Abstract
In this study was proposed a new analytical technique for the extraction of two organophosphorus pesticides (OPPS) in water samples followed by gas chromatography−flame ionization detector (GC−FID) analysis. This technique, called organic gas steam−liquid extraction (OGS−LE), was performed by using a special home-made extraction cell that was designed to extraction without emulsification and with high extraction efficiency by a small amount of organic solvent. In this method, 2 mL extraction solvent (n-heptane) was added to the assigned column in the extraction cell inside the heating chamber. The organic solvent column was warmed up at 78°C. The aqueous sample solution (25 mL) was injected into the assigned column in the extraction cell by a syringe. Using N2 flotation into the organic solvent, the gas steam of the organic solvent was transferred to the aqueous column. N2 bubbles in the aqueous column moved upward from the bottom and interacted with the aqueous phase. The organic gas steam, along with N2 bubbles, after desolations in the aqueous sample and supersaturating, was collected on the surface of the aqueous sample. By using N2 flotation, the organic solvent was collected on the top of the solution without emulsification. The organic solvent was collected by means of a microsyringe. Then, 1 μL of the collected organic solvent was injected into the GC−FID for analysis. One variable at a time (OVAT) was applied to investigate the optimum conditions of all the variables. The variables of interest in the OGS−LE were selected as extraction solvent type and volume, ionic strength, the temperature of the heating chamber, extraction time, flow rate of the carrier gas and volume of the sample solution. Using optimized variables in the extraction process, for chlorpyrifos and diazinon, the detection limits, the precisions and the linearity of the method were found 41.6 and 21.9 ng mL–1, 1.8 and 7.1 (RSD, n = 3), 50–5000 μg mL–1 and 50–5000 μg mL–1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Organophosphate pesticides (OPPs) such as chlorpyrifos and diazinon are widely used in agriculture to control pests and fruit and vegetable crops. They may persist in the soil, surface water and on the surface of the plants so rain can wash them on soil and plants into surface waters and they can enter to human food cycle. Therefore, the analysis of OPPs by a simple, sensitive and affordable method in food, water and environmental samples is significant [1]. Various methods have been developed by researchers to determine trace amounts of OPPs such as thin layer chromatography (TLC) [2], gas chromatography [3] and high pressure liquid chromatography [4]. In recent years, preparation techniques to analysis with less organic solvent consumption like dispersive liquid−liquid microextraction (DLLME) [5, 6], homogeneous liquid−liquid microextraction (HLLME) [7, 8] and homogeneous liquid−liquid microextraction by flotation assistance (HLLME-FA) [9, 10] were notable. In the present study, the organic gas steam−liquid extraction method (OGS−LE) was applied as a fast, simple, efficient, economic, environment friendly and selective extraction of two types of OPPs. Then, the determination takes place by GC−FID. Some main benefits of the OGS−LE system are: no need for dispersive solvent compared to HLLME-FA that caused to achieve bigger distribution coefficient and more extraction efficiency, elimination of centrifugation step compared to DLLME, possibility to use solvents with lower density than water compares to other microextraction methods. Extra extraction efficiency and less solvent consumption compared to the traditional liquid−liquid extraction method due to better interaction between the two phases.
EXPERIMENTAL
Materials
Two organophosphate pesticides were used in the present studies (chlorpyrifos and diazinon) obtained from Dr. Ehrenstorfer (Augsburg, Germany). To obtain concentration of 1000 mg L–1 of compounds, calculated amount of one (0.997 and 1.060 g, respectively) was dissolved in 100 mL Ethanol then stock solutions were prepared by diluting with water. Sodium chloride was also from Merck Co. (Darmstadt, Germany). n-Heptane and ethanol used were from Merck Co. (residue grade, Darmstadt, Germany). All other chemicals and solvents were of analytical grade or better from Merck Company.
Apparatus
The determination of compounds was carried out using gas chromatography (GC, Agilent 6890 N, Palo Alto, CA, USA). The features and operating conditions of GC-FID system were as follows: equipped with split/splitless injector with split ratio 1 : 1 and 250°C temperature, DB-5 MS (5% phenylmethyl polysiloxane fused silica capillary column, 30 m length, 0.25 mm i.d. and 0.25 μm film thickness) and helium (purity 99.999%, Farafan gas, Iran) as carrier gas at a constant flow rate of 1 mL min–1. The GC oven temperature was as follows: initial temperature 50°C for 3 min, 25°C min–1 to 100°C for 2 min, again with 30°C min–1 increasing to 280°C and holding for 10 min. The detector temperature was set at 250°C. The extracted organic solvent (1 μL) was injected using a 5 μL microsyringe (Hamilton, Reno, NV, Australia). Chemstation software (G1701EA Ver. E.02.01.1177) was used for data acquisition.
To control the N2 gas flow rate as evaporating gas (in OGS−LE procedure) a metering valve (Hock, Spartanburg, SC, USA) was used.
The weight process chemicals took place by using a balance (Mettler Toledo, AB204-S, USA).
The temperature of the organic solvent column (in OGS−LE cell) was maintained at the desired value (±0.1°C) by circulating thermostat a mixture of water and ethylene glycol (Wisecircu, Witeg Co., Germany).
OGS−LE Procedure
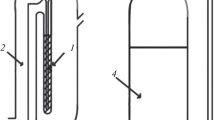
As shown in Fig. 1 the extraction cell consists of three columns: (1) First column named heated chamber that includes an organic solvent (2 mL n-heptane) tube. This column is warmed up by circulating thermostat a mixture of water and ethylene glycol to reach the adequate temperature for the organic solvent tube. Organic solvent and N2 gas inlet are on the top of this chamber while a liquid gas separator is in the bottom to prevent condensed liquid drops of the organic solvent entry into the second column. (2) The second column is a conical shape in at the top and is equipped with a drain valve in the bottom that includes the aqueous solution of analyte. (3) The third column is a narrow tube for addition of distilled water to enhance aqueous solution surface to easy collection of the organic solvent including analyte from the conical part in the second column.
Schematic representation of the experimental setup (OGS−LE Cell). (1) solvent inlet and gas inlet; (2) heated chamber; (3) liquid−gas separator; (4) cool water outlet; (5) solvent steam path; (6) hot water inlet; (7) organic solvent; (8) aqueous solution cell; (9) distilled water inlet; (10) N2 inlet; (11) N2 bubble contain solvent stream; (12) organic solvent condensation; (13) organic solvent contain extracted analyte.
25 mL of aqueous solution of pesticides was injected from the top of the second column. By using N2 flotation (flow rate 12 mL min–1) the organic gas steam (after setting the temperature at 78°C) from the first column transferred to the second column. N2 bubbles contain organic solvent steam interacted with aqueous sample solution and after desolvation and supersaturating of n-heptane, collected on the surface of aqueous solution. Finally, a little distilled water was added to raise organic solvent in the third column to facility draining by a syringe. Then, 1 μL of the collected organic solvent was injected into the GC-FID for analysis.
RESULTS AND DISCUSSION
Effects of parameters were studied and using one variable at a time (OVAT) method optimized, and the method performance was evaluated. In order to investigate the effect of parameters, the concentration of analyte, 500 μg L–1 was considered.
Effect of NaCl Concentration
The effect of NaCl concentration on extraction efficiency was investigated by a variable amounts of NaCl (0, 0.1, 0.5, 3, 6 mol L–1). By increasing ionic strength the peak area for both analyte (diazinon and chlorpyrifos) decreased which could because of increasing volume of collected organic solvent consequently dilution of analyte. The presence of salt prevents solvation and interaction of extracting solvent (n-heptane) in water samples. Maximum extraction was in zero concentration of NaCl, so we didn’t use NaCl in further steps.
Selection of Aqueous Phase Volume
To examine the effect of aqueous volume, different amounts of aqueous solution were evaluated (15, 20, 25 mL). By increasing the volume of the aqueous phase, the peak area of both analytes was increased because of increasing the amount of analyte in the aqueous phase, so the 25 mL was selected as the best one.
Effect of Extraction Temperature
In order to survey the temperature effect on extraction efficiency, the temperature of the heating chamber (73, 78, 83, 88, 93°C) was change by circulating thermostat a mixture of water and ethylene glycol by a circulator. At 73°C, the volume of collected organic solvent at the water sample surface was very small, as a result, there was no analysis possible. With increasing temperature, the peak area of analytes was decreased, because, the evaporation rate of the organic solvent was increased and the collected organic solvent volume was increased and concentration was decreased, thus, further extractions were performed at 78°C as optimum temperature (Fig. 2).
Time of Extraction
The time of extraction is one of the most important parameters in extraction efficiency. To check the time effect, the time range was varied between 10 to 30 min. Similar to extraction temperature, by increasing the time, the peak area decreased, because, evaporation amount of the organic solvent was increased and the collected organic solvent volume was increased and the concentration was decreased so, the extraction time of 10 min was achieved maximum efficiency. At the time of 10 min, the volume of collected organic solvent at the water sample surface was very small, as a result, there was no analysis possible.
Effect of Evaporating Gas Flow Rate
The survey of evaporating gas (N2) flow rate effect was investigated to obtain optimum ones in the range of 10–16 mL min–1. The maximum extraction was observed in 12 mL min–1. Similar to temperature and time of extraction effect, in upper flow rate the amount of collected organic solvent in conical part of the second column is going to increase so, the concentration of analytes will decrease. In a lower flow rate of 12 mL min–1 the amount of collected solvent on the aqueous phase surface isn’t noticeable for injection to GC.
Selection of Type and Volume of Extraction Solvent
The solvent selected should have a lower density of water. Benzene and n-heptane have this feature. According to the octanol−water partition coefficient (Kow), (Kow-n-heptane: 4.66; Kow-benzene: 2.13; Kow-diazinon: 3.81; Kow-chlorpyrifos: 4.96 [11]), the polarity of the analytes is similar to the n-heptane. Therefore, the extraction efficiency is expected to be higher by heptane. The experimental result showed that extraction efficiency by heptane was 4 times extracted by benzene. To select an appropriate n-heptane volume, different amounts of solvent were evaluated (1, 2, 3 and 4 mL). In this step, the goal was to gain the highest percentage of extraction with the lowest consumption of organic solvent. The maximum point was obtained by 2 mL of n-heptane, while in the upper volume of extraction solvent, the volume of the collected solvent increased and hence the concentration of the species decreased.
Analytical Evaluation
Under optimum conditions, regression equations, correlation coefficient (r2), dynamic linear range (DLR), limit of detection (LOD) for both analyte were calculated. DLRs were in the range of 50–5000 μg L–1 with 0.999 for r2. The LODs were evaluated as the analytes concentration equal to three times of the standard deviation of the blank signal divided by the slope of the calibration curve and LODs were 41.6 and 21.9 μg L–1 for chlorpyrifos and diazinon respectively. The reproducibility in peak response was investigated on three replicate experiments under the optimized conditions.
The preconcentration factor (PFs) were found as the ratio of the analytes peak area in the n-heptane contain 500 μg L–1 of each analyte to analytes peak area in the organic solvent after extraction from the aqueous solution contain 500 μg L–1 of each analyte, the peak area of the analyte was considered as a measure of concentration and PFs were 227 and 210 for chlorpyrifos and diazinon respectively.
OGS−LE technique and the other methods for the extraction and determination of chlorpyrifos and diazinon were compared in Table 1. The repeatability of this method is comparable to other methods. Even amount of the enrichment factor is better than other methods. Due to the higher sensitivity of the mass spectrometer detector, the methods used by this detector have a higher sensitivity to OGS−LE.
Analysis of Real Samples
The practical suitability of the developed OGS−LE method was confirmed by the determination of analytes in real samples (well, river and spring water), in Table 2 the results are presented. According to Table 2, no analyte was found in real samples. The spike method was applied to check the selectivity of OGS−LE for the analytes against the matrix effect and to determine the relative recovery, accuracy and the precision of the proposed extraction method. The water samples were spiked with 50 and 100 μg mL–1 for chlorpyrifos and diazinon then treated by OGS−LE method and determined by GC-FID. All samples were analyzed three times under optimized conditions. The results indicate a good agreement that could confirm the practical suitability of the method.
CONCLUSIONS
In the present research, the OGS−LE technique was developed as a simple, inexpensive, fast and efficient method for the extraction and preconcentration of two organophosphorus pesticides, chlorpyrifos and diazinon, from aqueous samples for the first time and followed by a gas chromatography-flame ionization detector (GC-FID) analysis. The developed method was convenient for the use of low-density extraction solvents. The new procedure of OGS−LE is distinguished from the DLLME methods, as it does not need centrifugation to separate the organic phase. Another advantage of this method, compared with the DLLMEand HLLE method, is that it does not require a third solvent to increase the contact area between the organic and aqueous phases.
REFERENCES
Hertz-Picciotto, I., Sass, J.B., Engel, S., Bennett, D.H., Bradman, A., Eskenazi, B., Lanphear, B., and Whyatt, R., Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms, PLoS Med., 2018, vol. 15, no. 10. https://doi.org/10.1371/journal.pmed.1002671
Yao, C., Cheng, F., Wang, C., Wang, Y., Guo, X., Gong, Z., Fan, M., and Zhang, Z., Separation, identification and fast determination of organophosphate pesticide methidathion in tea leavesby thin layer chromatography—surface-enhanced Raman scattering, Anal. Methods, 2013, vol. 5, pp. 5560–5564.
Rastrelli, L., Totaro, K., and De Simone, F., Determination of organophosphorus pesticide residues in Cilento (Campania, Italy) virgin olive oil by capillary gas chromatography, Food Chem., 2002, vol. 79, pp. 303–305.
Carabias Martinez, R., Rodriguez Gonzalo, E., Amigo Moran, M.J., and Mendez, J.H., Sensitive method for the determination of organophosphorus pesticides in fruits and surface waters by high-performance liquid chromatography with ultraviolet detection, J. Chromatogr., 1992, vol. 607, pp. 37–45.
Zhao, E., Zhao, W., Han, L., Jiang, S., and Zhou, Z., Application of dispersive liquid-liquid microextraction for the analysis of organophosphorus pesticides in watermelon and cucumber, J. Chrom. A, 2007, vol. 1175, pp. 137–140.
Chu, S.P., Huang, C.K., Chen, P.S., and Huang, S.D., Two dispersive liquid-liquid microextraction methodscoupled with gas chromatography-mass spectrometry for the determination of organophosphoruspesticides in field water, Environ. Chem., 2014, vol. 11, pp. 661–672.
Farajzadeh, M.A., Feriduni, B., and Afshar Mogaddam, M.R., Development of a new extraction method based on counter current salting-out homogenous liquid−liquid extraction followed by dispersive liquid–liquid microextraction: Application for theextraction and preconcentration of widely used pesticides from fruit juices, Talanta, 2016, vol. 146, pp. 772–779.
Berijani, S., Sadigh, M., and Pournamdari, E., Homogeneous liquid-liquid microextraction for determination of organophosphorus pesticides in environmental water samples prior to gas chromatography-flame photometric detection, J. Chromatogr. Sci., 2015, vol. 54, pp. 1061–1067.
Haji Hosseini, M., Rezaee, M., Mashayekhi, H.A., Akbarian, S., Mizani, F., and Pourjavid, M.R., Determination of polycyclic aromatic hydrocarbons in soil samples using flotation-assisted homogeneous liquid-liquid microextraction, J. Chromatogr. A, 2012, vol. 1265, pp. 52–56.
Haji Hosseini, M., Rezaee, M., Akbarian, S., Mizani, F., Pourjavid, M.R., and Arabieh, M., Homogeneous liquid-liquid microextraction via flotation assistance for rapid and efficient determination of polycyclic aromatic hydrocarbons in water samples, Anal. Chim. Acta, 2012, vol. 762, pp. 54–60.
Debruijn, J. and Hermens, J., Uptake and elimination kinetics of organophophorous pesticides in the guppy (Poecilla reticulata): correlations with the octanol/water partition coefficient, Environ. Toxicol. Chem., 1991, vol. 10, pp. 791–804.
Farajzadeh, M.A. and Abbaspour, M., Development of a new sample preparation method based on liquid–liquid–liquid extraction combined with dispersive liquid-liquid microextraction and its application on unfiltered samples containing high content of solids, Talanta, 2017, vol. 174, pp. 111–121.
Pano-Farias, N.S., Ceballos-Magaña, S.G., Muñiz-Valencia, R., Jurado, J.M., Alcázar, Á., and Aguayo-Villarreal, I.A., Direct immersion single drop micro-extraction method for multi-class pesticides analysis in mango using GC–MS, Food Chem., 2017, vol. 237, pp. 30–38.
Domínguez, I., Arrebola, F.J., Romero-González, R., Nieto-García, A., Martínez Vidal, J.L., and Garrido Frenich, A., Solid phase microextraction and gas chromatography coupled to magnetic sector high resolution mass spectrometry for the ultra-trace determination of contaminants in surface water, J. Chromatogr. A, 2017, vol. 1518, pp. 15–24.
Petrarca Fernandes, M.H., Godoy, H.T., and Cunha, S.C., Multiclass pesticide analysis in fruit-based baby food: A comparative study of sample preparation techniques previous to gas chromatography-mass spectrometry, Food Chem., 2016, vol. 212, pp. 528–536.
Wang, Y., Wang, Z., Zhang, H., Shi, Y., Ren, R., Zhang, H., and Yu, Y., Application of pneumatic nebulization single-drop microextraction for the determination of organophosphorous pesticides by gas chromatography-mass spectrometry, J. Sep. Sci., 2011, vol. 34, pp. 1880–1885.
Timofeeva, I., Kanashina, D., Moskvin, L., and Bulatov, A., An evaporation-assisted dispersive liquid–liquid microextraction technique as a simple tool for highperformance liquid chromatography tandem-mass spectrometry determination of insecticides in wine, J. Chromatogr. A, 2017, vol. 1512, pp. 107–114.
Wu, L., Song, Y., Hu, M., Zhang, H., Yu, A., Yu, C., Ma, Q., and Wang, Z., Application of magnetic solvent bar liquid-phase microextraction for determination of organophosphorus pesticides in fruit juice samples by gas chromatography mass spectrometry, Food Chem., 2015, vol. 176, pp. 197–204.
Nodeh, H.R., Ibrahim, W.A.W., Kamboh, M.A., and Sanagi, M.M., New magnetic graphene-based inorganic-organic sol–gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction, Chemosphere, 2017, vol. 166, pp. 21–30.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Majid Haji Hosseini, Jafarpanah, F., Sharifkhani, S. et al. Development of Organic Gas Steam–Liquid Extraction (OGS–LE) Method for the Extraction of Chlorpyrifos and Diazinon From Aqueous Samples and Determination by GC–FID. J. Water Chem. Technol. 43, 394–400 (2021). https://doi.org/10.3103/S1063455X21050088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X21050088