Abstract
Samples of four popular brands of canned fish consumed in Iran were analyzed for levels and determination of health risk assessment of Cd, Hg, Pb, Se, Sn and Zn after wet digestion with acids by ICP-OES and DMA. The results showed that the contents of Cd, Hg, Pb, Se, Sn and Zn (µg/g) in canned samples were 0.10 ± 0.04, 0.13 ± 0.05, 0.75 ± 0.65, 2.04 ± 0.43, 0.18 ± 0.03 and 12.61 ± 11.92, respectively. Evaluation of these levels showed that except for Cd and Pb, the average contents of other metals are significantly lower than adverse level for the human consumption. Also, HRI values were within the safe limits (HRI <1), and there are no potential health risk for adults and children via consumption of canned fish. Finally, the content of Cd and Pb was higher than MPL in the canned fish samples. Due to the discharge of pollutants into the aquatic environments, heavy metals content and pesticides residue monitoring in the foodstuff is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, due to the growing concern about the health benefits and risks of food consumption, much attention has been paid to the study of essential and toxic trace element concentrations in foodstuffs (Guérin et al. 2011). Trace metals are taken up and subsequently accumulate by organisms from contaminated urban, mining, agricultural and industrial effluents or wastewaters and natural sources, which enter the aquatic environment via direct discharges (Marsden and Rainbow 2004). Physicochemical and other processes such as diffusion, dilution, sorption, precipitation, uptake and elimination by marine organisms can regulated the distribution of heavy metals in the different components of the marine ecosystem (Arellano et al. 1999; Hussein and Khaled 2014). Also, ecological needs, seasonal changes, size, sex and moult of marine animals were found to affect metal accumulation in their tissues (Yilmaz and Yilmaz 2007). Metals such as Cu, Fe, Mn and Zn are essential metals since they play an important role in biological systems especially human physiology, whereas other metals such as As, Cd, Cr, Hg and Pb are non-essential metals, as they are toxic, even in trace amounts. It should be noted that essential metals could also produce toxic effects when the metal intake is excessively elevated (Türkmen et al. 2008; Hosseini et al. 2013a). However, like essential metals, nonessential metals are also taken up by fish and accumulate in their tissues especially muscle, gill and liver (Yılmaz et al. 2010). Therefore, accumulation of heavy metals is dependent on the content of the metal and the exposure period (Pourang et al. 2005; Sobhanardakani et al. 2011; Hosseini et al. 2013a, b). Fish consumption is known to be healthy in the human diet as they provide omega-3(n-3) fatty acids that reduce cholesterol levels and the incidence of stroke, heart disease and preterm delivery (Daviglus et al. 2002; Patterson 2002). Therefore, fish and fish products are widely consumed in many parts of the world because they have a high protein content rich in essential amino acids, micro and macro elements such as Ca, P, F, and I, liposoluble vitamins and polyunsaturated fatty acids with the benefit of a hypocholesterolic effect (anti-atheriosclerosis) (Ismail 2005; Ikem and Egiebor 2005). However, those benefits may be offset by the presence of pollutants, especially heavy metals (Burger and Gochfeld 2004; Hosseini et al. 2013a).
Risk analysis is a process that combines risk assessment, risk management and risk communication. Risk assessment that is based on scientific analyses, is a quantitative or qualitative explanation of the likelihood of harm associated with exposure to a chemical compounds. In this regard, the human health risk assessment requires identification, collection and integration of information on hazardous chemicals, their exposure to humans and the relationship between exposure, dose and adverse effects. On the other hand, a human potential health risk assessment is the process to estimate the nature and possibility of adverse health effects in humans who may be exposed to toxins and chemicals, now or in future. It includes hazard identification, dose–response assessment, exposure assessment and risk characterization steps (Sobhanardakani 2016).
Nowadays, canned food including canned fish are constitute affordable and inexpensive products and therefore, they are widely distributed and a popular food source in many countries especially in Libya, USA, Portugal, the Kingdom of Saudi Arabia, Turkey, and Iran (Storelli et al. 2010; Hosseini et al. 2013a). However, the content of heavy metals increases at subsequent levels of the food chain, as these metals tend to accumulate in tissues of marine animals and especially in fish species (Squadrone et al. 2013; Winiarska-Mieczan et al. 2015). In this regard, because metal pollution in aquatic environments can be harmful to human health, it is necessary to understand and control the hazard levels of pollution in seafood. Therefore, the aim of the current study was to determine both the content and human health risk assessment of Cd, Hg, Pb, Se, Sn and Zn via consumption of different brands commonly consumed canned fish in Iran.
2 Materials and methods
2.1 Sample collection
120 samples of four different brands of canned fish including tuna fish and common kilka [30 samples for each species: yellowfin tuna (Thunnus albacares); common Kilka (Clupeonella cultriventris caspia); Kawakawa (Euthynnus affinis); and longtail tuna (Thunnus tonggol)] were purchased from 10 different markets in Tehran and used for analysis of concentration of Cd, Hg, Pb, Se, Sn and Zn.
2.2 Chemical analyses
After opening, each can content was homogenized thoroughly with a food blender with stainless steel cutters (Boadi et al. 2011). Samples were digested with 10 ml of 1N nitric acid in closed Teflon vessels in a microwave oven (CEM MARS-5 closed vessel microwave digestion system) using the following microwave digestion program: pressure of 200 psi, ramp time of 25 min, temperature of 210 °C, maximum power of 300 W, and hold of time 10 min. Then, hydrogen peroxide (1.5 ml, 30%) was added to each digest to break down organic matter that may not be during the nitric acid digestion and the same heating program was applied. After cooling, residues were transferred to 25 ml volumetric flasks and diluted to level with deionized water. Before analysis, the samples were filtered through a 0.45-μm membrane filter. Sample blanks were prepared in the laboratory in a similar manner to the field samples. All metal concentrations (µg/g, ww) were determined with three replications using inductively coupled plasma–optical emission spectrometer (Optima 2100 DV, Perkin Elmer) (Türkmen et al. 2009). Hg was determined by cold vapor atomic absorption using a direct mercury analyzer (DMA-80) (Ikem and Egiebor 2005; Hosseini et al. 2013b). Standard solutions were prepared from stock solutions (Merck, multi-element standard). All the instrumental conditions applied for Cd, Pb, Se, Sn and Zn content determinations were set in accordance with general recommendations (wave length for Cd, Pb, Se, Sn and Zn: 226.50 nm, 220.35, 196.03, 284.0 and 206.20 nm, respectively).
2.3 Statistical analysis
The statistical analysis of the obtained results consisted in a first Kolmogorov–Smirnov normality test, followed by the study of the variance homogeneity using an ANOVA parametric test with a DMS post hoc and Duncan multiple range test. The mean levels of heavy metals were compared with international standard using a one-sample test. Probabilities less than 0.05 were considered statistically significant (p < 0.05). The statistical calculations were done using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) statistical package.
2.4 Potential health risk assessment
The human health risk posed by chronic exposure to the heavy metals was assessed. For computing potential health risk assessment, the average daily intake of metal (DIM) was calculated using the Eq. 1 (Guo et al. 2016):
Cmetal, Cfactor, Dfood intake, and Baverage weight represent the heavy metal concentrations in canned fish (µg/g), conversion factor (0.085), daily intake of canned fish (3.5E0−3 kg/person/day), and average body weight (70.0 kg for adults and 15.0 kg for children), respectively (Falco et al. 2006; Omar et al. 2013; Tang et al. 2015; Guo et al. 2016).
The health risk index (HRI) for the local population through the consumption of canned fish was assessed using the Eq. 2 (Guo et al. 2016):
Here, DIM and RfD represent daily intake of metal and reference dose of metal, respectively. The oral reference doses for Cd, Hg, Pb, Se, Sn and Zn were 0.001, 0.0007, 0.0035, 0.005, 0.20 and 0.30 mg/kg/day, respectively. An HRI < 1 means the exposed population is assumed to be safe (Xue et al. 2012; Nahar Jolly et al. 2013; Ru et al. 2013; Liang et al. 2015; Zeng et al. 2015; Guo et al. 2016).
The total HRI (THRI) of heavy metals for the canned fish was calculated as the mathematical sum of each individual metal HRI value according to Eq. 3 (Guo et al. 2016):
3 Results and discussion
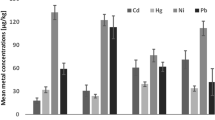
The concentrations of Cd, Hg, Pb, Se, Sn and Zn in the analyzed canned fish samples are presented in Table 1. The data show that the percentage of metals contamination of canned fish samples reached 100%. Among the analyzed canned fish samples, Cd levels ranged from 0.06 to 0.15, Hg from 0.09 to 0.18, Pb from 0.19 to 1.59, Se from 1.59 to 2.61, Sn from 0.14 to 0.20 and Zn from 5.77 to 30.47 µg/g, respectively.
Comparing the heavy metal concentrations in canned fish with the maximum permissible limits according to EU, ATSDR and FAO/WHO in µg/g (0.050 Cd, 0.50 for Hg and Pb, 2.0 for Se, 250 for Sn and 50.0 for Zn, respectively), the mean concentration of Pb was higher than MPL (Hosseini et al. 2013a, 2015; Sobhanardakani et al. 2017). Therefore, canned fish’s contribution to the total body burden of Cd, Hg, Se, Sn and Zn can be considered as negligibly small.
In addition, all the calculated HRI values of heavy metals were within the safe limits (HRI < 1) (Table 2). Furthermore, the THRI values, which varied from 4.11E−04 to 1.05E−03 for adults and from 1.92E−03 to 4.90E−03 for children, were also within the safe limit (THRI < 1). Therefore, we conclude that there is no potential significant health risk through only consuming canned fish from the Iran.
Cadmium is a non-essential heavy metal, very toxic to humans and acculmulates in the body in various tissues. The main accumulation of Cd in aquatic organisms is the liver and kidney, and also the gills, which might be a route of uptake (Yılmaz et al. 2010). The use of Cd in the food industry and agriculture has been identified as a major source of its wide dispersion in the environment and following that in food. The major route of exposure to Cd for nonsmokers is through food; the contribution from other pathways to total uptake is small (Hosseini et al. 2015). Our results show that the mean concentrations of Cd in canned fish samples were 0.10 ± 0.04 µg/g which is much higher than in the MPL. However, Cd levels were in similar ranges as described in the literature:
-
0.053 ± 0.006 µg/g in the muscle of 3 tuna species collected from the Alexandria, Egypt (Hussein and Khaled 2014),
-
0.06–0.25 μg/g in canned fish marketed in Turkey (Tuzen and Soylak 2007),
-
0.2–0.07 in canned tuna samples consumed in Iran (Taghipour and Aziz 2010),
-
0.16 ± 0.11 in canned tuna samples consumed in Saudi Arabia (Ashraf 2006),
-
0.13 ± 0.04 in canned bluefin tuna produced in Thailand and
-
0.53 ± 0.005 in canned bluefin tuna produced in Korea (Islam et al. 2010).
In another study, Boadi et al. (2011) reported that the mean concentrations of Cd (µg/g) in canned fish from Ghana were <0.001 and also Tuzen and Soylak (2007) reported that the levels of Cd in canned fish sold in Turkey ranged between 0.06–0.25 μg/g respectively. Ikem and Egiebor (2005) reported that the mean concentrations of Cd (µg/kg) in canned fish from Georgia and Alabama were 0.0 for pink salmon, 0.0 for red salmon, 1.90 for tuna, 0.0 for mackerel, 0.0 for sardines and 0.0 for herring. Also, the mean levels of Cd in canned sardines consumed in Nigeria were determined 0.20 ± 0.06 µg/g (Iwegbue et al. 2009). Mol (2011) reported that the levels of Cd in the canned bonito, sardines, and mackerel produced in Turkey were 0.01–0.02 µg/g. Mahalakshmi et al. (2012) reported that the contents of Cd (µg/g) in the canned tuna fish consumed in Canada were also 0.020–0.025 µg/g. The results of the study of Winiarska-Mieczan et al. (2015) showed that the content of Cd in canned fish marketed in Lublin, Poland do not exceed safe levels for adult men.
Mercury (Hg) is a toxic element that occurs widely in the biosphere and presenting occupational hazards associated with both inhalation and ingestion. No vital function for the Hg in living organisms had been found yet. The common use of Hg is in industry and agriculture, and the recognition that alkyl derivatives of this element are more toxic than most other chemical forms, can enter the food chain via the activity of microorganisms with the ability to methylate. Hg is present in industrial wastes. Many studies have shown that Hg is bioamplified in the food chain with high-trophic-level predatory species especially tuna, swordfish and shark that accumulation higher mercury compared with herbivorous species (Burger and Gochfeld 2004; Essa and Rateb 2011). Therefore, the primary sources of Hg contamination in humans are via eating fish (Stancheva et al. 2014). The results showed that the mean concentration of Hg in analyzed canned fish samples is 0.13 ± 0.05 µg/g and lower than MPL. In this regard, Boadi et al. (2011) reported that the mean concentration of Hg (µg/g) in canned fish was 0.16 ± 0.09. In the other studies, the mean levels of Hg (µg/g) in canned fish samples were 0.093 ± 0.098 (Ikem and Egiebor 2005), 0.31 ± 0.17 (Ashraf 2006), 0.60 ± 0.009 and 0.62 ± 0.012 in canned tuna fish produced in Canada and India, respectively (Mahalakshmi et al. 2012). Also, Islam et al. (2010) reported that the mean concentration of Hg (µg/g) was 1.07 ± 0.04 for canned longtail tuna produced in USA, 0.26 ± 0.03 for canned bluefin tuna produced in Thailand and 0.24 ± 0.02 for canned bluefin tuna produced in Korea.
Lead is the most common environmental metal poison and therefore the most common environmental health hazard (Şireli et al. 2006). Humans are exposed to Pb mainly through inadvertent ingestion of Pb paint, inhalation of traffic exhaust fumes, and consumption of Pb contaminated foods (Adekunle and Akinyemi 2004). However, the uptake of Pb via the food chain is of less importance since the content of this element in fish does not increase with age and trophic level but with increasing concentration in the water (Şireli et al. 2006). Pb poisoning in fish causes a decrease in growth rates, development, metabolism and survival, in addition to increased mucus formation (Burger et al. 2002). Pb was detected in all examined canned fish samples with concentrations ranging from 0.19 to 1.59 µg/g. Also, the results of present study showed that the mean concentration of Pb in analyzed samples were higher than MPL. In the literature, Pb levels range between 0.67 and 0.99 µg/g in muscle tissue in Thunnus thynnus (Hussein and Khaled 2014), 0.01–0.17 µg/g for canned fish (Boadi et al. 2011), 0.0–0.0017 µg/g for canned fish from the USA (Ikem and Egiebor 2005), 0.06–0.25 µg/g for canned fish kilka (Taghipour and Aziz 2010), 0.14–0.82 µg/g in tuna fish (Ashraf 2006), 0.11–0.29 µg/g for canned sardines (Iwegbue et al. 2009), 0.09–0.45 µg/g for canned bonito, sardines, and mackerel produced in Turkey (Mol 2011), 0.09–0.40 μg/g for canned fish marketed in Turkey (Tuzen and Soylak 2007), and 0.011–0.089 µg/g for canned tuna commercialized in Canada and India (Mahalakshmi et al. 2012). Also, Islam et al. (2010) reported that the mean contents of Pb (µg/g) were 0.74 ± 0.28 for canned longtail tuna produced in USA, 0.13 ± 0.05 for canned bluefin tuna produced in Thailand and 0.12 ± 0.01 for canned bluefin tuna produced in Korea. In another study Winiarska-Mieczan et al. (2015) reported that the content of Pb in canned fish marketed in Lublin, Poland do not exceed safe levels for adult men.
Selenium is a micronutrient element and humans and animals require this element for the functioning of a number of selenium-dependent enzymes, but in high content causes adverse health effects. Also, Se treats similarly to As, although it seems to concentrate least strongly (Miklavčič et al. 2011; Rezayi et al. 2011; Hosseini et al. 2013a). In the present study, the mean concentration of Se in canned fish samples was 2.04 ± 0.43 µg/g and there was no significant difference between mean levels for Se with MPL. Numerous studies have shown Se supplementation counteracts the negative impacts of exposure to Hg in all investigated species of mammals, birds and fish. Therefore, the protective effects of Se against Hg toxicity have been illustrated in many animals and in humans (Falnoga and Tusek-Znidaric 2007; Kaneko and Ralston 2007; Miklavčič et al. 2011). Thus, Se levels must also be considered when Hg is high or above the limits (Hosseini et al. 2013a). In this regard, Miklavčič et al. (2011) reported that the mean contents of Se (µg/g) were 0.47 in canned fish available on the Slovenian market.
Tin estimation in canned food is significant for both human health and quality assessment. A high Sn level indicates migration of this element from the container to food usually because of poor lacquering (Hosseini et al. 2013a). The results showed that the mean concentrations of Sn in canned fish samples were 0.18 ± 0.03 µg/g and was much lower than the MPL. This could be a result of improved quality assurance measures during processing or storage conditions of canned food especially canned fish (Khalafalla et al. 2016). Tin contents in the literature have been reported in the range of 0.0003–0.011 µg/g for canned fishes marketed in USA (Ikem and Egiebor 2005).
Zinc is responsible for some important biological functions and it is a constituent of all cells, and more than 90 zinc-containing enzymes and proteins. Therefore, relatively high level of Zn is necessary to maintain these biological functions. Too little Zn can cause problems, but too much Zn (above the limit of 50 µg/g) is also harmful to human health. It appears to have a protective effect against the toxicities of Cd and Pb (Hussein and Khaled 2014). The results of the present study showed that the mean level of Zn in canned fish samples were 12.61 ± 11.92 µg/g and was much lower than the MPL. In the literature, Zinc concentration has been reported as follows: 16.54–22.12 µg/g for muscle in Thunnus thynnus collected from Egypt (Hussein and Khaled 2014), 0.07–0.19 µg/g in canned fish marketed in Ghana (Boadi et al. 2011), 0.004–0.03 µg/g for canned fishes marketed in USA (Ikem and Egiebor 2005), 11.32–23.90 µg/g for canned fish kilka consumed in Iran (Taghipour and Aziz 2010), 0.09–4.63 µg/g canned sardines consumed in Nigeria (Iwegbue et al. 2009), 0.90–2.50 μg/g for canned fish marketed in Turkey (Tuzen and Soylak 2007), and 8.20–12.40 for the canned bonito, sardines, and mackerel produced in Turkey (Mol 2011). Also, Islam et al. (2010) reported that the mean contents of Zn (µg/g) were 41.38 ± 3.02 for canned longtail tuna produced in USA, 57.60 ± 10.81 for canned bluefin tuna produced in Thailand and 38.35 ± 10.13 for canned bluefin tuna produced in Korea.
As shown in Table 2, HRI values of Cd, Hg, Pb, Se, Sn and Zn for children and adults are <1. Here, the average HRI value was 6.73E−04 for adults and 3.14E−03 for children. Therefore, we conclude that target population might have no potential significant health risk through only consuming canned fish from the Iran. However, the non-carcinogenic risks were greater for children than for adults. In this regard, Hussein and Khaled (2014) after analyzed the heavy metals in muscle of Thunnus thynnus collected from Alexandria, Egypt reported that Cd, Pb and Zn THQ values were less than 1 and show a situation of no risk for the consumer of the investigated tuna species.
4 Conclusion
Based on the results, despite the mean contents of Cd and Pb were higher than MPL, but according of the HRI values there are no health risks with respect to the concentrations of these toxic elements in canned fishes analyzed in this study. Therefore, the body burden of these elements is highly dependent on the contents of various elements in fish consumed, the amount of food consumed, the frequency of consumption of fish, and the rate of detoxification of contaminants in the human body.
References
Adekunle IM, Akinyemi MF (2004) Lead levels of certain consumer products in Nigeria: a case study of smoked fish foods from Abeokuta. Food Chem Toxicol 42:1463–1468
Arellano JM, Ortiz JB, Capeta Da Silva D, González de Canales ML, Sarasquete C, Blasco J (1999) Levels of copper, zinc, manganese and iron in two fish species from salt marshes of Cadiz Bay (Southwest Iberian Peninsula). Bol. Inst. Esp. Oceanogr 15(1–4):485–488
Ashraf W (2006) Levels of selected heavy metals in tuna fish. Arab J Sci Eng 31(1A):89–92
Boadi NO, Twumasi SK, Badu M, Osei I (2011) Heavy metal contamination in canned fish marketed in Ghana. Am J Sci Ind Res 2(6):877–882
Burger J, Gochfeld M (2004) Mercury in canned tuna: white versus light and temporal variation. Environ Res 96:239–249
Burger J, Gaines KF, Boring CS, Stephens WL, Snodgrass J, Dixon C, McMahon M, Shikla S, Shukla T, Gochfeld M (2002) Metal levels in fish from the Savannah River: potential hazards to fish and other receptors. Environ Res 89:85–97
Daviglus M, Sheeshka J, Murkin E (2002) Health benefits from eating fish. Comment Toxicol 8:345–374
Essa HH, Rateb HZ (2011) Residues of some heavy metals in freshwater fish (Oreochromis niloticus and Labeo niloticus) in Assiut City markets. Ass Univ Bull Environ Res 14(1):31–39
Falco G, Ilobet J, Bocio A, Domingo JL (2006) Daily intake of arsenic, cadmium, mercury, and lead by consumption of edible marine species. J Agric Food Chem 54:6106–6112
Falnoga I, Tusek-Znidaric M (2007) Selenium–mercury interactions in man and animals. Biol Trace Elem Res 119(3):212–220
Guérin T, Chekri R, Vastel C, Sirot V, Volatier J-L, Leblanc J-C, Noël L (2011) Determination of 20 trace elements in fish and other seafood from the French market. Food Chem 127:934–942
Guo J, Yue T, Li X, Yuan Y (2016) Heavy metal levels in kiwifruit orchard soils and trees and its potential health risk assessment in Shaanxi. China. Environ Sci Pollut Res 23(14):14560–14566
Hosseini SV, Aflaki F, Sobhanardakani S, Tayebi L, Babakhani Lashkan A, Regenstein JM (2013a) Analysis of mercury, selenium and tin concentrations in canned fish marketed in Iran. Environ Monit Assess 185(8):6407–6412
Hosseini SM, Sobhanardakani S, Batebi Navaei M, Kariminasab M, Aghilinejad SM, Regenstein JM (2013b) Metal content in caviar of wild Persian sturgeon from the southern Caspian Sea. Environ Sci Pollution Res 20(8):5839–5843
Hosseini SV, Sobhanardakani S, Kolangi Miandare H, Harsij M, Regenstein JM (2015) Determination of toxic (Pb, Cd) and essential (Zn, Mn) metals in canned tuna fish produced in Iran. J Environ Health Sci Eng 13:59
Hussein A, Khaled A (2014) Determination of metals in tuna species and bivalves from Alexandria. Egypt. Egypt J Aquat Res 40:9–17
Ikem A, Egiebor NO (2005) Assessment of trace elements in canned fishes (Mackerel, Tuna, Salmon, Sardines and Herrings) marketed in Georgia and Alabama (United state of America). J Food Compos Anal 18:771–787
Islam MM, Bang S, Kim KW, Ahmed MK, Jannat M (2010) Heavy metals in frozen and canned marine fish of Korea. J Sci Res 2(3):549–557
Ismail HM (2005) The role of omega-3 fatty acids in cardiac protection: an overview. Front Biosci 10:1079–1088
Iwegbue CMA, Nwajei GE, Arimoro FO, Eguavoen O (2009) Characteristic levels of heavy metals in canned sardines consumed in Nigeria. Environ 29:431–435
Kaneko JJ, Ralston NVC (2007) Selenium and mercury in pelagic fish in the Central North Pacific near Hawaii. Biol Trace Elem Res 119(3):242–254
Khalafalla FA, Ali FHM, Hassan ARHA, Basta SE (2016) Residues of lead, cadmium, mercury and tin in canned meat products from Egypt: an emphasis on permissible limits and sources of contamination. J Consum Protect Food Safe 11(2):137–143
Liang Q, Xue ZJ, Wang F, Sun ZM, Yang ZX, Liu SQ (2015) Contamination and health risks from heavy metals in cultivated soil in Zhangjiakou City of Hebei Province. China. Environ Monit Assess 187(12):754
Mahalakshmi M, Balakrishnan S, Indira K, Srinivasan M (2012) Characteristic levels of heavy metals in canned tuna fish. J Toxicol Environ Health Sci 4(2):43–45
Marsden ID, Rainbow PS (2004) Does the accumulation of trace metals in crustaceans affect their ecology- the amphipod example? J Exp Mar Biol Ecol 300:373–408
Miklavčič A, Stibilj V, Heath E, Polak T, Tratnik JS, Klavž J, Mazej D, Horvat M (2011) Mercury, selenium, PCBs and fatty acids in fresh and canned fish available on the Slovenian market. Food Chem 124:711–720
Mol S (2011) Levels of heavy metals in canned bonito, sardines, and mackerel produced in Turkey. Biol Trace Elem Res 143:974–982
Nahar Jolly Y, Islam A, Akbar S (2013) Transfer of metals from soil to vegetables and possible health risk assessment. Springerplus 2(1):385
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2013) Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch Environ Contam Toxicol 65(4):753–764
Patterson J (2002) Introduction-comparative dietary risk: balance the risks and benefits of fish consumption. Comment Toxicol 8:337–344
Pourang N, Tanabe S, Rezvani S, Dennis JH (2005) Trace elements accumulation in edible tissues of five sturgeon species from the Caspian Sea. Environ Monit Assess 100(1–3):89–108
Rezayi M, Esmaeli AS, Valinasab T (2011) Mercury and selenium content in Otolithes ruber, and Psettodes erumei from Khuzestan Shore. Iran. Bull Environ Contam Toxicol 86:511–514
Ru QM, Feng Q, He JZ (2013) Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chem Toxicol 53:256–262
Şireli UT, Göncüoğlu M, Yıldırım T, Gücükoğlu A, Çakmak O (2006) Assessment of heavy metals (cadmium and lead) in vacuum packaged smoked fish species (mackerel, Salmo salar and Oncorhynhus mykiss) marketed in Ankara (Turkey). Ege J Fish Aquat Scis 23(3–4):353–356
Sobhanardakani S (2016) Health risk assessment of As and Zn in canola and soybean oils consumed in Kermanshah. Iran. J Adv Environ Health Res 4(2):62–67
Sobhanardakani S, Tayebi L, Farmany A (2011) Toxic Metal (Pb, Hg and As) Contamination of muscle, gill and liver tissues of Otolithes rubber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson and Onchorynchus mykiss. World Appl Sci J 14(10):1453–1456
Sobhanardakani S, Hosseini SV, Kolangi Miandare H, Faizbakhsh R, Harsij M, Regenstein JM (2017) Determination of Cd, Cu. Mn and Zn concentrations in Iranian Caspian Sea caviar of Acipenser persicus using anodic stripping voltammetry. Iran J Sci Technol (Sci). doi:10.1007/s40995-017-0217-x
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzio T, Guarise S, Benedetto A, Abete MC (2013) Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 90(2):358–365
Stancheva M, Makedonski L, Peycheva K (2014) Determination of heavy metal concentrations of most consumed fish species from Bulgarian Black Sea coast. Bulg Chem Commun 46(1):195–203
Storelli MM, Barone G, Cuttone G, Giungato D, Garofalo R (2010) Occurrence of toxic metals (Hg, Cd, and Pb) in fresh and canned tuna: public health implications. Food Chem Toxicol 48(11):3167–3170
Taghipour V, Aziz S (2010) Determination of trace elements in canned Kilka fish marketed in Islamic Republic of Iran. World Appl Sci J 9(6):704–707
Tang W, Cheng J, Zhao W, Wang W (2015) Mercury levels and estimated total daily intakes for children and adults from an electronic waste recycling area in Taizhou, China: key role of rice and fish consumption. J Environ Sci 34:107–115
Türkmen M, Türkmen A, Tepe Y, Ates A, Gökkus K (2008) Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean Seas: twelve fish species. Food Chem 108:794–800
Türkmen M, Türkmen A, Tepe Y, Tore Y, Ates A (2009) Determination of metals in fish species from Aegean and Mediterranean seas. Food Chem 113:233–237
Tuzen M, Soylak M (2007) Determination of trace metals in canned fish marketed in Turkey. Food Chem 101(4):1395–1399
Winiarska-Mieczan A, Kwiecień M, Krusiński R (2015) The content of cadmium and lead in canned fish available in the Polish market. J Consum Protect Food Safe 10(2):165–169
Xue ZJ, Liu SQ, Liu YL, Yan YL (2012) Health risk assessment of heavy metals for edible parts of vegetables grown in sewage-irrigated soils in suburbs of Baoding City, China. Environ Monit Assess 184:3503–3513
Yilmaz AB, Yilmaz L (2007) Influences of sex and seasons on levels of heavy metals in tissues of green tiger shrimp (Penaeus semisulcatus de Hann, 1844). Food Chem 101:1664–1669
Yılmaz AB, Sangün MK, Yağlıoğlu D, Turan C (2010) Metals (major, essential to non-essential) composition of the different tissues of three demersal fish species from Iskenderun Bay, Turkey. Food Chem 123:410–415
Zeng X, Wang Z, Wang J, Guo J, Chen X, Zhuang J (2015) Health risk assessment of heavy metals via dietary intake of wheat grown in Tianjin sewage irrigation area. Ecotoxicol 24(10):2115–2124
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding has been received to conduct and complete of this study.
Conflict of interest
The author declare that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Sobhanardakani, S. Tuna fish and common kilka: health risk assessment of metal pollution through consumption of canned fish in Iran. J Consum Prot Food Saf 12, 157–163 (2017). https://doi.org/10.1007/s00003-017-1107-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-017-1107-z