Abstract
The present state of the art of studying whey is considered. The processes and methods of whey processing (thermal, chemical, physicochemical, biotechnological, and electrophysical) are presented. Thermal and isoelectric precipitation of proteins using reagents and coagulants, as well as the main membrane processing methods (reverse osmosis, diafiltration, microfiltration, ultrafiltration, and nanofiltration), are described. The possibilities of the effective separation of whey proteins by a combination of membrane and other methods are mentioned. Chromatographic methods for fractionation of whey proteins (simulated moving bed chromatography, high-gradient magnetic fishing chromatography, selective adsorption, displacement chromatography, membrane adsorption), which provide a high degree of separation, are described. Highly porous chromatographic materials providing a high flow rate and biotechnological processing methods—biosynthesis of lactulose, enzymatic hydrolysis of lactose and whey proteins, aerobic and anaerobic fermentation—are considered. The electrophysical methods of processing whey (electrodialysis, electroactivation), which include electrodialysis and electroactivation, as well as electrochemical activation as a phenomenon and technology, as a new promising processing method that allows the creation of a wasteless cycle for obtaining valuable components and useful derivatives from whey without the use of reagents are analyzed. It is emphasized that, depending on the used regimes, protein–mineral concentrates with a predetermined protein or mineral composition are obtained with the simultaneous isomerization of lactose into lactulose. It is stated that the efficiency of methods for processing whey is ensured by a significant increase in the efficiency of technological processes, a decrease in the labor costs, a reduction in the processing time and materials, and an improvement in the quality and functional properties of the final products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Increasingly effective engineering and technological solutions that ensure the rational and full utilization of whey components and make allowance for the ecological constituent associated with wasteless treatment are required to process whey. In order to increase the efficiency of whey processing and to maximize the recovery of valuable substances while ensuring high efficiency it is necessary to reasonably approach the choice of the method of processing of whey taking into account the technological, construction and environmental characteristics, and the capabilities of the process management [1].
Interest in the usage of whey protein is increasing due to its functional properties, such as water binding, solubility, gel formation, emulsification, foaming, etc. Research on the creation and development of new methods and technologies should ensure the optimal use of whey components, especially whey proteins, to obtain new derivatives and ensure a wasteless production.
METHODS AND PROCESSES OF WHEY TREATMENT
The most commonly used processes and methods of whey treatment are thermal, chemical, physicochemical, biotechnological, and electrophysical. The whey treatment methods used in various branches of industry are presented in Table 1; they make it possible to extract valuable components and produce new derivatives also intended for improving health.
Thermal and Isoelectric Precipitation
Whey proteins are precipitated due to high temperatures (thermal precipitation), varying pH up to the isoelectric point (pI) (isoelectric precipitation), the precipitation by calcium at medium temperatures (thermo-calcium precipitation) [22].
Thermal precipitation or thermocoagulation is a method based on the sensitivity of whey proteins to heating. It’s disadvantage is protein denaturation [23, 24].
Isoelectric precipitation is based on the precipitation at the corresponding values of pH and concentration of ions [23, 24].
Thermo-calcium precipitation is based on the formation of the aggregates of lipid-insoluble calcium phosphates at medium temperatures (50°C), neutral pH (7.3–7.5), and in the presence of calcium. Proteins are bound with the above-mentioned phosphate aggregates [23, 24]. This method is used in the production of lactalbumin, a mixture of denatured α-lactalbumin, β-lactoglobulin, and other whey proteins. The denaturation of whey proteins at thermal treatment leads to the production of derivatives with reduced functional properties [25, 26].
Precipitation Using Reagents
Precipitation using reagents is applied for the selective separation of proteins. Whey proteins can be separated by the method of aqueous two-phase separation (ATP) [27], which is an alternative to thermal processes and allows for the separation of proteins [28, 29]. In the ATP systems, liquid–liquid separation occurs through mixtures of two polymers or a polymer and a salt which at certain concentrations form a true solution in one phase, and in the other cause the formation of two immiscible phases, among which the biomolecules present in the mixture are subsequently also separated [30]. ATP was also used for the selective separation of proteins the concentration of which was determined in separate phases [27, 31, 32].
The aqueous two-phase system can include polyethyleneglycol (PEG-4000 and -6000), sodium sulphate, sodium carbonate, and sodium citrate as phase forming polymers. They are economically available and form a two-phase system with other neutral polymers as well as with salts. The solutions of polyethylene, calcium phosphate, sodium sulphate, and sodium chloride are prepared at the following ratios: 50, 40, 30, 35, and 40%, respectively. The aqueous two-phase systems were prepared mixing the respective amounts of PEG and salts, and they were centrifuged at 15 000 rpm for 10 min for two-phase separation. The concentration of protein in single phases was determined by the Folin reduction method. The coefficients of distribution and productivity were determined as well. The use of PEG-4000 with a concentration of 50% and salts with a concentration of 40% at pH 5.4 and a temperature of 35°С allows obtaining the maximum productivity (~90%) and a distribution coefficient of ~20% [33].
The Precipitation of Whey Proteins
The precipitation of whey proteins by application of coagulants, such as sodium polyphosphate and hexametaphosphate, iron salts, and polyelectrolytes, is an effective method for precipitation and extraction of whey proteins, but requires additional operations in order to separate proteins from the coagulant [22].
The use of natural polymer chitosan allows the precipitation of whey proteins to obtain pure lactose in the supernatant. This coagulant is a high molecular weight cationic linear polymer obtained by deacetylation of chitin (b (1-4)-N-acetyl-D-glucozamin) from the shell of crustaceans. Lactose from deproteinized whey protein treated with chitosan has a purity of 99.89% [34, 35].
Sternberg [36] compared the effectiveness of two coagulants based on whey precipitation with polyacrylic and trichloroacetic acids and found higher efficiency of the latter with a protein yield of 85.7–86.7% in comparison with the polyacrylic acid (62.2–68.4%). The recovery of proteins using polyacrylic acid makes it possible to obtain a white precipitate of protein -polyacrylate at pH 3.8–4.2, the rest of the dry matter amount to ~30% of the initial volume [22, 36].
Membrane Processes
The main membrane methods to process whey are reverse osmosis (RO), diafiltration (DF), microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF). More effective separation of whey proteins is possible due to a combination of membrane and other methods [37]. The driving force of all membrane processes is a difference in pressure and concentration gradients which causes the separation of substances through the semipermeable membrane.
The use of membrane methods in the processing of whey allows: concentration of whey to a content of 24% dry matter using RO and NF before evaporation and drying; obtaining whey protein isolate (WPI) with a content of 90% (WPI90); isolation of whey protein concentrate (35–80% of protein); transformation of lactose into more valuable products by fermentation (for example, ethanol or lactic acid) or enzymatic hydrolysis in continuous membrane reactors; fractionation of whey to obtain additives of improved quality; MF of whey as a preliminary treatment for further UF; subsequent concentration and demineralization of whey and UF permeate by NF (Fig. 1) [37–39].
Modes of membrane filtration according to types of filtrated elements [38].
Reverse osmosis (RO) is the process of filtration of solutions under high pressure through a semi-permeable membrane, the pore size allows only a small amount of solutes with a very low molecular weight to pass through, allowing the solvent to pass through and retaining molecules or ions of solutes [40]. The method is based on the phenomenon of osmosis—a spontaneous passage of the solvent through the semipermeable membrane into the solution. The pressure at which equilibrium occurs is called osmotic. If a pressure exceeding the osmotic one is applied from the side of the solution, then the transfer of the solvent will occur in the opposite direction, which is reflected in the name of the process “reverse osmosis” [41, 42]. RO is used to concentrate milk and whey, to extract solid particles from various dispersed media, including milk and whey. The liming factors of RO are osmotic pressure, viscosity and solubility of lactose and calcium salts in whey, which can precipitate, especially if temperature and pH are not controlled [37].
In the dairy industry, RO allows the concentration of process liquid from 10 to 25% of solids content, and it is a cheaper method than common evaporation. The low temperatures at which the process takes place prevent the loss of volatile, valuable components, and denaturation during heating, for example, denaturation of proteins. The application of reverse osmosis allows the repeated usage of water that helps to save expenses considerably. RO in the dairy industry has become indispensable for whey concentration for cheese making, milk concentration, whey desalination, and wastewater treatment [43].
RO for the concentration of whey is used in combination with other membrane processes. For example, it is used in combination with UF to isolate lactose-containing whey permeate. After this treatment, the level of the biological and chemical consumption of oxygen (BCO and CCO) greatly decreases in the by-products. The plate and frame RO modules are also used to concentrate lactose. After UF, the permeate is passed through two parallel RO lines where it is demineralized, and the third RO line reduces and purifies water for the UF system [43].
Diafiltration (DF) is a variant of membrane separation when one or several components of the solution are purified from the impurities of some other components. During DF, some solvent (usually water) is introduced into the solution whose flow rate is equal to the amount of the removed permeate. The component for which the membrane is highly selective is retained by it and is purified from the component for which the membrane is low selective, since it passes with the solvent into the permeate [44, 45].
DF is used for better purification from lactose of the protein concentrate received due to UF. This is a particular case of UF when the received protein concentrate is diluted with demineralized water and subjected again to UF up to the initial weight fraction of dry matter. Furthermore, some part of lactose and mineral substances is washed away from the protein concentrate and passes through the membrane together with the solvent. The whey-protein concentrate (WPC) produced via the UF method has a high solubility in water and high emulsifying, foaming, and gel formation properties [46]. DF is used to obtain a WPC with a high protein content, to eliminate high concentrations of residual products, to ensure a high degree of purification and at the same time high productivity of the process [47, 48].
Microfiltration (MF) is a method of membrane filtration under low pressure. Ceramic tubular or polysulfonamide spiral membranes are usually used to process whey. MF is effectively used for preliminary processing of raw materials to reduce bacterial contamination of whey and to remove fat. The pores of MF ceramic membranes provide an effective retention of the casein dust, fat, bacteria, and spores [49].
MF is preprocessing for UF, and it is a process similar to UF but with a membrane whose pores are bigger to let the particles with a size from 0.2 to 2 μm to pass through it. Whey usually contains a small amount of fat and casein, and, since the centrifugal separation does not remove them completely, MF is used to decrease the fat to protein ratio to 0.001–0.003. In addition, some precipitated salts can be removed with the help of MF, which also allows a considerable reduction in the microbial load [50].
Separation of whey proteins using MF (pore size from 0.05 to 0.2 μm) can be carried out in the periodic or continuous mode. Industrial processes use continuous filtration due to an easier control and a longer filtration time. Milk MF has traditionally used ceramic membranes that can withstand high temperatures, low and high pH values. MF is conventionally carried out at 50–55°C with a transmembrane pressure of 0.1–1.0 bar and high tangential flow rates (>6 m/s). Tubular ceramic membranes are capable of providing a permeate flow rate of 55–65 L/(m2 h) with an average concentration factor of 1 to 4. Lower filtration temperatures and higher transmembrane pressures result in a reduced permeate flow and a higher whey protein retention. In industrial microfiltration of milk, ceramic membranes (0.05–0.2 μm) require a high tangential flow to achieve high permeate flows [39, 51, 52]. The use of ceramic membranes with pores from 0.2 to 1.8 μm allows bacteria to be removed from milk.
The content of bacteria in milk filtered through the membranes with a pore size of 1.4 microns decreases by the two orders of magnitude without a noticeable retention of proteins. This technology makes it possible to significantly increase the level of milk pasteurization and to reduce protein denaturation in comparison with conventional heat pasteurization [53]. MF has also been studied to remove residual lipids from whey before UF and sterilize it. This often includes thermal treatment and/or correction of pH to aggregate lipids and calcium phosphates [54].
Ultrafiltration (UF) is a variation of membrane filtration during which the suspended solid particles and dissolved substances with a high molecular weight are preserved in the so-called retentate, while water and dissolved substances with a low molecular weight can pass through the membrane into the permeate (filtrate). This separation process is used in industry and scientific investigations to purify and concentrate protein solutions. UF and MF are based on different capacities of absorption and different rates of diffusion. UF is used for more effective separation of complex mixtures [55].
In the dairy industry, UF is carried out with polymer and ceramic membranes receiving a flow of retentate that can be additionally processed by means of evaporation, drying, or spraying. UF allows varying the ratio of concentrations between the components of whey, thanks to the retention of proteins and the selective permeability of lactose, minerals, water, and compounds with a low molecular weight [48].
In the case of UF, the whey is simultaneously fractionated, purified, and concentrated producing a high-quality WPC. The UF is controlled by a pressure gradient of approximately 275.9 kPa, and the temperature is maintained within the range of 50–60°С using polysulfone membranes with a pore dimension from 10–1–10–2 μm. UF membrane modules are available in four basic configurations: tubular, bare fiber, spirally wound, and flat plates [39].
The UF methods make it possible to produce WPC with a content of protein within the range of 34–90%, which can be increased modifying the process in combination with DF that allows maintaining the content of lactose and mineral substances. The WPC obtained in this way can be used to increase the protein content in products, avoiding denaturation, which is beneficial for using them as food additives [39].
Nanofiltration (NF) is a newish method of membrane filtration for separation of dry substances with low solubility in water, such as natural and synthetic organic substances with application of nanometer pores (1–10 nm) smaller than at MF and UF. NF is becoming widely used in the food industry, in particular, in the dairy industry, for simultaneous concentration and partial demineralization. The used membranes are mainly prepared of polymer films. The materials that are usually used include polyethyleneterephthalate or metals, such as aluminum. The pore dimensions are controlled by the values of pH, temperature, and time at the pore density from 1 to 106 at cm2 [39, 54]. The membranes for NF are manufactured of polyethyleneterephthalate and other similar materials by the treatment of a thin polymer film with particles under the action of high energies; thus, the pores are “engraved” into the membrane. The membranes made of a metal, say, of aluminum oxide, are manufactured electrochemically to apply a thin layer of aluminum oxide from metallic aluminum in an acid medium [56].
The use of NF is widespread in various industries, including the dairy industry, and plays a special role in the pharmaceutic industry, primarily for molecular separation [57]. NF allows the treatment of large volumes and in a continuous flow. Its main disadvantages are a high cost and complexity of maintenance [58].
NF is an important process in the dairy industry for separation of low molecular substances and minerals, which makes it possible to concentrate whey up to 20–24% of the content of fat and permeate after the UF treatment of whey [54]. A partial demineralization and concentration of the product is achieved at NF. It improves the organoleptic parameters, allows for a decrease in the volumes of the received whey reducing transportation costs and expenses for the production of dry whey [59].
Chromatographic Fractionation of Whey Proteins
Chromatographic methods are called multistage separation methods in which the components are distributed between the stationary and mobile phases. The stationary phase can be a solid substance, liquid, or gel applied to a solid carrier and it can be placed into the column, applied in the form of a thin layer or film, etc. [60]. The mobile phase can be gas, liquid, or supercritical gas (fluid). The separation is based on the adsorption, distribution (separation) of masses, and ion exchange, which rests on the distinctions in the physicochemical properties of molecules, such as size, mass, volume, etc. There are different forms of chromatography depending on the main carrier and purposes of the investigations: paper, thin layer, gaseous, liquid, etc. The processes of the chromatographic separation of substances are improved depending on the goals of research [60].
The ion exchange chromatography (IEC) is one of the methods used for production of whey protein isolates based on the reversible interaction between the functional groups of proteins and the ion exchange resins. The separation of whey proteins, due to their properties to be precipitated at different pIs, allows the assignment of two groups of whey proteins: the main proteins (β-lactoglobulin (β-Lg), α-lactalbumin (α‑La), and bovine serum albumin (BSA)), which are charged negatively at pH 6.2–6.4, and the minor proteins lactoferrin (LF) and lactoperixodase (LP), which have a positive charge [61].
The cation resins (charged negatively) are used to capture positively charged proteins (LF and LP). At the same pH, the basic proteins are negatively charged, so they are not retained by the resin, resulting in a fraction rich in these proteins. LF and LP are separated at the elution by alkaline solutions. Then, the fractions are washed and dried by spraying [62]. The ion exchange chromatography can work under two conditions: the selective adsorption of whey proteins and the selective elution of the proteins captured by the ion exchange resin. The separation of whey proteins using anion exchange chromatography consists in creating conditions in which whey proteins are bound and subsequently eluted from the column, usually by changing the pH of the buffer solution or increasing the salt concentration. The primary task in the separation of whey proteins is the separation of two main protein fractions: α-La from β-Lg [63].
Cation exchange chromatography is a technique used to isolate and separate whey proteins, especially, LF and LP, and to isolate immunoglobulins (Ig). These proteins are charged positively within a very wide range of pH, which allows the optimization of the conditions when α-La and β-Lg are not absorbed on the matrix. It is of particular importance, since the concentration of Ig, LP, and LF in whey is not high unlike α-La and β-Lg [63].
Simulated moving bed chromatography is based on the simulation of a true counter current operation between the solid and the liquid phase, by valves switching over a series of columns or a column movement in a carousel. The input of the initial material and the removal of the substance under analysis are simultaneous and continuous, which gives the impression of a moving bed. The counterflow mode makes possible a more effective application of the adsorbent and liquid flows with such advantages as higher productivity and product concentration, low consumption of the buffer, effective usage of raw materials, and higher purity of the product (Fig. 2) [64, 65].
Mechanism of simulated moving bed chromatography used to separate lactose; zones 1 and 4 include one column each, while zones 2 and 3 include three columns each [65].
High-gradient magnetic fishing chromatography (HGMF) is based on the adsorption of protein on the superpamagnetic, i.e., magnetosensitive particles that are drawn into the nonporous substrates with micron dimensions without any “magnetic memory” when a magnetic field is applied [66]. Due to a very small size and tough material used to make these supports, these matrices provide the shortest adsorption times and are less prone to fouling than porous adsorbents [66–60]. Rapid collection of carriers in a highly magnetized filter and subsequent easy desorption and extraction of bound protein from the carriers can significantly increase the processing speed [70].
Heeboll–Nielsen et al. [67] developed superparamagnetic cation exchangers and used HGMF to separate main proteins from sweet whey. They then described the production of superparamagnetic anion exchangers and the application of them together with superparamagnetic cation exchangers at fractionation of whey. The initial whey was processed with the superparamagnetic cation exchanger to adsorb main proteins with the following treatment of supernatant with the superparamagnetic anion exchanger [69].
In the initial stage of cation exchange, the quantitative removal of LF and LP was achieved with some simultaneous binding of Ig. The Ig were separated from two other proteins by means of desorption with a low concentration of NaCl (≤0.4 М) while LF and LP were then coeluated in a much purer form. The anion exchanger adsorbed β-Lg selectively providing the separation from other proteins; however, a little amount of high purity β-Lg was detached during elution [67, 71].
Selective adsorption. In selective chromatography, the separation of substances occurs due to their selective adsorption in the stationary phase. The selective adsorption is caused by the affinity of a compound to a solid adsorbent, which is determined by polar interactions of their molecules. Ion exchange, liquid, paper, thin layer, and gas adsorption chromatography are referred to adsorption chromatography [72].
Selective adsorption has not been used until recently. The ion exchangers themselves are not selective matrices since they can adsorb a lot of proteins with similar values of the pI. The improvement of selective adsorption on the ion exchanger can be achieved by using the specific properties of the target protein, one of which is their size. However, there have been no carriers for ion exchangers until now that could allow the selective adsorption of low molecular proteins [73].
Bolivar et al. [74] developed an anion exchanger substrate for the selective adsorption of low molecular proteins by activation of the amine-based substrate (MANAE–Sepharose preparation) by glutaraldehyde and additional coating of the substrate surface by the BSA. In this case, the “wells” are generated by two neighboring molecules of BSA. At the bottom of these “wells”, some of the monoaminoethyl-N-aminoethyl groups are ready to bind small molecules that are small enough to fit between two BSA molecules on an existing substrate. Since the BSA surface is capable of adsorbing many proteins, which reduces the selectivity of the system, the coating of BSA immobilized with dextran molecules reduces protein adsorption on BSA surface (Fig. 3). This new substrate (matrix) has been evaluated for selective adsorption of low molecular weight whey proteins, β-Lg, and α-La, while other larger proteins are retained in the supernatant [75, 76].
Schematic diagram of aminoglutaraldehyde substrate coated with proteins modified with dextrane: only small proteins can interact with the substrate surface [74].
Displacement chromatography is a method of separation of substances, which allows to obtain higher concentrations of a certain component due to displacement by a dissolved substance, which is sorbed more intensively than the components of the initial mixture [77]. The principle is that the sample is loaded onto the top of the column and transported with a solution that is a stronger sorbent than the components of the original mixture. As a result, the components are separated into successive “rectangular” zones of highly concentrated pure substances [78]. The displacement chromatography produces products with higher concentration, higher purity, and increased throughput compared to other chromatographic separation methods [78, 79].
The basic principle of displacement chromatography: the matrix has only a finite number of binding sites for solutes (stationary phase), and if the site is occupied by one molecule, it is not accessible to others. As in any chromatography, equilibrium is established between molecules of a given type, bound to the matrix, and molecules of the same type, which are not present in a solution. Since the number of binding sites is finite, the concentration of free molecules in a solution is high relative to the dissociation constant for these sites, and they will be mostly filled. The molecule with a high affinity of “displacement” to the matrix compete more effectively for the binding sites enriching the moving phase with the dissolved substance with a lower affinity. The mobile phase flow through the column preferentially carries away the lower affinity solution; thus, at high concentrations, a higher affinity solution eventually displaces the lower affinity molecules [80, 81].
The disadvantages of the displacement chromatography are: an overlap between each pair of components; the mixed area must be treated separately to maintain the purity of the separated components; difficult interpretation of adjacent zones, especially if the displacement mechanism is not fully developed; the time it takes to regenerate limits the throughput [81].
The displacement chromatography is used to separate amino acids, rare earth elements, and isotopes, to clean proteins from complex mixtures, and to isolate substances with low molecular weight for purifying proteins in the systems of ion exchange [82]. It is well suited for the quantitative separation of purified proteins from complex mixtures. It is also used for separation of whey proteins, particularly, with hydroxyapatite—a very useful ion exchange matrix with many advantages, such as simple fabrication, nontoxicity, possible regeneration, and low cost. The hydroxyapatite chromatography of whey is mainly based on the displacement of ions when the displacers (phosphate or organic acids) form stronger complexes with calcium than the carboxyl groups of the adsorbed proteins [83, 84].
Rossano et al. [83] presented the method based on the application of an improvised hydroxyapatite for a single stage separation of lactin (nonabsorbed) from whey proteins (adsorbed). The total fraction of protein can be eluted with 0.4 M phosphate at pH 7.0. Approximately 56% of proteins, mainly α-La and IgG, were eluted with 0.4 M phosphate at pH 5.0. The fractions were applied to the Superdex 75 column for final purification by gel filtration. The main advantages of this procedure are as follows: (a) single-stage quantitative separation between lactin and proteins, (b) ease of flexibility in the extraction of proteins from certain proteins to the total protein fractions, and (c) purification of whey proteins in the native form [71].
Schlatterer et al. [85] conveniently separated β-Lg from other whey proteins via the hydroxyapatite chromatography (Macro-Prep Ceramic hydroxyapatite Bio-Rad, Munich, Germany) with the gradient of fluoroidiones with the phosphate buffer solution as a displacing agent. β-Lg was fully eluted at the peak at the fluoride concentration of approximately 0.6 mole/L. The purity of β-Lg in this fraction was at least 96%, with the traces of IgG, BSA, and LF, which were conveniently removed with the help of Superdex.
Ng and Yoshitake [86] described the method of ceramic chromatography in the mixed mode (ceramic hydroxyapatite CHT, Bio-Rad, Munich, Germany) for the fractionation of LF from whey in one column. They managed to remove LP that usually contaminates LF purified with other methods due to the similarity in the molecular weight and their isoelectric properties. LP was originally desorbed from the matrix under the isocritical conditions. LF was prepared without any activity of LP and other main whey proteins, such as α-Lа and β-Lg. The process has all productivity characteristics necessary for an advantageous application in bio-industry (simplicity, high efficiency, and low costs) [87].
High-porosity chromatographic materials providing high flow rate. Macroporous chromatographic materials are used to extract proteins from food flows at high rates with no need for some stages of treatment and, thus, for high expenses. These materials ensure a slight decrease in the pressure in the column; therefore, high flows are easily achieved [71]. The main advantage of macroporous media is the fact that the functional groups, which are even present on the internal surfaces, adsorb proteins with the least diffusion limitations since they are in the convective flow [88–90].
The limitations of the classic chromatography, which appear due to the diffusion of molecules to the internal surfaces where they can be bound, can be reduced by introducing materials with high porosity. In addition, these materials ensure a slight pressure drop in the column; therefore, high flow rates are easily achieved. The basic advantage of macroporous media is the fact that even functional groups, which are present on the internal surfaces, adsorb proteins with the least diffusion limitations since they are inside the convection flow [71]. The types of chromatography on the basis of these materials are membrane convective liquid chromatography, perfusion chromatography, and continuous bed chromatography.
Membrane convective liquid chromatography is a method when porous cellulose membranes with a pore size of 1.2 μm are used to separate proteins [91].
In perfusion chromatography, the packed columns are used where the particles have a bidispersed porous structure: (a) with a transverse pore size of 600–800 nm for the crossing particles and (b) the diffusion pores of 50–150 nm which smooth out the transverse pores [92].
Continuous bed chromatography is based on polymerization of the newest monomers and ionomers directly within the chromatographic column [93]. The polymer chains are united in a dense network of nodes which consist of microparticles with an average diameter of 200 nm, with the channels between the nodes being large enough (a diameter of 7–15 μm) to provide a high convection rate of the flow [71].
Membrane adsorption. The common chromatographic processes demonstrate enormous gaps for the quick and reliable processing of whey because long cycles and complicated systems for the process control are required in the case of large volumes. Some of these limitations can be overcome with the help of the membrane chromatography when the adsorption membrane is used as a stationary phase that combines the principles of chromatography and membrane separation in one device. Chromatographic membranes are modified by specific ligands or functional groups to bind the molecules–targets and to ensure a quick binding since the adsorption ligands are on the way of the convection flow through the membrane pores [71].
The chromatographic membrane systems usually have pore sizes within the range of MF (0.02–10 μm) since they are not separated on the base of molecular size and can ensure high flow rates, low pressure drops, easy packing of the column, and a low degree of pollution, which makes them perfect agents to separate proteins under the production conditions [94, 95].
The commercial membrane systems for laboratory and industrial scales manufactured on the basis of ion exchange are systems with strongly acidic (sulfonic acid), strongly alkaline (quaternary ammonium), weakly acidic (carbonic acid), and weakly alkaline (diethylamine) bases [96, 97].
Plate et al. [98] developed a new procedure for isolating serum LF and LP from sweet whey under laboratory conditions using cation exchange membrane systems (SartobindS, Sartorius, Goettingen, Germany). This fast, reliable and efficient procedure has been expanded to an industrial scale module to purify the sweet whey concentrate with over 90% LF recovery (7 g of LF during a cycle for modules with 2 m2) [98, 99].
The Biotechnological Methods of Whey Processing
The biotechnological methods of whey processing are biosynthesis of lactulose, enzymatic hydrolysis of lactose, and aerobic and anaerobic fermentation. The biosynthesis of lactulose consists of the enzymatic hydrolysis of lactose disaccharide to monosaccharides of glucose and galactose, and it can be performed with the help of hydrolysis and the reactions of transfer by catalyzed glycosidase (for example, β-galactosidase) or direct isomerization of lactose into lactulose with cellobiose-2-epimerase [22, 100–104].
The hydrolysis of whey can be performed by two methods: enzymatic (fermentation) and acidic. The chemical or acidic hydrolysis is carried out by adding an acid, such as sulfuric acid, but it has some disavantages: it causes denaturation of protein, requires preliminary demineralization of whey as the mineral salts inactivate the acid, leads to the formation of brown color (the result of the Maillard reactions, which requires discoloration with activated carbon), and favors the formation of unintended waste products.
Enzymatic hydrolysis is performed using the lactase enzyme (β-galactosidase, EC 3.2.1.32), which is found in animals, plants, bacteria, fungi, and yeasts [105].
The enzymatic synthesis of lactulose from lactose is determined by the composition, the concentration of the substrate and enzyme, the temperature, the medium pH, and the treatment mode. The enzyme concentration considerably affects the selective character of the reactions; at a higher concentration of enzymes (≥15 U/mL), the primary hydrolysis of lactose and the secondary hydrolysis (disintegration of lactulose) are quicker [106]. The controlled enzymatic hydrolysis of proteins can improve the functional properties of the hydrolisate and ensure more pleasing taste, and the allergenic properties can be eliminated [107, 108].
The enzymatic hydrolysis of whey proteins consists in the hydrolysis of the WPC, 80%, at the optimal temperatures (50°C–60°C) and pH 7–8 with the help of different enzymes (proteases secreted from Bacillus licheniformis, Aspergillus oryzae, and Aspergillus sojae, etc.) resulting in whey protein hydrolysates. After the enzymatic hydrolysis, the peptide bounds of protein are split, new amino groups are formed, and an amino group for every “broken” peptide bond is found. The number of the formed amino groups causes a linear growth in amine nitrogen [109, 110].
Aerobic fermentation can be classified in accordance with different criteria by the type and conditions of treatment, such as the feed mode (periodic, semiperiodic, or continuous reactors), temperature (psychrofilic, mesophilic, and thermophilic reactors), solids content (reactors with a high or low solids content), complexity (single stage reactors, multistage reactors), reactor form (horizontal, vertical), the mode of retention of microorganisms in the reactor (fixed film, suspended growth, or hybrid), and the substrate humidity (wet or dry splitting) [111–115].
Aerobic fermentation is characterized by a relatively quick degradation of the organic substance at room temperature (22°C–24°C) requiring a certain interval of the hydraulic retention time. However, a high organic load of raw whey makes the aerobic fermentation not sufficient to manufacture final products. The optimum carbohydrate/nitrogen/phosphor (C/N/P) ratio in the aerobic processes is approximately 100 : 5 : 1 in comparison with 500 : 5 : 1 in the anaerobic processes [116].
Anaerobic fermentation is a three-stage processing of whey: reduction in pollution emission, power generation, and separation of nutritional substances [117]. The anaerobic fermentation depends on the physicochemical composition of whey (organic composition, pH values of the medium, and oxidizing properties), the type of inoculum (maintenance of a stable buffer), and the reactor configuration (wastewater recirculation capabilities) [118]. It is performed by different microorganisms, such as obligate anaerobic strains of the species Clostridium (Clostridium butyricum, Clostridium pasteurianum, and Clostridium beijerinkii) and facultative anaerobic species, such as Enterobacter, Citrobacter sрp., and Escherichia coli, as well as the mixed microbiological communities under the mesophilic (30°С–38°С) or thermophilic (55°С) conditions [22].
Anaerobic fermentation includes degradation and stabilization of an organic substance by microorganisms under anaerobic conditions and contributes to the formation of biogas (a mixture of carbon dioxide and methane) and biomass. This complex process consists of three successive stages: hydrolysis of lactose (and proteins), fermentation, and methanogenesis. It is performed during the interaction of some mixed species of bacteria. In the process of methanogenesis, approximately 90% of the hydrolyzed organic substance evolves into biogas. It is estimated that 45 L of biogas, which contains 55% of methane, can be obtained from 1 L of whey. From a liter of whey, 20 L of methane can be produced, which is equivalent to 739 kJ [22]. Anaerobic fermentation is not widely used in the dairy industry due to low reaction rates and relative instability of the process in conventional reactors [119–125].
Ethanol fermentation. In addition to whey, the solution of whey powder, the permeate after UF, and the deproteinized whey can be used as raw materials to generate ethanol. Their fermentation to obtain ethanol requires a certain group of microorganisms, such as Torula cremoris, Kluyveromyces fragilis, Kluyveromyces marxianus, Candida pseudotropicalis, and Saccharomyces cerevisiae. The reaction that describes the biotransformation of lactose into ethanol theoretically shows a maximum value of 0.538 kg of ethanol per a kilogram of the consumed lactose. The alcoholic fermentation of lactose from whey or whey permeate is not economically competitive in comparison with other substrates, such as cane sugar, corn flour, and lignocellulosic biomass [125].
Hydrogen fermentation. The anaerobic fermentation processes of whey, diluted whey, the solution of whey powder, and whey permeate powder made it possible to obtain hydrogen. Theoretically, the process makes it possible to obtain a yield of 8 mol of hydrogen per 1 mol of lactose. The biogas mixture that is formed during the generation of hydrogen contains methane and carbon dioxide as well. The generation of hydrogen can be increased controlling the main parameters, such as pH in neutral or slightly acidic pH conditions (4.0–7.5); dominant microorganisms; substrate composition; temperature; humidity; hydraulic retention time; and addition of metals, yeast extract, nutritional substances, etc. The methanogenic microorganisms that consume hydrogen decrease the generation of it. In this process, the reactors with a continuous mixer, the periodic reactors, and the reactors with an inclined anaerobic sludge are mainly used. In the reactor with a continuous mixer, the hydraulic retention time is varied from 6 to 84 h in comparison with 12 h for the reactors with an inclined anaerobic sludge and 24–280 h for the periodic reactors. As a rule, the periodic reactors lead to a higher percentage of the generated hydrogen (50–88%) than other configurations (20–60%) [125].
Lactic acid fermentation. The permeate with a low content of protein and high concentration of lactose and mineral salts allows for the production of lactic acid mainly from the whey ultrafiltrate.
To produce lactic acid, the following microorganisms are used: Lactobacillus casei, Lactobacillus helveticus, Lactobacillus acidophilus, Lactobacillus delbrueckii, Streptococcus thermophilus, Lactococcus lactis, Lactobacillus salivarius, Pediococcus, etc. Some investigations also point to the application of mixed cultures with a synergetic effect. The production of lactic acid from whey and permeate without food additives is limited on an industrial scale because of low productivity (the lactic acid production is 3.8–12 kg/m3, the hydraulic retention time is 48–56 h, the temperature is 23°С–37°С). The food additives are a key factor that limits the process efficiency. The substitution of the additives (yeast extract, peptone, soy flour, whey protein, MgSO4, MnSO4, and (NH4)2SO4) for proteolytic enzymes or microorganisms doubles the yield of lactic acid [125].
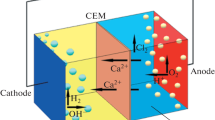
Electrophysical Methods of Whey Processing (Electrodialysis, Electroactivation)
Electrodialysis is an electrochemical process of transfer of ions through the membrane under the action of an electric field. The ion transfer rate in electrodialysis systems is affected by the temperature, the flow rate, the working liquid composition, and the applied voltage. Electrodialysis processing ensures the 90% degree of whey demineralization without any substantial change in the quantitative composition of other components. The product undergoes well the processes of vacuum concentration, crystallization, and drying, and it has improved technological and organoleptic characteristics and a wider range of application, including whole milk products [126]. Electrodialysis makes it possible to reduce the content of mineral substances in whey due to its passage through a weak electric field of the electrolysis modules equipped with the ion-selective membranes. In the case of electrodialysis (depending on the purposes) various ion-selective membranes are used: biselective, cation-exchange, and anion-exchange [127].
The cation exchange membranes contain covalent-negative bound groups such as sulfinic acid. They allow cations to pass and retain anions [126, 128].
The opposite phenomenon takes place on the anion membranes. In this case, quarternary amines are the positively charged groups. Thus, passing the direct electric current, the cations of the salts in the whey and working solution move to the cathode and the anions move to the anode. The electrodialysis of whey consists in its alternative passage through the blocks separated by the ion-exchange membranes with the transfer of whey salts to the reference solutions in the adjacent chambers [127, 129]. Owing to ion migration, whey is desalted, and the reference solution is concentrated. The processes of desalination and concentration by electrodialysis proceed simultaneously and are closely interconnected. The electric current direction changed, and the process proceeds contrarily. The same takes place at the application of the cation- and ion-exchange membranes [128, 129].
Electrochemical activation (electroactivation). The electrochemical activation (ECA) as a physicochemical process is a combination of electrochemical and electrophysical impacts on water containing ions and molecules of dissolved substances performed under the conditions of the lowest heat release in the area of a spatial charge at the surface of the electrode (anode or cathode) of the electrochemical system during the nonequilibrium transfer by the electrons through the electrode–electrolyte interface [130].
As a result of the electrochemical activation, water passes into a metastable (activated) state, which is characterized by the abnormal values of the physicochemical parameters, including the redox potential associated with the activity of electrons in water, electrical conductivity, pH, and other parameters. Spontaneously changing in time, the parameters and properties of water, perturbed by the previous external influence, gradually reach equilibrium values as a result of relaxation [131].
The process of producing electrochemically activated water and solutions refers to extremely nonequilibrium ones, and it is an object under study in a new rapidly developing field of chemistry—synergetics in chemical processes and chemical technology. If the main problem in the conventional applied electrochemistry is to determine the parameters of the optimum approaching of the electrochemical process to equilibrium conditions, then it is important for the electrochemical activation to define the parameters of the optimum displacement from the conditions of the equilibrium proceeding of electrochemical reactions [132].
Electroactivation is a new direction in the treatment of water and liquids, including whey, developing new technological directions. One of them, based on the electrolysis of aqueous solutions, makes it possible to transform lactose into lactulose by electroactivation of whey [133–135]. A new product enriched in lactulose is used as a valuable ingredient with the proven prebiotic effect.
The electroactivation method makes it possible to isomerize lactose into lactulose by both mechanisms simultaneously, known in biochemistry of carbohydrates: L-A-transformation and Amadori rearrangement. Although electroactivation is a chemical-free method, the rearrangement mechanism of L-A transformation is due to the accumulation of hydroxyl ions, which are formed as a result of the dissociation of water. The presence of the activated amino groups, both upon activation of protein compounds and nitrogen-containing compounds of non-protein origin, present in whey, leads to isomerization by Amadori rearrangement, in which lactose reacts with amines due to the presence of amino acids in whey, as well as creatine, creatinine, carbamide, and uric acid that promote the formation and hydrolysis of lactulosamine. The resulting lactulosylamine rearranges and turns into lactulosamine, which is then disintegrated into lactulose and amines [136, 137]. In the case of electric isomerization of lactose to produce lactulose, the reactors with different design and geometrical parameters are used [138, 139].
The treatment of whey requires more effective engineering and technological solutions with the account for both rational and more complete use of its components and the ecological constituent associated with wasteless processing. One of the successful technological solutions is the electrophysical processing of whey with the recovery of the protein mineral concentrate (PMC) and simultaneous isomerization of lactose into lactulose on the base of ECA of the liquid [87]. The PMC has a high biological value due to the presence of all essential and nonessential amino acids.
To increase the efficiency of whey protein recovery and reduce specific energy consumption, it is necessary to reasonably approach the choice of an electrochemical reactor, taking into account such design characteristics as the distance between the electrodes and between the electrodes and the membrane. The geometric form must exclude any nonfunctional zones ensuring a high productivity of whey processing in a suitable electrochemical reactor [140].
The advantages of ECA are the possibility of complete exclusion or a significant reduction of chemical reagents in technological processes of the activation of aqueous solutions for various purposes, as well as the exclusion or drastic reduction of the need for wastewater treatment. The economical feasibility is predefined by a considerable growth in the effeciency of technological processes due to both a decrease in the labor cost, time, and materials and the improvement of the quality and functional properties of final products. The recovery of PMC depends on the electrophysical processing modes (in particular, on the electric current density) and the specific energy consumption per unit volume, on the variation in pH, on the redox potential, and on temperature [87, 141].
When electric current is applied to the dry matter content of whey (about 6%), which includes more than 200 components, many inter- and intramolecular processes occur that generate changes in the recovery of whey proteins in the PMC. The main mechanisms of the formation of protein compounds at the electroactivation are the ion flotation and salinization of proteins due to the action of coagulation and complex formation. The electric fractionation (or electrophysical treatment) of whey allows the simultaneous separation of two main fractions: protein mineral concentrates and deproteinized whey. In turn, depending on the treatment modes, the PMCs are enriched with different protein fractions: β-Lg or α-La. Under certain processing regimes, PMCs enriched with LF and Igs are obtained. The method is wasteless, and it makes it possible to simultaneously isomerize lactose into lactulose, which is contained in the deproteinized whey enriched in minerals, amino acids, and oligosaccharides. The investigations of the geometric and design peculiarities of the electrolyzes for various purposes of electrofractionation of whey and reduction in energy consumption during the electrophysical processing are of the growing interest, and they should be continued [87, 141].
CONCLUSIONS
Thus, analyzing a lot of published works and the accumulated authors’ experience concerning the chemical composition, classification, and derivatives, it should be emphasized the obviousness and expediency of using whey for the extraction and widespread consumption of the contained valuable components of dry matter. A diverse protein composition makes it possible to produce many protein derivatives from whey, such as dry whey/whey powder; dry demineralized whey of 25, 50, and 90%; whey powder without lactose; WPC with a protein content of 34, 50, 60, 75, and 80%; WPI; whey protein hydrolysates;PMCs; lactose (industrial, food, pharmaceutic); derivatives of lactose—lactitol, lactulose, galacto-oligosaccharides; separate proteins (LF, LP, glycomacropeptides); milk minerals; and permeate.
Numerous functional and nutritional properties of whey proteins and products on their basis make it possible to provide the application of whey derivatives in many branches of the food-processing industry.
The healing properties of whey and its derivatives, such as antioxidant, immunomodulatory, stimulatory, and antitumor, due to various mechanisms of action associated with their functional properties, make it possible to obtain a diverse range of biologically active additives used in the pharmaceutical industry.
The separation and production of valuable products from whey predetermine the possibility to apply different methods based on the certain techniques of whey processing or a combination of them for an effective extraction of valuable components, development of the wasteless cycles of treatment at the simultaneous reduction in the energy consumptions, and the compliance with high ecological requirements (see Part I).
The described processes and methods of whey processing, such as thermal, chemical, physicochemical, biotechnological, and electrophysical, make it possible to diversify the range of the obtained valuable derivatives from whey.
The membrane methods, which include the RO, DF, MF, UF, and NF, allow the performance of an effective separation of whey proteins due to a combination of the membrane and other methods. The chromatographic fractionation methods, such as simulated moving bed chromatography, high-gradient magnetic fishing chromatography, and selective adsorption, provide a high degree of separation. The biotechnological methods (biosynthesis of lactulose; enzymatic hydrolysis of lactose, whey proteins; aerobic and anaerobic fermentation) are used to produce different biologically active food additives. The controlled fermentation processes are considered during the production of lactic acid, ethanol, hydrogen, and protein hydrolysis.
The electrophysical methods of whey processing include electrodialysis and electroactivation. ECA is a new promising method of processing that makes it possible to develop a wasteless treatment cycle with generation of valuable components to form useful derivatives from whey without any reagents. Depending on the applied treatment modes, the PMCs with a predetermined protein and mineral composition at the simultaneous isomerization of lactose into lactulose are received.
The economical efficiency of the chosen whey processing methods is ensured by a considerable growth in the effectiveness of technological processes due to the decrease in labor costs, reduction in time, and savings in material, and the improvement of the quality and functional properties of final products.
REFERENCES
Sprinchyan, E.G., Optimization of protein extraxtion during electrophysis of whey, Elektron. Obrab. Mater., 2010, vol. 46, no. 6, p. 81.
Castro-Rosas, J., Guerrero-Rodríguez, W., Rodríguez-Miranda, J., Páez-Lerma, J., et al., Optimization of thermal protein precipitation from acid whey, J. Food Process. Preserv., 2013, vol. 37, no. 5, p. 924. https://doi.org/10.1111/j.1745-4549.2012.00728
Pessato, T.B., de Carvalho, N.C., de Figueiredo, D., et al., Complexation of whey protein with caffeic acid or (–)-epigallocatechin-3-gallate as a strategy to induce oral tolerance to whey allergenic proteins, Int. Immunopharmacol., 2019, vol. 68, p. 115.
Ghadermazi, R., Asl, A.K., and Tamjidi, F., Optimization of whey protein isolate-quince seed mucilage complex coacervation, Int. J. Biol. Macromol., 2019, vol. 131, p. 368.
Perumalsamy, M. and Murugesan, T., Extraction of cheese whey proteins (α-lactalbumin and β-lactoglobulin) from dairy effluents using environmentally benign aqueous biphasic system, Int. J. Chem. Environ. Eng., 2012, vol. 3, no.1, p. 50.
Domínguez-Puerto, R., Valle-Guadarrama, S., Guerra-Ramírez, D., and Hahn-Schlam, F.F., Purification and concentration of cheese whey proteins through aqueous two-phase extraction, CyTA–J. Food, 2018, vol. 16, p. 452.
Arunkumar, A. and Etzel, M.R., Negatively charged tangential flow ultrafiltration membranes for whey protein concentration, J. Membr. Sci., 2015, vol. 475, p. 340.
Wang, W., Han, X., Yi, H., and Zhang, L., The ultrafiltration efficiency and mechanism of transglutaminase enzymatic membrane reactor (EMR) for protein recovery from cheese whey, Int. Dairy J., 2018, vol. 80, p. 52.
Choi, J., Im, S., and Jang, A., Application of a volume retarded osmosis–low pressure membrane hybrid process for treatment of acid whey, Chemosphere, 2019, vol. 219, p. 261.
Marx, M., Bernauer, S., and Kulozik, U., Manufacturing of reverse osmosis whey concentrates with extended shelf life and high protein nativity, Int. Dairy J., 2018, vol. 86, p. 57.
Heidebrecht, H.J., Kainz, B., Schopf, R., Godl, K., et al., Data concerning the chromatographic isolation of bovine IgG from milk- and colostral whey, Data Brief, 2018, vol. 21, p. 527. https://doi.org/10.1016/j.dib.2018.09.115
Fuciños, C., Fuciños, P., Estévez, N., Pastrana, L.M., et al., One-step chromatographic method to purify α-lactalbumin from whey for nanotube synthesis purposes, Food Chem., 2019, vol. 275, p. 480. https://doi.org/10.1016/j.foodchem.2018.09.144
Pagliano, G., Ventorino, V., Panico, A., Romano, I., et al., The effect of bacterial and archaeal populations on anaerobic process fed with mozzarella cheese whey and buttermilk, J. Environ. Manage., 2018, vol. 217, p. 110. https://doi.org/10.1016/j.jenvman.2018.03.085
Escalante, H., Castro, L., Amaya, M.P., Jaimes, L., et al., Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries, Waste Manage., 2018, vol. 71, p. 711. https://doi.org/10.1016/j.wasman.2017.09.026
Rico, C., Muñoz, N., and Rico, J.L., Anaerobic co-digestion of cheese whey and the screened liquid fraction of dairy manure in a single continuously stirred tank reactor process: Limits in co-substrate ratios and organic loading rate, Bioresour. Technol., 2015, vol. 189, p. 327. https://doi.org/10.1016/j.biortech.2015.04.032
Merkel, A., Ashrafi, A.M., and Ečera, J., Bipolar membrane electrodialysis assisted pH correction of milk whey, J. Membr. Sci., 2018, vol. 555, p. 185.
Merkel, A., Ashrafi, A.M., and Ondrušek, M., The use of electrodialysis for recovery of sodium hydroxide from the high alkaline solution as a model of mercerization waste water, J. Water Process. Eng., 2017, vol. 20, p. 123.
Kareb, O., Gomaa, A., Champagne, C., Jean, J., et al., Electro-activation of sweet defatted whey: Impact on the induced Maillard reaction products and bioactive peptides, Food Chem., 2017, vol. 221, p. 590. https://doi.org/10.1016/j.foodchem.2016.11.134
Kareb, O., Champagne, C., Jean, J., Gomaa, A., et al., Effect of electro-activated sweet whey on growth of Bifidobacterium, Lactobacillus, and Streptococcus strains under model growth conditions, Food Res. Int., 2018, vol. 103, p. 316. https://doi.org/10.1016/j.foodres.2017.10.060
Vrabie, E., Bologa, M., Paladii, I., Stepurina, T., et al., Electrical processing of whey. Role of construction, technological and energy characteristics of reactors, Surf. Eng. Appl. Electrochem., 2019, vol. 55, p. 197.
Simova, H., Kysela, V., and Černín, A., Demineralization of natural sweet whey by electrodialysis at pilot-plant scale, Desalin. Water Treat., 2010, vol. 14, p. 170.
Prazeres, A.R., Carvalho, F., and Rivas, J., Cheese whey management: A review, J. Environ. Manage., 2012, vol. 110, p. 48. https://doi.org/10.1016/j.jenvman.2012.05.018
Misun, D., Curda, L., and Jelen, P., Batch and continuous hydrolysis of ovine whey proteins, Small Ruminant Res., 2008, vol. 79, no. 1, p. 51.
Pereira, C.D., Diaz, O., Cobos, A., Valorization of by-products from ovine cheese manufacture: Clarification by thermocalcic precipitation/microfiltration before ultrafiltration, Int. Dairy J., 2002, vol. 12, no. 9, p. 773.
Toro-Sierra, J., Tolkach, A., and Kulozik, U., Frac-tionation of α-lactalbumin and β-lactoglobulin from whey protein isolate using selective thermal aggregation, an optimized membrane separation procedure and resolubilization techniques at pilot plant scale, Food Bioprocess Technol., 2013, vol. 6, p. 1032.
Wolz, M. and Kulozik, U., Thermal denaturation kinetics of whey proteins at high protein concentrations, Int. Dairy J., 2015, vol. 49, p. 95.
Joyce, A.M., Kelly, A., and O’Mahony, J., Controlling denaturation and aggregation of whey proteins during thermal processing by modifying temperature and calcium concentration, Int. J. Dairy Technol., 2018, vol. 71, no. 2, p. 446.
Asenjo, J. and Andrews, B., Aqueous two-phase systems for protein separation: Phase separation and applications, J. Chromatogr. A, 2012, vol. 1238, p. 1. https://doi.org/10.1016/j.chroma.2012.03.049
Glyk, A., Scheper, T., and Beutel, S., PEG-salt aqueous two-phase systems: An attractive and versatile liquid-liquid extraction technology for the downstream processing of proteins and enzymes, Appl. Microbiol. Biotechnol., 2015, vol. 99, no. 16, p. 6599. https://doi.org/10.1007/s00253-015-6779-7
Raja, S., Murty, V. R., Thivaharan, V., Rajasekar, V., et al., Aqueous two phase systems for the recovery of biomolecules—A review, Sci. Technol., 2011, vol. 1, p. 7. https://doi.org/10.5923/j.scit.20110101.02
Gai, Q., Qu, F., Zhang, T., and Zhang, Y., Integration of carboxyl modified magnetic particles and aqueous two-phase extraction for selective separation of proteins, Talanta, 2011, vol. 85, p. 304. https://doi.org/10.1016/j.talanta.2011.03.055
Nitsawang, S., Hatti-Kaul, R., and Kanasawud, P., Purification of papain from Carica papaya latex: Aqueous two-phase extraction versus two-step salt precipitation, Enzyme Microb. Technol., 2006, vol. 39, p. 1103. https://doi.org/10.1016/j.enzmictec.2006.02.013
Ponni, M.M. and Shamnamol, G.K., Extraction of cheese whey protein from dairy effluent by using polyethylene glycol and sodium sulphate, Int. J. Innovative Res. Sci. Eng. Technol., 2015, vol. 4, no. 3, p. 868.
Su, H., Wang, Z., and Tan, T., Preparation of a surface molecular-imprinted adsorbent for Ni2 based on Penicillium chrysogenum, J. Chem. Technol. Biotechnol., 2005, vol. 80, no. 4, p. 439.
Su, H., Zhao, Y., Li, J., and Tan, T., Biosorption of Ni2+ by the surface molecular imprinting adsorbent, Process Biochem., 2006, vol. 41, no. 6, p. 1422.
Sternberg, M., Chiang, J.P., and Eberts, N.J., Cheese whey proteins isolated with polyacrylic acid, J. Dairy Sci., 1975, vol. 59, no. 6, p. 1042.
Kowalik-Klimczak, A., The possibilities of using membrane filtration in the dairy industry, J. Mach. Constr. Maint., 2017, vol. 105, no. 2, p. 99.
Mikhailenko, I.G. and Budrik, V.G., Membrane technologies and whey processing, Materialy III Mezhdunarodnoi nauchno-prakticheskoi konferentsii “Innovatsionnye issledovaniya i razrabotki dlya nauchnogo obespecheniya proizvodstva i khraneniya ekologicheski bezopasnoi sel’skokhozyaistvennoi i pishchevoi produktsii” (Proc. III Int. Sci.-Pract. Conf. “Innovative Researches and Developments for Scientific Support of the Production and Storage of Environmentally Healthy Agricultural and Food Products”), Krasnodar: Vseross. Nauchno-Issled. Inst. Tab., Makhorki Tab. Izd., 2019, part 2, p. 608.
Akpinar-Bayizit, A., Ozcan, T., and Yilmaz-Ersan, L., Membrane processes in production of functional whey components, Mljekarstvo, 2009, vol. 59, no. 4, p. 282.
Yorgun, M.S., Balcioglu, I.A., and Saygin, O., Performance comparison of ultrafiltration, nanofiltration and reverse osmosis on whey treatment, Desalination, 2008, vol. 229, nos. 1–3, p. 204.
Zolotoreva, M.S. and Topalov, V.K., Membrane processes in whey processing technology, Pererab. Moloka, 2014, no. 5, p. 10.
Garud, R.M., Kore, S.V., Kore, V.S., and Kulkarni, G.S., A short review on process and applications of reverse osmosis, Univ. J. Environ. Res. Technol., 2011, vol. 1, no. 3, p. 233.
Velpula, S., Umapathy, K.S., Thyarla, A., Srikanth, K., et al., Dairy wastewater treatment by membrane systems. A review, Int. J. Pure App. Biosci., 2017, vol. 5, no. 6, p. 389.
Gavazzi-April, C., Benoit, S., Doyen, A., Britten, M., et al., Preparation of milk protein concentrates by ultrafiltration and continuous diafiltration: Effect of process design on overall efficiency, J. Dairy Sci., 2018, vol. 101, no. 11, p. 9670.
Tan, R. and Franzreb, M., Continuous ultrafilt-ration/diafiltration using a 3D-printed two membrane single pass module, Biotechnol. Bioeng., 2019, vol. 117, p. 654.
Nambiar, A., Li, Y., and Zydney, A.L., Countercurrent staged diafiltration for formulation of high value proteins, Biotechnol. Bioeng., 2018, vol. 115, no. 1, p. 139.
Ebersold, M.F. and Zydney, A.L., The effect of membrane properties on the separation of protein charge variants using UF, J. Membr. Sci., 2004, vol. 243, p. 379.
Baldasso, C., Barros, T.C., and Tessaro, I.C., Concentration and purification of whey proteins by ultrafiltration, Desalination, 2011, vol. 278, p. 381.
Evdokimov, I.A., Volodin, D.N., Somov, V.S., Chablin, B.V., et al., Membrane technologies in dairy industry, Molochn. Prom-st., 2013, no. 9, p. 15.
Steinhauer, T., Marx, M., Bogendörfer, K., and Kulozik, U., Membrane fouling during ultra- and microfiltration of whey and whey proteins at different environmental conditions: The role of aggregated whey proteins as fouling initiators, J. Membr. Sci., 2015, vol. 489, p. 20. https://doi.org/10.1016/j.memsci.2015.04.002
Baruah, G.L., Venkiteshwaran, A., and Belfort, G., Global model for optimizing crossflow microfiltration and ultrafiltration processes: A new predictive and design tool, Biotechnol. Prog., 2005, vol. 21, no. 4, p. 1013. https://doi.org/10.1021/bp050184r
Nelson, B.K. and Barbano, D.M., A microfiltration process to maximize removal of serum proteins from skin milk before cheese making, J. Dairy Sci., 2005, vol. 88, p. 1891.
García, L.F., Blanco, S., and Rodríguez, F., Microfiltration applied to dairy streams: Removal of bacteria, J. Sci. Food Agric., 2013, vol. 93, no.2, p. 187.
Barukčić, I., Božanić, R., and Kulozik, U., Influence of process temperature and microfiltration pre-treatment on flux and fouling intensity during cross-flow ultrafiltration of sweet whey using ceramic membranes, Int. Dairy J., 2015, vol. 51, p. 1.
Iltchenco, S., Preci, D., Bonifacino, C., Franco Fragua, E., et al., Whey protein concentration by ultrafiltration and study of functional properties, Ciên. Rural, 2018, vol. 48, no. 5, p. 1.
Hinkova, A., Zidova, P., Pour, V., Bubník, Z., et al., Potential of membrane separation processes in cheese whey fractionation and separation, Procedia Eng., 2012, vol. 42, p. 1425. https://doi.org/10.1016/j.proeng.2012.07.536
Kumar, P., Sharma, N., Ranjan, R., Kumar, S., et al., Perspective of membrane technology in dairy industry: a review, Asian-Aust. J. Anim. Sci., 2013, vol. 26, no. 9, p. 1347. https://doi.org/10.5713/ajas.2013.13082
Atra, R., Vatai, G, Bekassy-Molnar, E., and Balint, A., Investigation of ultra- and nanofiltration for utilization of whey protein and lactose, J. Food Eng., 2005, vol. 67, p. 325.
Rice, G., Kentish, S., O’Connor, A., Stevens, G., et al., Fouling behavior during the nanofiltration of dairy ultrafiltration permeate, Desalination, 2006, vol. 199, p. 239.
Ismail, B. and Nielsen, S.S, Basic Principles of Chromatography, Food Analysis, Nielsen, S.S., Ed., Boston, MA: Springer-Verlag, 2010, pp. 473–498. https://doi.org/10.1007/978-1-4419-1478-1_27
Etzel, M.R., Manufacture and use of dairy protein fractions, J. Nutr., 2004, vol. 134, no. 4, p. 996.
El-Sayed, M.M.H. and Chase, H.A., Purification of the two major proteins from whey concentrate using a cation-exchange selective adsorption process, Biotechnol. Prog., 2010, vol. 26, no. 1, p. 192.
Santos, M.J., Teixeira, J., and Rodrigues, L.R., Fractionation of the major whey proteins and isolation of β-lactoglobulin variants by anion exchange chromatography, Sep. Purif. Technol., 2012, vol. 90, p. 133.
Andersson, J. and Mattiasson, B. Simulated moving bed technology with a simplified approach for protein purification. Separation of lactoperoxidase and lactoferrin from whey protein concentrate, J. Chromatogr. A, 2006, vol. 1107, nos. 1–2, p. 88.
Geisser, A., Hendrich, T., Boehm, G., et al., Separation of lactose from human milk oligosaccharides with simulated moving bed chromatography, J. Chromatogr. A, 2005, vol. 1092, no. 1, p. 17.
Sturaro, A., Marchi, M.D., Masi, A., and Cassandro, M., Quantification of whey proteins by reversed phase-HPLC and effectiveness of mid-infrared spectroscopy for their rapid prediction in sweet whey, J. Dairy Sci., 2016, vol. 99, no. 1, p. 68.
Heeboll-Nielsen, A., Justesen, S.F.L., Hobley, T.J., and Thomas, O.R.T., Super paramagnetic cation-exchange adsorbents for bioproduct recovery from crude process liquors by high-gradient magnetic fishing, Sep. Sci. Technol., 2004, vol. 39, no. 12, p. 2891. https://doi.org/10.1081/SS-200028791
Heeboll-Nielsen, A, Choewsmiddelberg, A.P.J., and Thomas, O.R.T., Efficient inclusion body processing using chemical extraction and high-gradient magnetic fishing, Biotechnol. Prog., 2003, vol. 19, no. 3, p. 887.
Heeboll-Nielsen, A., Justesen, S.F.L., and Thomas, O.R.T., Fractionation of whey proteins with high-capacity super paramagnetic ion-exchangers, J. Biotechnol., 2004, vol. 113, nos. 1–3, p. 247.
Hubbuch, J.J., Matthiesen, D.B., Hobley, T.J., et al., High-gradient magnetic separation versus expanded bed adsorption: A first principle comparison, Bioseparation, 2001, vol. 10, p. 99.
Stanic, D., Radosavljevic, J., Stojadinovic, M., and Velickovic, T.C., Application of ion exchanger in the separation of whey proteins and lactin from milk whey, in Ion Exchange Technology II, Inamuddin, D. and Luqman, M., Eds., Dordrecht: Springer-Verlag, 2012, pp. 35–63.
Tsonev, L.I. and Hirsh, A.G., Theory and applications of a novel ion-exchange chromatographic technology using controlled ph gradients for separating proteins on anionic and cationic stationary phases, J. Chromatogr. A, 2008, vol. 1200, no. 2, p. 166.
Pessela, B. C., Munilla, R., Betancor, L., Fuentes, M., et al., Ion-exchange using poorly activated supports, an easy way for purification of large proteins, J. Chromatogr. A, 2004, vol. 1034, nos. 1–2, p. 155.
Bolivar, J.M., Batalla, P., Mateo, C., Carrascosa, A.V., et al., Selective adsorption of small proteins on large pore anion-exchangers coated with medium size proteins, Colloid Surf. B, 2010, vol. 78, no. 1, p. 140.
Blanc, F., Bernard, H., Alessandri, S., Bublin, M., et al., Update on optimized purification and characterization of natural milk allergens, Mol. Nutr. Food Res., 2008, vol. 52, suppl. 2, p. 166.
Li, X., Luo, Z.L., Chen, H.B., and Cao, Y.S., Isolation and antigenicity evaluation of beta-lactoglobulin from buffalo milk, Afr. J. Biotechnol., 2008, vol. 7, no. 13, p. 2258.
Pinto, N.D. and Frey, D., Displacement chromatography of proteins using a retained pH front in a hydrophobic charge induction chromatography column, J. Chromatogr. A, 2015, vol. 1387, p. 53.
Tugcu, N., Purification of proteins using displacement chromatography, Methods Mol. Biol. (Clifton, NJ), 2008, vol. 421, p. 71. https://doi.org/10.1007/978-1-59745-582-4_6
Kalász, H., Displacement chromatography, J. Chromatogr. Sci., 2003, vol. 41, p. 281. https://doi.org/10.1093/chromsci/41.6.281
Zhao, G. and Sun, Y., Displacement chromatography of proteins on hydrophobic charge induction adsorbent column, J. Chromatogr. A, 2007, vol. 1165, nos. 1–2, p. 109.
Srajer Gajdosik, M., Clifton, J., and Josic, D., Sample displacement chromatography as a method for purification of proteins and peptides from complex mixtures, J. Chromatogr. A, 2012, vol. 1239, p. 1.
Giovannini, R. and Freitag, R., Continuous separation of multicomponent protein mixtures by annular displacement chromatography, Biotechnol. Prog., 2002, vol. 18, no. 6, p. 1324.
Rossano, R., D’Elia, A., and Riccio, P., One-step separation from lactose: Recovery and purification of major cheese-whey proteins by hydroxyapatite—a flexible procedure suitable for small- and medium-scale preparations, Protein Expression Purif., 2001, vol. 21, no. 1, p. 165.
Cummings, L.J., Snyder, M.A., and Brisack, K., Protein chromatography on hydroxyapatite columns, Method Enzymol., 2009, vol. 463, p. 387.
Schlatterer, B., Baeker, R., and Schlatterer, K., Improved purification of β-lactoglobulin from acid whey by means of ceramic hydroxyapatite chromatography with sodium fluoride as a displacer, J. Chromatogr. B, 2004, vol. 807, no. 2, p. 223.
Ng, P.K. and Yoshitake, T. Purification of lactoferrin using hydroxyapatite, J. Chromatogr. B, 2010, vol. 878, no. 13–14, p. 976.
Tercinier, L., Ye, A., Anema, S., Singh, A., et al., Characterization of milk protein adsorption onto hydroxyapatite, Int. Dairy J., 2017, vol. 66, p. 27.
Dainiak, M.B., Kumar, A., Plieva, F.M., Galaev, I.Y., et al., Integrated isolation of antibody fragments from microbial cell culture fluids using supermacro porous cryogels, J. Chromatogr. A, 2004, vol. 1045, nos. 1–2, p. 93.
Jungbauer, A. and Hahn, R., Monoliths for fast bioseparation and bioconversion and their applications in biotechnology, J. Sep. Sci., 2004, vol. 27, nos. 10–11, p. 767.
Lozinsky, V., Plieva, F., Galaev, I., et al., The potential of polymeric cryogels in bioseparation, Bioseparation, 2001, vol. 10, no. 4, p. 163.
Gerstner, J.A., Hamilton, R., and Cramer, S.M., Membrane chromatographic systems for high through put protein separations. J. Chromatogr. A, 1992, vol. 596, no. 2, p. 173.
Neuville, B.C., Lamprou, A., Morbidelli, M., and Soos, M., Perfusive ion-exchange chromatographic materials with high capacity, J. Chromatogr. A, 2014, vol. 1374, p. 180.
Urban, J. and Jandera, P., Polymethacrylate monolithic columns for capillary liquid chromatography, J. Sep. Sci., 2008, vol. 31, no. 14, p. 2521.
Kawai, T., Saito, K., and Lee, W., Protein binding to polymer brush, based on ion-exchange, hydrophobic, and affinity interactions, J. Chromatogr. B, 2003, vol. 790, nos. 1–2, p. 131.
Lalli, E., Silva, J., Boi, C., and Sarti, G., Affinity membranes and monoliths for protein purification, Membranes, 2019, vol. 10, p. 1.
Voswinkel, L. and Kulozik, U., Fractionation of whey proteins by means of membrane adsorption chromatography, Procedia Food Sci., 2011, vol. 1, p. 900.
Leeb, E., Holder, A., Letzel, T., Cheison, S.C., et al., Fractionation of dairy based functional peptides using ion-exchange membrane adsorption chromatography and cross-flow electro membrane filtration, Int. Dairy J., 2014, vol. 38, p. 116.
Plate, K., Beutel, S., Buchholz, H., Demmerc, W., et al., Isolation of bovine lactoferrin, lactoperoxidase and enzymatically prepared lactoferricin from proteolytic digestion of bovine lactoferrin using adsorptive membrane chromatography, J. Chromatogr. A, 2006, vol. 1117, no. 1, p. 81.
Wang, H., Yang, R., Hua, X., Zhao, W., et al., Enzymatic production of lactulose and 1-lactulose: Current state and perspectives, Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 14, p. 6167.
Panesar, P.S. and Kumari, S., Lactulose: Production, purification and potential applications, Biotechnol. Adv., 2011, vol. 29, no. 6, p. 940.
Song, Y.S., Lee, H.U., Park, C., and Kim, S.W., Optimization of lactulose synthesis from whey lactose by immobilized β-galactosidase and glucose isomerase, Carbohydr. Res., 2013, vol. 369, p. 1.
Song, Y.S., Lee, H.U., Park, C., and Kim, S.W., Batch and continuous synthesis of lactulose from whey lactose by immobilized β-galactosidase, Food Chem., 2013, vol. 136, no. 2, p. 689.
Illanes, A., Whey upgrading by enzyme biocatalysis, Electron. J. Biotechnol., 2011, vol. 14, no. 6, p. 9.
Ryan, M. and Walsh, G., The biotechnological potential of whey, Rev. Environ. Sci. Biotechnol., 2016, vol. 15, p. 479.
Sitanggang, A.B., Drews, A., and Kraume, M., Development of a continuous membrane reactor process for enzyme-catalyzed lactulose synthesis, Biochem. Eng. J., 2016, vol. 109, p. 65.
Bhattacharjee, S. and Sarker, D., Kinetic study of enzymatic hydrolysis of lactose in whey, Int. J. Chem. Eng. Res., 2017, vol. 9, no. 2, p. 223.
Mota, M.V.T., Ferreira, I.M.P.L.V.O., Oliveira, M.B.P., Rocha, C., et al., Enzymatic hydrolysis of whey protein concentrates: Peptide HPLC profiles, J. Liq. Chromatogr. Relat. Technol., 2004, vol. 27, no. 16, p. 2625.
Ghosh, B.C., Prasad, L.N., and Saha, N.P., Enzymatic hydrolysis of whey and its analysis, J. Food Sci. Technol., 2017, vol. 54, no. 6, p. 1476.
Morais, H.A., Silvestre, M.P.C., Silva, M.R.V., Silva, D.M., et al., Enzymatic hydrolysis of whey protein concentrate: Effect of enzyme type and enzyme: substrate ratio on peptide profile, J. Food Sci. Technol., 2015, vol. 52, no. 1, p. 201.
Stamatelatou, K., Giantsiou, N., Diamantis, V., Alexandridis, C., et al., Biogas production from cheese whey wastewater: Laboratory-, and full-scale studies, Water Sci. Technol., 2014, vol. 69, no. 6, p. 1320.
Goli, A., Shamiri, A., Khosroyar, S., Talaiekhozani, A., et al., A review on different aerobic and anaerobic treatment methods in dairy industry wastewater, J. Environ. Treat. Tech., 2019, vol. 6, no. 1, p. 113.
Bajpai, P., Anaerobic reactors used for waste water treatment, in Anaerobic Technology in Pulp and Paper Industry, Singapore: Springer-Verlag, 2017, pp. 37–53.
Tatoulis, T.I., Tekerlekopoulou, A.G., Akratos, C., Pavlou, S., et al., Aerobic biological treatment of second cheese whey in suspended and attached growth reactors, J. Chem. Technol. Biotechnol., 2015, vol. 90, p. 2040.
Aspasia, A., Chatzipaschali, A., and Stamatis, G., Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects, Energies, 2012, vol. 5, p. 3492. https://doi.org/10.3390/en5093492
Kataki, S., West, H., Clarke, M., and Baruah, D., Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments, Waste Manage., 2016, vol. 49, p. 437.
Escalante, H., Castro, L., Besson, V., and Jaimes-Estevez, J., Feasibility of the anaerobic process of cheese whey in a plug flow reactor (PFR), Ing., Invest. Tecnol., 2017, vol. 8, no. 3, p. 265.
Hassan, A. and Nelson, B., Invited review: anaerobic fermentation of dairy food wastewater, J. Dairy Sci., 2012, vol. 95, no. 11, p. 6188.
Chen, Y., Cheng, J.J., and Creamer, K.S., Inhibition of anaerobic digestion process: A review, Bioresour. Technol., 2008, vol. 99, no. 10, p. 4044. https://doi.org/10.1016/j.biortech.2007.01.057
Najafpour, G.D., Hashemiyeh, B.A., Asadi, M., and Ghasemi, M.B., Biological treatment of dairy wastewater in an up flow anaerobic sludge-fixed film bioreactor, Am. Eurasian J. Agric. Environ. Sci., 2008, vol. 4, p. 251.
Kelleher, B.P., Leahy, J.J., Henihan, A.M., O’Dwyer, T.F., et al., Advances in poultry litter disposal technology—A review. Bioresour. Technol., 2002, vol. 83, p. 27.
Audic, J.L., Chaufer, B., and Daufin, G., Non-food applications of milk components and dairy co-products: A review, Lait, 2003, vol. 83, p. 417.
Dereli, R.K., Zee, F.P., Ozturk, I., and Lier, J.V., Treatment of cheese whey by a cross-flow anaerobic membrane bioreactor: Biological and filtration performance, Environ. Res., 2019, vol. 168, p. 109.
Frigon, J., Breton, J., Bruneau, T., Moletta, R., et al., The treatment of cheese whey wastewater by sequential anaerobic and aerobic steps in a single digester at pilot scale, Bioresour. Technol., 2009, vol. 100, no. 18, p. 4156.
Gannoun, H., Khelifi, E., Bouallagui, H., Touhami, Y., et al., Ecological clarification of cheese whey prior to anaerobic digestion in up flow anaerobic filter, Bioresour. Technol., 2008, vol. 99, p. 6105.
Pilarska, A., Pilarski, K., Witaszek, K., Waliszewska, H., et al., Treatment of dairy waste by anaerobic co-digestion with sewage sludge, Ecol. Chem. Eng., 2016, vol. 23, p. 115.
Garshina, T.I., Processing of milk whey by electrodialysis, Molochn. Prom-st., 2012, no. 11, p. 55.
Merkel, A. and Ashrafi, A.M., An investigation on the application of pulsed electrodialysis reversal in whey desalination, Int. J. Mol. Sci., 2019, vol. 20, no. 8, p. 1918. https://doi.org/10.3390/ijms20081918
Greiter, M., Novalin, S., Wendland, M., and Kulbe, K., Desalination of whey by electrodialysis and ion exchange resins: Analysis of both processes with regard to sustainability by calculating their cumulative energy demand, J. Membr. Sci., 2002, vol. 210, no. 1, p. 91.
Khomenkov, A., Il’ina, S.Yu., and Kaigorodova, O., The use of electrodialysis for whey processing, Nauchn. Dialog: Molodoi Uch., 2018, p. 31. https://doi.org/10.18411/spc-22-01-2018-09
Bakhir, V.M., Zadorozhnii, Yu.G., Leonov, B.I., Panicheva, S.A., et al., Elektrokhimicheskaya aktivatsiya: ochistka vody i poluchenie poleznykh rastvorov (Electrochemical Activation: Water Treatment and Production of Useful Solutions), Moscow: Vseross. Nauchno-Issled. Ispyt. Inst. Med. Tekh., 2001, p. 176.
Bakhir, V.M., Electrochemical activation: The key to sustainable water treatment technologies, Vodosnabzh. Kanaliz., 2012, nos. 1–2, p. 89.
Bologa, M., Sprinchan, E., and Bologa, Al., Recovery of lactulose products and protein-mineral concentrate, Surf. Eng. Appl. Electrochem., 2008, vol. 44, p. 410. https://doi.org/10.3103/S1068375508050128
Aider, M. and Gimenez-Vidal, M., Lactulose synthesis by electroisomerization of lactose: Effect of lactose concentration and electric current density, Innovative Food Sci. Emerging Technol., 2012, vol. 16, p. 163.
Khramtsov, A.G., Evdokimov, I.A., Ryabtseva, S.A., and Suyuncheva, B.O., Isomerization of lactose into lactulose during the electroactivation of milk whey, Khranenie Pererab. Sel’khozsyr’ya, 2004, no. 10, p. 25.
Sara, C., Silverio, E.A., Macedo, J.A.T., and Rodrigues, R., Biocatalytic approaches using lactulose: End product compared with substrate, comprehensive review, Food Sci. Food Saf., 2016, vol. 15, p. 878. https://doi.org/10.1111/1541-4337.12215
Sinel’nikov, B.M., Khramtsov, A.G., Evdokimov, I.A., Ryabtseva, S.A., et al., Laktoza i ee proizvodnye (Lactose and Its Derivatives), St. Petersburg: Professiya, 2007.
Permyakov, S., Uversky, V., Veprintsev, D., Cherskaya, A., et al., Mutating aspartate in the calcium-binding site of α-lactalbumin: Effects on the protein stability and cation binding, Protein Eng., 2001, vol. 14, no. 10, p. 785.
Ait Aissa, A. and Aider, M., Lactose isomerization into lactulose in an electro-activation reactor and high-performance liquid chromatography (HPLC) monitoring of the process, J. Food Eng., 2013, vol. 119, p. 115.
Djouab, A. and Aider, M., Whey permeate integral valorisation via in situ conversion of lactose into lactulose in an electro-activation reactor modulated by anion and cation exchange membranes, Int. Dairy J., 2019, vol. 89, p. 6.
Sprinchan (Vrabie), E., Bologa, M., Stepurina T., Bologa Al., et al., Peculiarities of the electric activation of whey, Surf. Eng. Appl. Electrochem., 2011, vol. 47, p. 66.
Bologa, M.K., Vrabie, E.G., and Stepurina, T.G., Peculiarities of mineralization of concentrates at electrophysical treatment of milk whey, Surf. Eng. Appl. Electrochem., 2013, vol. 49, no. 6, p. 504. https://doi.org/10.3103/S1068375513060057
ACKNOWLEDGMENTS
The authors thank Dr. Olga Iliasenco for the technical assistance in preparing this paper.
Funding
This work has been performed in the framework of the ANCD project no. 20.80009.5007.06 (2020–2023) “Intensification of the Processes of Transfer and Treatment in Electric, Electromagnetic, Cavitation Fields; Practicality.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Myshkina
About this article
Cite this article
Paladii, I.V., Vrabie, E.G., Sprinchan, K.G. et al. Whey: Review. Part 2. Treatment Processes and Methods. Surf. Engin. Appl.Electrochem. 57, 651–666 (2021). https://doi.org/10.3103/S1068375521060119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375521060119