Abstract
Traumatic brain injury (TBI) is a leading cause of disability worldwide, triggering chronic neurodegeneration underlying cognitive and mood disorder still without therapeutic prospects. Based on our previous observations that guanosine (GUO) attenuates short-term neurochemical alterations caused by TBI, this study investigated the effects of chronical GUO treatment in behavioral, molecular, and morphological disturbances 21 days after trauma. Rats subject to TBI displayed mood (anxiety-like) and memory dysfunction. This was accompanied by a decreased expression of both synaptic (synaptophysin) and plasticity proteins (BDNF and CREB), a loss of cresyl violet-stained neurons, and increased astrogliosis and microgliosis in the hippocampus. Notably, chronic GUO treatment (7.5 mg/kg i.p. daily starting 1 h after TBI) prevented all these TBI-induced long-term behavioral, neurochemical, and morphological modifications. This neuroprotective effect of GUO was abrogated in the presence of the adenosine A1 receptor antagonist DPCPX (1 mg/kg) but unaltered by the adenosine A2A receptor antagonist SCH58261 (0.05 mg/kg). These findings show that a chronic GUO treatment prevents the long-term mood and memory dysfunction triggered by TBI, which involves adenosinergic receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) constitutes a major cause of mortality and morbidity; it involves a primary mechanical insult that initiates a cascade of secondary injuries eventually leading to long-term cellular and behavioral deficits [1, 2]. The secondary injury involves the abrupt depolarization of neurons and massive release of glutamate resulting in glutamate excitotoxicity, mitochondrial dysfunction, inflammatory events, and neuronal cells loss [2, 3], which underlie long-term cognitive and psychiatric complications [1, 2]. Indeed, the vulnerability of the hippocampus, with deficits of synaptic plasticity [4, 5], provides an explanation for the high prevalence of cognitive deficits following TBI [6], which are aggravated over days to weeks by astrocytic scars and microglia-related neuroinflammation [2, 7,8,9].
There are currently few therapeutic options to mitigate the long-term consequences of TBI since most drugs failed translation to clinics, probably as a result of a single mechanism of action [10]. Targeting the purinergic modulation system may offer a unique benefit in view of its multi-target neuroprotective effects in neurons and glia in different models of neurodegenerative diseases [11]. Among the several purinergic signals, guanosine (GUO) has been less seldom explored, although it affords long-term benefits to control brain neurodegeneration [12, 13] as well as mood [14,15,16] and memory impairments [17,18,19]. Additionally, we have previously shown that GUO prevents several short-term TBI-induced modifications [3, 20], but it is currently unknown if a chronic GUO treatment might be effective to control TBI-induced long-term alterations.
A major open question is the mechanism of GUO-mediated neuroprotection, since no membrane receptor for GUO has been identified [12, 13]. The similarity of GUO and adenosine has entrained the hypothesis that GUO could act as a modulator of adenosine receptors, namely A1 and A2A receptors [14, 21, 22]. These two main adenosine receptors in the brain are well established to be involved in the initiation and spreading of degeneration following different insults to the brain [23, 24].
We now used a moderate fluid percussion injury (FPI) affecting the hippocampus to explore the impact of chronic GUO treatment on the long-term behavioral, neurochemical, and morphological alterations following TBI and further tested if the main adenosine receptors in the brain would be involved in the effects of GUO.
Materials and Methods
This manuscript was written in accordance with the ARRIVE guidelines.
Animals
Male adult Wistar rats (n = 166; 120 days old; 280–320 g) were obtained from our animal house colony. The animals were housed in groups of four animals per cage (polypropylene, 41 × 34 × 16 cm L × W × H, 1394 cm2) with the floor covered with autoclaved shavings under controlled conditions (12:12-h light-dark cycle, lights turned on at 07:00 a.m.; 22 ± 2 °C; 45–65% relative humidity) on a ventilated rack with access to water and food (Puro Lab 22 PB) ad libitum. All animals were acclimatized to laboratory conditions for 1 week before the experiments. This study was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Maria, Brazil (Permit Number: 153/2014) and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Ethics Committee imposed the selection of representative drug concentrations, based on our previous experience in using all tested drugs, to reduce the number of animals used in the experiments, while making all efforts to minimize animal suffering.
Chemicals
The following drugs were used: guanosine (GUO) dissolved in 0.9% saline; 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; adenosine A1 receptor antagonist) and 2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1, 2, 4]triazolo[1,5-c]pyrimidin-5-amine (SCH58261; adenosine A2A receptor antagonist) dissolved in saline with 0.1% DMSO (Sigma, St. Louis, MO, USA). These drugs were administered intraperitoneally (i.p.) at a constant volume of 1 ml/kg. Trizol® reagent, iScript™ cDNA synthesis Kit (Biorad®, Hercules, CA, USA), and SYBR® Select Master Mix were from Thermoscientific® (Waltham, MA, USA). All other chemicals of analytical grade were obtained from standard commercial suppliers.
Experimental Design
The animals were submitted to a surgical procedure to fix the TBI cannula in the right hemisphere and 24 h after the TBI was performed. To test the neuroprotective effect of GUO, rats were randomly divided into four groups: sham-vehicle, sham-GUO, TBI-vehicle, and TBI-GUO. Sham rats underwent all procedures identical to TBI rats, with the exception of the fluid percussion injury (FPI). The dose of GUO was chosen based on previous in vivo studies of excitotoxicity, which demonstrated an optimal effect of guanosine at 7.5 mg/kg i.p. [3, 20]. Rats received a single dose of GUO or vehicle per day and the first administration was performed 1 h after TBI.
To assess the involvement of the adenosine modulation system in the neuroprotective effects of GUO, rats were divided in four groups: sham-vehicle, TBI-DPCPX, sham-DPCPX-GUO and TBI-DPCPX-GUO to test A1 receptors and sham-vehicle, TBI-SCH 58261, sham-SCH 58261-GUO and TBI-SCH 58261-GUO to test A2A receptors. Rats received vehicle or doses previously validated as effective [25, 26] of DPCPX (1 mg/kg) or SCH 58261 (0.05 mg/kg) every day (i.p.) 30 min before GUO. The analysis of all behavioral data showed that the SCH58261 and DPCPX administration did not demonstrate per se effect (not shown; parallel experiments). In order to use a minimum number of animals, the same rats of sham-vehicle group were used for both different adenosine antagonist’s treatments at the same time. Drug treatments lasted 21 days after TBI (Fig. 1).
Experimental procedure. In the period of pharmacological intervention, the GUO (7.5 mg/kg) treatment started 1 h after TBI and continued during the 21 days of the experimental protocol. The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. The short-term memory (STM) and long-term memory (LTM) was performed by object recognition
After behavioral analysis, a part of the animals under deep anesthesia (thiopental sodium, 100 mg/kg i.p.) was perfused for the histochemistry and immunohistochemistry assays (n = 7 per group). For qPCR analysis (n = 5 per group), the animals were euthanized by decapitation to quickly dissect the ipsilateral hippocampus on an ice-cold Petri dish.
Surgical Procedure and Induction of TBI
The model of FPI was carried out as previously described [27] with minor modifications. In brief, rats were anesthetized with a single i.p. injection of Equithesin (4 ml/kg), a mixture containing sodium pentobarbital (58 mg/kg), chloral hydrate (60 mg/kg), magnesium sulfate (127.2 mg/kg), propylene glycol (42.8%), and absolute ethanol (11.6%) and placed in a rodent stereotaxic apparatus. A 3-mm-diameter craniotomy was drilled on the right convexity, 2 mm posterior to the bregma and 3 mm lateral to the midline, ensuring that the dura mater remained intact. A plastic injury cannula was fixed with cyanoacrylate, stabilized with dental cement. When dental cement has hardened, the cannula was filled with chloramphenicol, closed with a proper plastic cap, and the animal removed from the stereotaxic device and returned to its home cage. After 24 h, animals were anesthetized (isoflurane, 1% inhaled) and the injury cannula was attached to the fluid percussion device developed in our laboratory. During surgery and FPI, body temperature (37 °C) was monitored rectally and maintained with a heating pad and an overhead incandescent bulb. A brief (10–15 ms) transient pressure fluid pulse (1.55 ± 0.09 atm) was applied against the exposed dura in the right hemisphere. Pressure pulses were measured extracranially by a transducer (fluid control hydraulic automation, Belo Horizonte, MG, Brazil) and recorded on a storage oscilloscope (Tektronix TDS 210, Beaverton, OR, USA). The fluid percussion caused apnea (30–70 s), unconsciousness (7–10 min) measured through the righting reflex restoration [28]. Overall mortality after TBI was 20.48% (34 out of 167 animals). Twenty-five of these animals died before restoring consciousness, and ten animals (six TBI-saline and three TBI-guanosine animals) died in their home cages before the end of the protocol [7, 28]. Sham animals underwent an identical procedure, with the exception of the FPI.
Behavioral Task
Locomotor Behavior
Distance, speed, and rearing were measured 4 min a day for 4 days, to avoid the possible interferences on locomotion caused by the tested drugs [29, 30]. These parameters were analyzed in an activity-monitoring chamber with 50 × 48 × 50 cm (Insight Ltda, Ribeirão Preto, SP, Brazil).
Elevated Plus-Maze
To evaluate anxiety, rats were exposed to an elevated plus maze, as previously described [31] to measure (a) the time spent in the open arms relative to the total time spent in the plus-maze, expressed as percentage; (b) the number of entries into the open arms; and (c) the number of entries into the closed arms. These parameters were defined as placing all four paws within the boundaries of the arm. The sessions lasted for 5 min, and after each trial, the maze was cleaned with an ethanol solution (20%).
Inhibitory Avoidance Task
The step-down inhibitory avoidance task (IA) has been used to evaluate aversive memory, as we have previously described [32]. Rats were trained using a 50 × 25 × 25-cm plexiglass box with a 5-cm-high, 8-cm-wide, and 25-cm-long platform on the left end of a series of bronze bars which made up the floor of the box. For the IA training session (acquisition), rats were gently placed on the platform facing the left rear corner of the training box. When they stepped down and placed their four paws on the grid, a foot shock was delivered (2 s, 0.5 mA). The retention trial (test) was performed 24 h after training. Each rat was placed again on the platform, and the transfer latency time (i.e., time took to step down from the platform) was measured in the same way as in the acquisition trial, but foot shock was not delivered and the transfer latency time was recorded to a ceiling of 600 s. The criterion for learning was taken as an increase in the transfer latency time on retention (second) trial as compared to the acquisition (first) trial. So, short transfer latencies indicate poor retention. To avoid confounds by lingering olfactory stimuli, the arena was cleaned with 20% ethanol after each animal was tested.
Object Recognition Memory Task
Recognition memory was measured using the object recognition task [33]. Rats first underwent a training session in which they were exposed to two identical objects (duple Lego toys) for 5 min. After 90 min (short-term memory; STM) or 24 h (long-term memory; LTM), two dissimilar objects were presented (a familiar and a novel one), and we measured the time spent exploring each object during 5 min, as previously described. A recognition index calculated for each animal in STM task was expressed by the ratio TB/(TA + TB) [TA = time spent exploring the familiar object A; TB = time spent exploring the novel object B] and in LTM task was expressed by the ratio TC/(TA + TC) [TA = time spent exploring the familiar object A; TC = time spent exploring the novel object C]. The experiments were performed by a blind observer for the treatment of animals. To avoid confounds by lingering olfactory stimuli and preferences, the objects and the arena were cleaned with 20% ethanol after each animal was tested. Exploration was defined as sniffing or touching the objects with the nose and/or forepaws. Sitting on or turning around the objects was not considered exploratory behaviors.
Ex Vivo Analysis
Analysis of mRNA Expression
Total RNA was isolated from hippocampus using Trizol® reagent (Invitrogen®, Carlsbad, CA, USA) immediately after euthanasia. Conversion of total RNA to cDNA was performed with the iScript™ cDNA synthesis Kit (Biorad®), accordingly to the manufacturer’s instructions. Quantitative real-time PCR was performed in 20 μl PCR mixture containing 1 μl of cDNA products as template and a SYBR® Select Master Mix (Applied Biosystems, Forster City, CA, USA). Gene-specific primer sequences were based on published sequences in GenBank Overview (http://www.ncbi.nlm.nih.gov/genbank/) designed with Primer3 program version 0.4.0 (http://frodo.wi.mit.edu/primer3/) and custom made by Invitrogen® (Table 1). The mixtures were heated at 95 °C for 5 min followed of 40 cycles of 15 s at 95 °C, 15 s at annealing temperature appropriated to each primer sequence, and 25 s at 72 °C for extension in a Thermocycler StepOne Plus (Applied Biosystems, Foster City, CA, USA). All samples were analyzed as quadruplicates with a non-template control also included. Samples were quantified using the ΔΔCq method [34], with tubulin and GAPDH serving as reference genes, according the MIQE guidelines [35].
Histochemistry and Immunohistochemistry
For the brain fixation, animals under deep anesthesia (sodium thiopental, 100 mg/kg i.p.) were transcardially perfused. The heart was exposed and, after clamping the descending aorta, a catheter was inserted in the ascending aorta. The animal was then perfused with 600 mL of heparinized saline while opening the right atria to allow the outflow of the perfusate. Rats were then perfused with 600 mL of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) at pH 7.4. The brains were removed and immersed in the same fixative overnight and then kept in 30% sucrose in physiological saline (0.9% NaCl) for at least 48 h before sectioning, as previously described [36]. The dorsal hippocampus of frozen brains was sectioned (30 μm coronal slices) with a Leica CM1850 cryostat (Leica Microsystems, Wetzlar, Germany) and hippocampal sections were mounted on slides coated with 2% gelatin with 0.08% chromalin and stored at − 20 °C until use. Neuronal morphology in dorsal hippocampal sections was evaluated by cresyl violet staining of Nissl bodies, as previously described [31, 36]. Briefly, sections were incubated for 10 min with cresyl violet (Sigma-Aldrich) solution (0.5% in acetate buffer). Sections were then washed twice with acetate buffer, twice in 100% ethanol, cleared with xylene, and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). The number of cresyl violet positive neurons in the CA1 region was determined using a light microscope (Leica DM 1000) to collect computerized image analyzed with NIH ImageJ software to count the number of cells with round, obvious nuclei and visible nucleoli in eight sections per rat.
Immunohistochemical detection of Iba-1 (a marker of reactive microglia) and of glial fibrillary acidic protein (GFAP) (a marker of astrocytes) was performed to gauge microgliosis and astrogliosis, as previously described [37]. After washing, the sections were permeabilized and blocked before incubation with the anti-Iba-1 rabbit polyclonal antibody (1:1000; 019-19741; RRID: AB_839504; Wako Chemicals, Richmond, VA, USA) and anti-GFAP mouse monoclonal antibody (1:1000; ab4648; RRID: AB_449329; Abcam, Inc., Cambridge, MA, USA) for 48 h at 4 °C. Slices were subsequently incubated with goat anti-mouse or goat anti-rat secondary antibody conjugated with a fluorophore (Alexa Fluor 488; Alexa Fluor 525; Invitrogen) (1:500) for 2 h at room temperature. The mean fluorescence intensities of GFAP and Iba-1 were then semi-quantitatively determined using NIH ImageJ software in images obtained with a Leica (TCS SP5 II) microscope.
Statistical Analysis
The normality of the data was analyzed using the D’Agostino and Pearson’s omnibus normality test. Data are mean ± SEM except for the inhibitory avoidance memory where data are expressed as median ± interquartile range. Data were analyzed by two-way analysis of variance (ANOVA) followed by post hoc comparisons using Newman-Keuls multiple test, except inhibitory avoidance memory test, which were analyzed by unpaired t test and a Scheirer-Ray-Hare test (an extension of the Kruskal–Wallis test) followed by Mann-Whitney post hoc test. Differences between groups were considered statistically significant when p < 0.05.
Results
Locomotor Activity
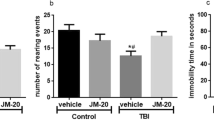
Locomotor activity was evaluated during 4 days (6–9th day of protocol; Fig. 1) to discard its interference in the behavioral of animals. We did not observe alterations in the pattern of locomotion, in particular distance (Fig. 2c) and rearing (Fig. 2d), between sham and TBI animals at any day of analysis.
Prevention by Guanosine of TBI-Induced Anxiety Involving Adenosinergic Receptors
The percentage of time spent in the open arms (F(7,88) = 10.54; p < 0.05; Fig. 3a) and the number of entries in the closed arms (F(7,88) = 6.45; p < 0.05; Fig. 3c) of the plus maze were significantly altered in TBI as compared to sham animals. The treatment with GUO (7.5 mg/kg) inhibited the anxiogenic effect of TBI, as testified by the higher percentage of time spent in the open arms (F(7,88) = 8.02; p < 0.05, Fig. 3a) and lower number of entries in the closed arms (F(7,88) = 8.24; p < 0.05, Fig. 3c). Notably, the administration of the A1 receptor antagonist DPCPX (1 mg/kg) inhibited this anxiolytic-like effect of GUO (F(7,88) = 8.80; p < 0.05, Fig. 3a and F(7,88) = 7.12; p < 0.05, Fig. 3c, respectively) and was devoid of effects in the behavior of animals subjected to TBI. Interestingly, it was demonstrated that the administration of A2A receptor antagonist SCH58261 (0.05 mg/kg) also attenuated the anxiogenic effect of TBI, by the higher percentage of time spent in the open arms (F(7,88) = 4.29; p < 0.05, Fig. 3a) and lower number of entries in the closed arms (F(7,88) = 7.23; p < 0.05, Fig. 3c). The administration of the A2A receptor antagonist SCH58261 did not affect the anxiolytic-like effect of GUO. Indeed, it showed a similar effect displayed by GUO treatment, with higher percentage of time spent in the open arms (Fig. 3a) and lower number of entries in the closed arms (Fig. 3c). This indicates an ability of GUO to attenuate the impact of TBI on anxiety, which seems to involve modulation of adenosine receptors. No differences were observed in the number of entries in open arms (Fig. 3b).
Prevention by guanosine of TBI-induced anxiety involving adenosinergic receptors. Time spent in open arms (a), number of entries in open arms (b), and in closed arms (c) were evaluated in elevated plus maze test (14 days after TBI). The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. Data were expressed as mean ± S.E.M. (n = 12) and analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups
Prevention by Guanosine of TBI-Induced Memory Deterioration Involving Adenosinergic Receptors
The training in the step-down inhibitory avoidance task did not show differences between the groups (Fig. 4a). Analysis of aversive memory revealed a significant decrease on learning of rats submitted to TBI (F(7,88) = 6.45; p < 0.05; Fig. 4b) and GUO treatment avoided this impairment (F(7,88) = 14.41; p < 0.05; Fig. 4b) on retention time. Notably, the administration of DPCPX attenuated this anti-amnesic-like effect of GUO (F(7,88) = 4.96; p < 0.05, Fig. 4b). Interestingly, SCH58261 (0.05 mg/kg) also attenuated the amnesic effect of TBI (F(7,88) = 4,15; p < 0.05, Fig. 4a) on retention time. The administration of this antagonist did not affect anti-amnesic-like effect of GUO (Fig. 4b). This indicates an ability of GUO to attenuate the deterioration of aversive memory after TBI.
Prevention by guanosine of TBI-induced memory deterioration involving adenosinergic receptors. The aversive memory was evaluated by inhibitory test. The training trials required to reach the acquisition criterion (a) and retention (test) (b) were evaluated at 15° and 16° days respectively after TBI. Data were presented as median and interquartile ranges, (n = 12) per group and analyzed by Scheirer-Ray-Hare test followed by Mann–Whitney post hoc test when appropriated. The work memory was evaluated by object recognition test. Training (c) and short- (d) and long-term memory (e) were performed at 20° and 21° days after TBI. Data were expressed as mean ± S.E.M. (n = 12) and analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. In both memory tests, the modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups
Different parameters of memory can be observed as the functional working memory, in which the animals have a tendency to avoid areas that they have just been and explore novel areas or objects. We first established that none of the treatments affected the exploration of objects during the training section (Fig. 4c). However, TBI significantly impaired both the short-term (F(7,88) = 7.66; p < 0.05; Fig. 4d) and long-term object recognition memory tasks (F(7,88) = 7.34; p < 0.05; Fig. 4e), since TBI animals displayed a lower exploration of a novel object in comparison to sham animals. GUO treatment attenuated this TBI-induced impairment of both short- and longer-term memory tasks (F(7,88) = 4.72; p < 0.05; Fig. 4d and F(7,88) = 4.57; 4E, respectively). The administration of DPCPX blocked the protective effect of GUO in short- (F(7,88) = 9.86; p < 0.05; Fig. 4d) and longer-term recognition memory (F(7,88) = 6.98; p < 0.05; Fig. 4e) in TBI-induced animals.
Newsworthy, SCH58261 (0.05 mg/kg) also reduced the TBI-induced impairment of both short- and longer-term memory tasks (F(7,88) = 4.06; p < 0.05; Fig. 4d and F (7,88) = 4.19; Fig. 4e, respectively). Moreover, SCH58261 did not modify the effects displayed by GUO (Fig. 4d and 4e, respectively). These results reinforce the importance of adenosinergic system in GUO effects.
Ex Vivo Analysis
Prevention by Guanosine of TBI-Induced Decrease of Plasticity Genes (BDNF and CREB) and Synaptic Markers (Synaptophysin) Involving Adenosinergic Receptors
BDNF (brain-derived neurotrophic factor) and CREB (cyclic AMP response element-binding protein) are major players in the regulation of functional and structural neuroplasticity underlying hippocampal-dependent memory [38]. In accordance with the deterioration of plasticity, TBI decreased the expression in the hippocampus of both BDNF (F(1,32) = 19.23; p < 0.05; Fig. 5a) and CREB (F(1,32) = 6.22; p < 0.05; Fig. 5b). Treatment with GUO fully prevented this TBI-induced alteration of BDNF (F(1,32) = 8.47; p < 0.05; Fig. 5a) and CREB expression (F(1,32) = 8.43; p < 0.05; Fig. 5b). As was observed for the alterations of memory, DPCPX administration prevented the effects of GUO on TBI-induced decrease of BDNF and CREB gene expression (F(1,32) = 12.42; p < 0.05, Fig. 5a and F(1,32) = 4.50 p < 0.05, Fig. 5b, respectively), confirming the involvement of A1 receptors in the neuroprotective effects of GUO. The SCH58261 administration also prevented this TBI-induced alteration of BDNF (F(1,32) = 10.1; p < 0.05; Fig. 5a) and CREB expression (F(1,32) = 5.77; p < 0.05; Fig. 5b) and did not alter effects of GUO treatment BDNF (Fig. 5a) and CREB (Fig. 5b) gene expression.
Prevention by guanosine of TBI-induced decrease of plasticity genes (BDNF and CREB) and synaptic markers (synaptophysin) involving adenosinergic receptors. Expression of BDNF (a), CREB (b), synaptophysin (c), and GAP-43 (d) were evaluated in qPCR test (21 days after TBI). The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. Data are expressed as mean ± S.E.M. (n = 5) and were analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups
Memory impairment is also associated with a synaptotoxicity typified by altered synaptic organization and expression of synaptic proteins [39]. Thus, we investigated the impact of TBI on the expression of two synaptic proteins—a constitutive protein, synaptophysin, and a synaptic protein associated with synaptic regeneration, growth associated protein 43 (GAP-43). Compared to sham rats, TBI led to a decreased expression of synaptophysin in the hippocampus (F(1,32) = 6.92 p < 0.05, Fig. 5c), whereas no difference in the expression of GAP-43 was found 21 days after traumatic injury (Fig. 5d). Treatment with GUO prevented the TBI-induced decrease of synaptophysin gene expression (F(1,32) = 6.29 p < 0.05, Fig. 5c) and this synapto-protective effect of GUO was blocked in presence of DPCPX (F(1,32) = 7.53; p < 0.05, Fig. 5c) but unaltered in the presence of SCH 58261 (Fig. 5c).
Prevention by Guanosine of TBI-Induced Neuronal Loss in the Hippocampus (CA1)
We assessed neuronal loss by histological analysis of coronal sections collected at 21 days post-injury and stained with cresyl violet. We observed a significant neuronal loss in the CA1 hippocampal regions 21 days after TBI (F(7,48) = 5.81 p < 0.05, Fig. 6) as compared to sham. Treatment with GUO reduced this TBI-induced hippocampal neurodegeneration (F(7,48) = 5.17 p < 0.05, Fig. 6) and this effect of GUO was prevented in the presence of DPCPX (F(7,48) = 7.03 p < 0.05, Fig. 6). However, SCH 58261 administration also diminished this TBI-induced hippocampal neurodegeneration (F(7,48) = 4.27 p < 0.05, Fig. 6) and did not alter the effects of GUO treatment (Fig. 6).
Prevention by guanosine of TBI-induced neuronal loss in the hippocampus (CA1). Representative images (a) and number of neuronal cells (b) were performed using cresyl violet (21 days after TBI). The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. Data are expressed as mean ± S.E.M. (n = 7) and were analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups. Scales 50× and 200×
Prevention by Guanosine of TBI-Induced Astrogliosis and Microglial Activation in the Hippocampus (CA1)
The analysis of GFAP immunoreactivity at 21 days post-TBI injury revealed a significant larger astrocytosis in the hippocampal CA1 region after TBI compared to sham (F(7,48) = 11.63 p < 0.05, Fig. 7). Treatment with GUO prevented astrogliosis (F(7,48) = 7.77 p < 0.05, Fig. 7) and the administration of DPCPX blocked this glioprotective effect (F(7,48) = 8.38 p < 0.05, Fig. 7).
Prevention by guanosine of TBI-induced astrogliosis in the hippocampus (CA1). The representative images of astrogliosis (a; scale 200 μm) and (b; scale 25 μm) are demonstrated as the expression of reactive astrocytes performed by GFAP fluorescence intensity (c; 21 days after TBI). The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. The mean fluorescence intensity (F.I.) of GFAP was semi-quantitatively determined using NIH ImageJ software. Data are expressed as mean ± S.E.M. (n = 7) and were analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups
We also assessed microgliosis at 21 days post-TBI injury. IBA-1 immunoreactivity in the hippocampal CA1 region was significantly larger 21 days after TBI compared to sham (F(7,48) = 13.18 p < 0.05, Fig. 8). Treatment with GUO prevented microgliosis (F(7,48) = 7.75 p < 0.05, Fig. 8) and DPCPX administration blocked this glioprotective effect (F(7,48) = 10.16 p < 0.05, Fig. 8). In accordance with the results above, SCH 58261 administration prevented both astrogliosis (F(7,48) = 5.11; p < 0.05; Fig. 7) and microglial activation (F(7,48) = 4.95; p < 0.05; Fig. 8) 21 days post-TBI injury as well as did not modify the effects of GUO treatment (Figs. 7 and 8, respectively).
Prevention by guanosine of TBI-induced microglial activation in the hippocampus (CA1). The representative images of microglial activation cells (a; scale 200 μm) and (b; scale 25 μm) are demonstrated as the expression of microglial activation performed by IBA-1 fluorescence intensity (c; 21 days after TBI). The modulation of adenosinergic system by GUO was evaluated through the administration of DPCPX (1 mg/kg) and SCH 58261 (0.05 mg/kg) 30 min before GUO. The mean fluorescence intensities (F.I.) of IBA-1 were semi-quantitatively determined using NIH ImageJ software. Data are expressed as mean ± S.E.M. (n = 7) and were analyzed by two-way ANOVA, followed by Newman–Keuls test when appropriated. Differences were considered significant (*p < 0.05) when compared to sham and TBI groups
Discussion
The present study shows that a daily treatment with guanosine (GUO) beginning after moderate percussion brain injury attenuates the long-term development of anxiety and memory deficits. This probably involves an ability of GUO to control aberrant plasticity at excitatory synapses since GUO prevented the TBI-induced long-term reduction of the expression of a synaptic marker (synaptophysin) and of genes associated with synaptic plasticity, namely BDNF and CREB. Additionally, GUO also attenuated TBI-induced long-term astrogliosis and microgliosis that compromise synaptic function and negatively impact on mood and memory [8, 40, 41]. Finally, we concluded that this neuroprotective effect of GUO required functional adenosine receptors.
The traumatic event triggering TBI initiates a cascade of excitotoxic events leading to long-term cellular and behavioral deficits, among which stem mood and memory deficits [1, 2]. There are currently no effective strategies to mitigate these long-term sequelae of TBI, probably because most candidate strategies mostly target one of the multiple excitotoxic cascades engaged in this pathology [10]. Our previous observations that GUO attenuated at least two of the excitotoxic processes engaged in TBI, namely the glutamatergic system and mitochondrial function [3, 20], prompted testing if GUO would dampen chronic neurological abnormalities associated with TBI, such as cognitive deficits and emotional disturbances [30, 42]. The present results confirmed that our model of fluid percussion brain injury caused a long-term development of anxiety as well as of memory impairment, as reported for different animal models of TBI [8, 43, 44]. Most importantly, we found that the GUO treatment after FPI was effective to reduce these delayed anxiety-like and reference memory deficits. This is in agreement with the previously reported ability of GUO to interfere with memory deficits [16,17,18,19] as well as with the anxiolytic-like effects of GUO and its derivatives in rodents [14, 15], which also produces anti-depressive-like effects in predictive tasks [13,14,15], the main comorbidity of anxiety.
The mechanism underlying this ability of GUO to attenuate the long-term mood and memory dysfunction after TBI still needs to be defined. The present study unravels the possibility that GUO might control the synaptic dysfunction that has been documented to underlie both mood dysfunction as well as memory impairments [39, 45]. Indeed, we now show that GUO prevented the TBI-induced decrease of the expression in the hippocampus of two genes that are paramount to sustain plastic changes, namely BDNF and CREB. Accordingly, previous studies documented the ability of GUO to control BDNF levels in a different functional context [46]. Furthermore, GUO also prevented the TBI-induced decrease of the expression of a well-established synaptic marker, synaptophysin [47], in agreement with the previously reported ability of GUO to control neurite outgrowth in PC12 cell [48] and to induce synaptogenesis in the adult brain [49]. Notably, GUO failed to affect the expression of GAP-43, which is more associated with the repair of synapses [50]. This suggests that GUO might attenuate hippocampal synaptotoxicity, which underlies memory impairment in different neurodegenerative disorders [39, 45].
Synaptic function is also critically dependent on astrocytic support [41, 51] and on microglia reactivity [40, 52] and, accordingly, both astrogliosis and microgliosis have been proposed as alternative or concurrent mechanisms to explain the deterioration of memory performance as well as of mood alterations upon TBI [8]. This contention is in agreement with the presently observed long-term modification of the morphology of astrocytes and of microglia in the hippocampus. Furthermore, we observed that GUO attenuated this TBI-induced gliosis, in accordance with previous demonstrations of protective effects of GUO on astrocytes [53, 54] and on microglia reactivity [55].
A major conclusion of the present study is the requirement of adenosine A1 receptors, but it cannot be discarded the involvement of adenosine A2A receptors for the long-term neuroprotection afforded by GUO. The SCH 58621 has showed strong neuroprotective effects against TBI and demonstrated the importance of the adenosinergic system in the progression of this pathology. Previous studies had already documented an ability of caffeine and adenosine receptor antagonists to be associated with neuroprotective effects of GUO, in conditions which the effects of GUO appear to be attenuated by adenosine receptor antagonists [13, 14, 21, 56]. In some instance, a preferential involvement of A2A receptors was reported [21], whereas in others, A1 receptors were selectively associated with the neuroprotective effects of GUO [14, 56]. Likewise, the mitogenic effects of GUO [57] or the impact on GUO on the synaptosomal K+-stimulated glutamate release [14] was partially dampened upon blockade of A1 receptors and unaffected by manipulation of A2A receptors. This dependency on A1 rather than A2A receptors is somewhat surprising since A2A receptor blockade affords a robust neuroprotection against different noxious brain insults [23], whereas the blockade of A1 receptors has paradoxical effects on neuroprotection [24] and a continuous activation (or over-expression) of A1 receptors has deleterious effects on mood and synaptic plasticity [43, 58]. In spite of the presently concluded critical requirement for A1 receptors for the neuroprotective effects of GUO, the exact relation between GUO and A1 receptor function still remains to be elucidated. In fact, it cannot be excluded that GUO might directly interact with A1 receptors, eventually as an allosteric modulator [59] since GUO binds with low affinity to adenosine receptors [60] and GUO binding is unaffected by caffeine [61]; another possibility might be an interaction with A1 receptor-containing heteromers, such as A1-A2A heteromers [21, 62] or A1-P2Y1 heteromers [63, 64]; alternatively, it is also possible that GUO might bolster the extracellular levels of adenosine through an increased outflow or inhibition of reuptake through nucleoside transporters (Fig. 9) [57, 65].
Schematic illustration of chronic guanosine treatment against neurotoxicity induced by TBI in rats. TBI induced a secondary insult (e.g., glutamate excitotoxicity and uptake impairment) (1); neuron death (2); astrogliosis (3); microglial activation; and (4) behavioral impairment. Guanosine (GUO) was able to block the injuries caused by the trauma through the modulation of purinergic system. The signal (?) indicates a possible mechanism of action involved. (A1, P2 receptors; A1/A2A, A1/P2Y1 receptors oligomerization)
The literature data describes that the glutamatergic system regulation by GUO can involve the modulation of A1 or even a putative oligomeric interaction resulting in A1R activation and A2AR blockage [21, 66] and the consequent activation of the PI3K/Akt and MAPK signaling cascades [21]. In agreement, these signaling pathways have been associated with the regulation of astrocytic glutamate transporters [13, 67, 68].
Neuroprotection event caused by GUO is believed to result from the inhibition of the secondary TBI cascade mechanisms (glutamatergic excitotoxicity, mitochondrial dysfunction, calcium homeostasis disruption, oxidative stress, inflammatory process and neuron death) as previously demonstrated by our group [3, 20]. These events im\plicate in the decrease of cellular damage (neuron death, astrogliosys and microglia activation), which is correlated to synaptic plasticity (expression of BDNF and CREB) and learning/memory (Fig. 9).
In summary, we report an ability of GUO to prevent the delayed anxiety and memory deficits following TBI, which might result from the pleiotropic impact of GUO on the expression of genes governing synaptic plasticity, on processes of synaptotoxicity, and on the control of astrogliosis and microgliosis in a manner involving adenosine A1 and A2A receptors (Fig. 9). Altogether, these findings posit GUO as a candidate post-traumatic therapy to alleviate the long-term consequences of TBI.
References
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurology 7(8):728–741. https://doi.org/10.1016/S1474-4422(08)70164-9
Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci 5:29. https://doi.org/10.3389/fnagi.2013.00029
Dobrachinski F, Gerbatin RD, Sartori G, Marques NF, Zemolin AP, Silva LF, Franco JL, Royes LF et al (2017) Regulation of mitochondrial function and glutamatergic system are the target of guanosine effect in traumatic brain injury. J Neurotrauma 34:1318–1328. https://doi.org/10.1089/neu.2016.4563
Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ (2000) Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res 861(1):69–76
Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS (2006) Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 16(6):541–550. https://doi.org/10.1002/hipo.20183
Scheff SW, Baldwin SA, Brown RW, Kraemer PJ (1997) Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma 14(9):615–627. https://doi.org/10.1089/neu.1997.14.615
Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI (2010) Fluid-percussion-induced traumatic brain injury model in rats. Nat Protoc 5(9):1552–1563. https://doi.org/10.1038/nprot.2010.112
Sajja VS, Hlavac N, VandeVord PJ (2016) Role of glia in memory deficits following traumatic brain injury: biomarkers of glia dysfunction. Front Integr Neurosci 10:7. https://doi.org/10.3389/fnint.2016.00007
Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP (2012) Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma 29(2):281–294. https://doi.org/10.1089/neu.2011.2123
McConeghy KW, Hatton J, Hughes L, Cook AM (2012) A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26(7):613–636. https://doi.org/10.2165/11634020-000000000-00000
Rodrigues RJ, Tome AR, Cunha RA (2015) ATP as a multi-target danger signal in the brain. Front Neurosci 9:148. https://doi.org/10.3389/fnins.2015.00148
Di Liberto V, Mudo G, Garozzo R, Frinchi M, Fernandez-Duenas V, Di Iorio P, Ciccarelli R, Caciagli F et al (2016) The guanine-based purinergic system: the tale of an orphan neuromodulation. Front Pharmacol 7:158. https://doi.org/10.3389/fphar.2016.00158
Bettio LE, Gil-Mohapel J, Rodrigues AL (2016) Guanosine and its role in neuropathologies. Purinergic Signal 12(3):411–426. https://doi.org/10.1007/s11302-016-9509-4
Almeida RF, Comasseto DD, Ramos DB, Hansel G, Zimmer ER, Loureiro SO, Ganzella M, Souza DO (2017) Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol 54(1):423–436. https://doi.org/10.1007/s12035-015-9660-x
Bettio LE, Neis VB, Pazini FL, Brocardo PS, Patten AR, Gil-Mohapel J, Christie BR, Rodrigues AL (2016) The antidepressant-like effect of chronic guanosine treatment is associated with increased hippocampal neuronal differentiation. Eur J Neurosci 43(8):1006–1015. https://doi.org/10.1111/ejn.13172
Lanznaster D, Mack JM, Coelho V, Ganzella M, Almeida RF, Dal-Cim T, Hansel G, Zimmer ER et al (2017) Guanosine prevents anhedonic-like behavior and impairment in hippocampal glutamate transport following amyloid-beta1-40 administration in mice. Mol Neurobiol 54(7):5482–5496. https://doi.org/10.1007/s12035-016-0082-1
Ganzella M, de Oliveira ED, Comassetto DD, Cechetti F, Cereser VH Jr, Moreira JD, Hansel G, Almeida RF et al (2012) Effects of chronic guanosine treatment on hippocampal damage and cognitive impairment of rats submitted to chronic cerebral hypoperfusion. Neurol Sci 33(5):985–997. https://doi.org/10.1007/s10072-011-0872-1
Paniz LG, Calcagnotto ME, Pandolfo P, Machado DG, Santos GF, Hansel G, Almeida RF, Bruch RS et al (2014) Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab Brain Dis 29(3):645–654. https://doi.org/10.1007/s11011-014-9548-x
Petronilho F, Perico SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO et al (2012) Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 26(6):904–910. https://doi.org/10.1016/j.bbi.2012.03.007
Gerbatin RDR, Cassol G, Dobrachinski F, Ferreira APO, Quines CB, Pace IDD, Busanello GL, Gutierres JM et al (2017) Guanosine protects against traumatic brain injury-induced functional impairments and neuronal loss by modulating excitotoxicity, mitochondrial dysfunction, and inflammation. Mol Neurobiol 54(10):7585–7596. https://doi.org/10.1007/s12035-016-0238-z
Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, Lopez MG, Tasca CI (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126(4):437–450. https://doi.org/10.1111/jnc.12324
Oliveira KA, Dal-Cim TA, Lopes FG, Nedel CB, Tasca CI (2017) Guanosine promotes cytotoxicity via adenosine receptors and induces apoptosis in temozolomide-treated A172 glioma cells. Purinergic Signal 13(3):305–318. https://doi.org/10.1007/s11302-017-9562-7
Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem 139(6):1019–1055. https://doi.org/10.1111/jnc.13724
de Mendonca A, Sebastiao AM, Ribeiro JA (2000) Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev 33(2–3):258–274
Prediger RD, Batista LC, Takahashi RN (2005) Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging 26(6):957–964. https://doi.org/10.1016/j.neurobiolaging.2004.08.012
Simoes AP, Machado NJ, Goncalves N, Kaster MP, Simoes AT, Nunes A, Pereira de Almeida L, Goosens KA et al (2016) Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology 41(12):2862–2871. https://doi.org/10.1038/npp.2016.98
D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW (2004) Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 127(Pt 2):304–314. https://doi.org/10.1093/brain/awh038
Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK (2005) Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma 22(1):42–75. https://doi.org/10.1089/neu.2005.22.42
Halldner L, Aden U, Dahlberg V, Johansson B, Ledent C, Fredholm BB (2004) The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology 46(7):1008–1017. https://doi.org/10.1016/j.neuropharm.2004.01.014
El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM (2000) The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129(7):1465–1473. https://doi.org/10.1038/sj.bjp.0703170
Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Muller CE et al (2015) Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A 112(25):7833–7838. https://doi.org/10.1073/pnas.1423088112
Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR (2007) Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol 203(1):241–245. https://doi.org/10.1016/j.expneurol.2006.08.008
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1(3):1306–1311. https://doi.org/10.1038/nprot.2006.205
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Cunha GM, Canas PM, Oliveira CR, Cunha RA (2006) Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience 141(4):1775–1781. https://doi.org/10.1016/j.neuroscience.2006.05.024
Rebola N, Simoes AP, Canas PM, Tome AR, Andrade GM, Barry CE, Agostinho PM, Lynch MA et al (2011) Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 117(1):100–111. https://doi.org/10.1111/j.1471-4159.2011.07178.x
Benito E, Barco A (2010) CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33(5):230–240. https://doi.org/10.1016/j.tins.2010.02.001
Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM (2017) Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci 40(6):347–357. https://doi.org/10.1016/j.tins.2017.04.002
Rial D, Lemos C, Pinheiro H, Duarte JM, Goncalves FQ, Real JI, Prediger RD, Goncalves N et al (2015) Depression as a glial-based synaptic dysfunction. Front Cell Neurosci 9:521. https://doi.org/10.3389/fncel.2015.00521
Zorec R, Horvat A, Vardjan N, Verkhratsky A (2015) Memory formation shaped by astroglia. Front Integr Neurosci 9:56. https://doi.org/10.3389/fnint.2015.00056
Bramlett HM, Dietrich WD (2015) Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma 32(23):1834–1848. https://doi.org/10.1089/neu.2014.3352
Chen Z, Xiong C, Pancyr C, Stockwell J, Walz W, Cayabyab FS (2014) Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J Neurosci 34(29):9621–9643. https://doi.org/10.1523/JNEUROSCI.3991-13.2014
Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O'Brien TJ (2008) Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma 25(11):1367–1374. https://doi.org/10.1089/neu.2008.0641
Lepeta K, Lourenco MV, Schweitzer BC, Martino Adami PV, Banerjee P, Catuara-Solarz S, de La Fuente RM, Guillem AM et al (2016) Synaptopathies: synaptic dysfunction in neurological disorders - a review from students to students. J Neurochem 138(6):785–805. https://doi.org/10.1111/jnc.13713
Su C, Wang P, Jiang C, Ballerini P, Caciagli F, Rathbone MP, Jiang S (2013) Guanosine promotes proliferation of neural stem cells through cAMP-CREB pathway. J Biol Regul Homeost Agents 27(3):673–680
Masliah E, Terry R (1993) The role of synaptic proteins in the pathogenesis of disorders of the central nervous system. Brain Pathol 3(1):77–85
Gysbers JW, Rathbone MP (1996) GTP and guanosine synergistically enhance NGF-induced neurite outgrowth from PC12 cells. Int J Dev Neurosci 14(1):19–34
Gerrikagoitia I, Martinez-Millan L (2009) Guanosine-induced synaptogenesis in the adult brain in vivo. Anat Rec 292(12):1968–1975. https://doi.org/10.1002/ar.20999
Denny JB (2006) Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr Neuropharmacol 4(4):293–304
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81(4):728–739. https://doi.org/10.1016/j.neuron.2014.02.007
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME (2017) Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 595(6):1929–1945. https://doi.org/10.1113/JP272134
Giuliani P, Ballerini P, Buccella S, Ciccarelli R, Rathbone MP, Romano S, D'Alimonte I, Caciagli F et al (2015) Guanosine protects glial cells against 6-hydroxydopamine toxicity. Adv Exp Med Biol 837:23–33. https://doi.org/10.1007/5584_2014_73
Quincozes-Santos A, Bobermin LD, de Souza DG, Bellaver B, Goncalves CA, Souza DO (2013) Gliopreventive effects of guanosine against glucose deprivation in vitro. Purinergic Signal 9(4):643–654. https://doi.org/10.1007/s11302-013-9377-0
Hansel G, Tonon AC, Guella FL, Pettenuzzo LF, Duarte T, Duarte M, Oses JP, Achaval M et al (2015) Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol Neurobiol 52(3):1791–1803. https://doi.org/10.1007/s12035-014-8978-0
Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Tavares RG, Wofchuk ST, Elisabetsky E, Souza DO (2010) Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol 159(6):1247–1263. https://doi.org/10.1111/j.1476-5381.2009.00597.x
Ciccarelli R, Di Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP (2000) Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia 29(3):202–211
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A et al (2015) Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87(3):549–562. https://doi.org/10.1016/j.neuron.2015.07.010
Guo D, Venhorst SN, Massink A, van Veldhoven JP, Vauquelin G, IJzerman AP, Heitman LH (2014) Molecular mechanism of allosteric modulation at GPCRs: insight from a binding kinetics study at the human A1 adenosine receptor. Br J Pharmacol 171(23):5295–5312. https://doi.org/10.1111/bph.12836
Muller CE, Scior T (1993) Adenosine receptors and their modulators. Pharm Acta Helv 68(2):77–111
Traversa U, Bombi G, Camaioni E, Macchiarulo A, Costantino G, Palmieri C, Caciagli F, Pellicciari R (2003) Rat brain guanosine binding site. Biological studies and pseudo-receptor construction. Bioorg Med Chem 11(24):5417–5425
Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N et al (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26(7):2080–2087. https://doi.org/10.1523/JNEUROSCI.3574-05.2006
Ballerini P, Di Iorio P, Caciagli F, Rathbone MP, Jiang S, Nargi E, Buccella S, Giuliani P et al (2006) P2Y2 receptor up-regulation induced by guanosine or UTP in rat brain cultured astrocytes. Int J Immunopathol Pharmacol 19(2):293–308
Yoshioka K, Saitoh O, Nakata H (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci U S A 98(13):7617–7622. https://doi.org/10.1073/pnas.121587098
Jackson EK, Cheng D, Jackson TC, Verrier JD, Gillespie DG (2013) Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol 304(5):C406–C421. https://doi.org/10.1152/ajpcell.00212.2012
Bettio LE, Gil-Mohapel J, Rodrigues AL (2016) Guanosine and its role in neuropathologies. Purinergic Signal 12:411–426. https://doi.org/10.1007/s11302-016-9509-4
Matos M, Augusto E, Agostinho P, Cunha RA, Chen JF (2013) Antagonistic interaction between adenosine A2A receptors and Na+/K+-ATPase-alpha2 controlling glutamate uptake in astrocytes. J Neurosci 33(47):18492–18502. https://doi.org/10.1523/JNEUROSCI.1828-13.2013
Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H (2010) PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun 393(3):514–518. https://doi.org/10.1016/j.bbrc.2010.02.038
Acknowledgments
We are deeply thankful to Sofia Ferreira for the technical assistance.
Funding
This study was supported by INCT for excitotoxicity and neuroprotection—MCT/CNPq. C.W.N., L.F.R., M.R.F., L.O.P., and F.A.A.S. received a fellowship by CNPq; F.D., R.R.G., and G.S. received a fellowship by CAPES. RAC received funding from Maratona da Saúde, GAI-FMUC and Banco Santander-Totta, ERDF, through Centro 2020 (project CENTRO-01-0145-FEDER-000008: BrainHealth 2020), and through FCT (projects POCI-01-0145-FEDER-007440 and PTDC/NEU-NMC/4154/2016). Additional support was given by FAPERGS/PRONEM 11/2029-1. F.D. received a fellowship from CNPq Brasil (Programa Ciência sem Fronteiras).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Maria, Brazil (Permit Number: 153/2014) and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Ethics Committee imposed the selection of representative drug concentrations, based on our previous experience in using all tested drugs, to reduce the number of animals used in the experiments, while making all efforts to minimize animal suffering.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dobrachinski, F., Gerbatin, R.R., Sartori, G. et al. Guanosine Attenuates Behavioral Deficits After Traumatic Brain Injury by Modulation of Adenosinergic Receptors. Mol Neurobiol 56, 3145–3158 (2019). https://doi.org/10.1007/s12035-018-1296-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1296-1