesis induced by polyvinylpyrrolidone-coated silver nanoparticles. Toxicol Lett 205:227–234. doi:10.1016/j.toxlet.2011.05.1033
Katz D, Ito E, Lau KS, Mocanu JD, Bastianutto C, Schimmer AD, Liu FF (2008) Increased efficiency for performing colony formation assays in 96-well plates: novel applications to combination therapies and high-throughput screening. Bio Technol 44:ix–xiv. doi:10.2144/000112757; 10.2144/000112757
Kittelson DB et al (2008) Effect of fuel and lube oil sulfur on the performance of a diesel exhaust gas continuously regenerating trap. Environ Sci Technol 42:9276–9282
Kumagai Y, Koide S, Taguchi K, Endo A, Nakai Y, Yoshikawa T, Shimojo N (2002) Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem Res Toxicol 15:483–489
LaGier AJ, Manzo ND, Dye JA (2013) Diesel exhaust particles induce aberrant alveolar epithelial directed cell movement by disruption of polarity mechanisms. J Toxic Environ Health A 76:71–85. doi:10.1080/15287394.2013.738169
Laumbach R et al (2009) Quantification of 1-aminopyrene in human urine after a controlled exposure to diesel exhaust. J Environ Monit: JEM 11:153–159
Li N, Hao M, Phalen RF, Hinds WC, Nel AE (2003a) Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol (Orlando, Fla) 109:250–265
Li N et al (2003b) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111:455–460
Liang C-C, Park AY, Guan J-L (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. NatProtocols 2:329–333
Lichtveld KM, Ebersviller SM, Sexton KG, Vizuete W, Jaspers I, Jeffries HE (2012) In vitro exposures in diesel exhaust atmospheres: resuspension of PM from filters versus direct deposition of PM from air. Environ Sci Technol 46:9062–9070. doi:10.1021/es301431s; 10.1021/es301431s
Liu P-L, Chen Y-L, Chen Y-H, Lin S-J, Kou YR (2005) Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: role of AIF and EndoG. Am J Physiol Lung Cell Mol Physiol 289:L739–L749. doi:10.1152/ajplung.00099.2005
Liu Y-Y, Lin T-C, Wang Y-J, Ho W-L (2009) Carbonyl compounds and toxicity assessments of emissions from a diesel engine running on biodiesels. J Air Waste Manage Assoc 59:163–171. doi:10.3155/1047-3289.59.2.163
Lloyd AC, Cackette TA (2001) Diesel engines: environmental impact and control. J Air Waste Manage Assoc 51:809–847
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2013) The role of oxidative stress during inflammatory processes. Biol Chem. doi:10.1515/hsz-2013-0241
Martin A, Clynes M (1993) Comparison of 5 microplate colorimetric assays for in vitro cytotoxicity testing and cell proliferation assays. Cytotechnology 11:49–58
Mattingly KA, Klinge CM (2012) Diesel exhaust particulate extracts inhibit transcription of nuclear respiratory factor-1 and cell viability in human umbilical vein endothelial cells. Arch Toxicol 86:633–642. doi:10.1007/s00204-011-0778-y
McConnachie LA et al (2013) Heme oxygenase expression as a biomarker of exposure to amphiphilic polymer-coated CdSe/ZnS quantum dots. Nanotoxicology 7:181–191. doi:10.3109/17435390.2011.648224
McDonald JD, Barr EB, White RK (2004a) Design, characterization, and evaluation of a small-scale diesel exhaust exposure system. Aerosol Sci Technol 38:62–78. doi:10.1080/02786820490247623
McDonald JD, Harrod KS, Seagrave J, Seilkop SK, Mauderly JL (2004b) Effects of low sulfur fuel and a catalyzed particle trap on the composition and toxicity of diesel emissions. Environ Health Perspect 112:1307–1312
McDonald JD, Campen MJ, Harrod KS, Seagrave J, Seilkop SK, Mauderly JL (2011) Engine-operating load influences diesel exhaust composition and cardiopulmonary and immune responses. Environ Health Perspect 119:1136–1141. doi:10.1289/ehp.1003101
Miller-Schulze JP et al (2010) Exposures to particulate air pollution and nitro-polycyclic aromatic hydrocarbons among taxi drivers in Shenyang. China Environ Sci Technol 44:216–221. doi:10.1021/es802392u
Moller P, Folkmann JK, Forchhammer L, Brauner EV, Danielsen PH, Risom L, Loft S (2008) Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett 266:84–97. doi:10.1016/j.canlet.2008.02.030
Napierska D et al (2009) Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 5:846–853. doi:10.1002/smll.200800461
Ntziachristos L, Froines JR, Cho AK, Sioutas C (2007) Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Particle Fibre Toxicol 4:5. doi:10.1186/1743-8977-4-5
Okayama Y, Kuwahara M, Suzuki AK, Tsubone H (2006) Role of Reactive Oxygen Species on Diesel Exhaust Particle-Induced Cytotoxicity in Rat. Cardiac Myocytes J Toxicol Environ Health, Part A 69:1699–1710. doi:10.1080/15287390600631078
Pan C-JG, Schmitz DA, Cho AK, Froines J, Fukuto JM (2004) Inherent redox properties of diesel exhaust particles: catalysis of the generation of reactive oxygen species by biological reductants. Toxicol Sci 81:225–232. doi:10.1093/toxsci/kfh199
Peplow PV, Chatterjee MP (2013) A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 62:1–21. doi:10.1016/j.cyto.2013.02.015
Poss J et al (2013) Diesel exhaust particles impair endothelial progenitor cells, compromise endothelial integrity, reduce neoangiogenesis, and increase atherogenesis in mice. Cardiovasc Toxicol 13:290–300. doi:10.1007/s12012-013-9208-0
Robinson AL et al (2007) Rethinking organic aerosols: semivolatile emissions and photochemical aging. Science 315:1259–1262. doi:10.1126/science.1133061
Rudell B, Sandstrom T, Hammarstrom U, Ledin ML, Horstedt P, Stjernberg N (1994) Evaluation of an exposure setup for studying effects of diesel exhaust in humans. Int Arch Occup Environ Health 66:77–83. doi:10.1007/BF00383361
Sawant AA et al (2008) Generation and characterization of diesel exhaust in a facility for controlled human exposures. J Air Waste Manage Assoc 58:829–837
Scheepers PT, Bos RP (1992) Combustion of diesel fuel from a toxicological perspective. I. Origin of incomplete combustion products. Int Arch Occup Environ Health 64:149–161
Schuetzle D, Perez JM (1983) Factors influencing the emissions of nitrated-polynuclear aromatic hydrocarbons (Nitro-PAH) from diesel engines. J Air Pollut Control Assoc 33:751–755. doi:10.1080/00022470.1983.10465636
Sexton KG, Jeffries HE, Jang M, Kamens RM, Doyle M, Voicu I, Jaspers I (2004) Photochemical products in urban mixtures enhance inflammatory responses in lung cells. Inhal Toxicol 16(Suppl 1):107–114. doi:10.1080/08958370490443196
Sharma M, Agarwal AK, Bharathi KVL (2005) Characterization of exhaust particulates from diesel engine. Atmos Environ 39:3023–3028. doi:10.1016/j.atmosenv.2004.12.047
Shi JP, Mark D, Harrison RM (2000) Characterization of particles from a current technology heavy-duty diesel engine. Environ Sci Technol 34:748–755. doi:10.1021/es990530z
Shinyashiki M et al (2009) Electrophilic and redox properties of diesel exhaust particles. Environ Res 109:239–244. doi:10.1016/j.envres.2008.12.008
Sjogren M, Li H, Banner C, Rafter J, Westerholm R, Rannug U (1996) Influence of physical and chemical characteristics of diesel fuels and exhaust emissions on biological effects of particle extracts: a multivariate statistical analysis of ten diesel fuels. Chem Res Toxicol 9:197. doi:10.1021/tx950095w
Sobus JR, Pleil JD, Madden MC, Funk WE, Hubbard HF, Rappaport SM (2008) Identification of Surrogate Measures of Diesel Exhaust Exposure in a Controlled Chamber Study. Environ SciTechnol 42:8822–8828. doi:10.1021/es800813v
Sylvester PW (2011) Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol (Clifton, NJ) 716:157–168. doi:10.1007/978-1-61779-012-6_9
Tau J, Novaes P, Matsuda M, Tasat DR, Saldiva PH, Berra A (2013) Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Invest Ophthalmol Vis Sci 54:4759–4765. doi:10.1167/iovs.12-10541
Totlandsdal AI et al (2012) Differential effects of the particle core and organic extract of diesel exhaust particles. Toxicol Lett 208:262–268. doi:10.1016/j.toxlet.2011.10.025
Vedal S et al (2013) National Particle Component Toxicity (NPACT) Initiative report on cardiovascular effects. Health Effects Institute, Boston
Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ (2012) Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-kappaB, and MAPK pathways. Arch Toxicol 86:879–896. doi:10.1007/s00204-012-0845-z
Weitkamp EA, Sage AM, Pierce JR, Donahue NM, Robinson AL (2007) Organic aerosol formation from photochemical oxidation of diesel exhaust in a smog chamber. Environ Sci Technol 41:6969–6975. doi:10.1021/es070193r
Weldy CS, Wilkerson HW, Larson TV, Stewart JA, Kavanagh TJ (2011) Diesel particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol vitro: an Int J Publ Assoc BIBRA 25:2064–2073. doi:10.1016/j.tiv.2011.08.008
World Health Organization (2006) Air Quality Guidelines: Global Update 2005. Druckpartner Moser, Germany
Yin F et al (2013) Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 33:1153–1161. doi:10.1161/ATVBAHA.112.300552
You Y et al (2012) Anti-atherosclerotic function of Astragali Radix extract: downregulation of adhesion molecules in vitro and in vivo. BMC Complement Altern Med 12:54
Zhang J, McCreanor JE, Cullinan P, Chung K, Ohman-Strickland P, Han I-K JL, Nieuwenhuijsen MJ (2009) Health effects of real-world exposure to diesel exhaust in persons with asthma vol 138. Health Effects Institute, Boston
Acknowledgments
Funding for this study was provided by NIH/NIEHS Grants T32ES015459, T32ES007032, P50ES015915, and P30ES007033, and the Amgen Scholar’s Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 555 kb)
Rights and permissions
About this article
Cite this article
Fox, J.R., Cox, D.P., Drury, B.E. et al. Chemical characterization and in vitro toxicity of diesel exhaust particulate matter generated under varying conditions. Air Qual Atmos Health 8, 507–519 (2015). https://doi.org/10.1007/s11869-014-0301-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-014-0301-8