Abstract
In this study, for the first time, the organic gas steam-liquid extraction by a special hand-made cell was used as a simple and inexpensive preconcentration technique for the determination of benzene, toluene, ethylbenzene and xylene in aqueous samples by gas chromatography-flame ionization detection. In this technique, the organic solvent vapor produced in the evaporator unit is introduced into the aqueous sample by using nitrogen as a carrier gas to extract target analytes. By entering the vapor bubbles of the organic solvent into the aqueous sample, the organic solvent dissolves in water and the organic solvent concentration in water reaches supersaturation. At this time, equilibrium occurs between the dissolved organic solvent and the insoluble organic solvent, and it is collected in the conical part of the cell. Using a microliter syringe, a certain volume of the collected organic solvent is injected into the gas chromatograph. Face central composite design was used to evaluate the effect of various factors including organic solvent volume, ionic strength, evaporating gas flow rate and heated chamber temperature. Under the optimized conditions, the detection limit, relative standard deviation and linear dynamic range of the method were 10 µg/L, 5.3–8.7% (n = 4) and 50–5000 µg/L, respectively. The proposed method was successfully applied to the determination of benzene, toluene, ethylbenzene and xylene in real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Benzene, toluene, ethylbenzene and xylene (BTEX) are among the important water pollutants [1–3]. BTEX contamination of water stems from release or spill of petroleum products such as gasoline, diesel fuel, jet fuel, crude oil and hydraulic oil [4]. Closeness to natural deposits of petroleum and natural gas is another way to introduce BTEX to the environment. Moreover, BTEX compounds are among the most abundantly produced chemicals, and this has increased the probability of water pollution caused by these chemicals [5]. These volatile organic compounds are also formed during the production of consumer goods such as paints, thinners, lacquers, adhesives, rubber products, inks, cosmetics and pharmaceutical products. Due to the toxicity of BTEX compounds, the development of simple and low-cost analytical techniques for their determination is of great importance. A number of sample preparation techniques [6–13] such as solid-phase microextraction (SPME) [14], ultrasound-assisted dispersive liquid-liquid microextraction (DLLME) [15] and headspace gas chromatography-mass spectrometry (GC-MS) [16] have been introduced to determine these species with advantages of less usage of organic solvents. However, these methods suffer from some inconveniencies such as fragile fiber with limited lifetime (in SPME method), the need to use solvents with a higher density than water in order to be sedimented by centrifugation and the need to use a water-soluble solvent (disperser solvent) which decreases the distribution coefficient of analyte (in DLLME method). To overcome these drawbacks, the present paper intends to describe and apply a sensitive, economical, simple and fast analytical technique for the determination of BTEX compounds in water samples. This is a unique and green development in the liquid-liquid extraction technique as it does not need stirring and a third solvent. Moreover, the presented technique does not need a centrifugation step, which makes it possible to use low-density organic solvents.
EXPERIMENTAL
Reagents. Benzene, toluene, ethyl benzene, xylene, n-hexane, ethanol and sodium chloride were of the highest purity available (Merck, Darmstadt, Germany). Stock solutions of BTEX were prepared daily by dissolving an appropriate amount of each compound in 20 mL of ethanol at room temperature. Standard aqueous solutions for calibration curve construction containing adequate amount of sodium chloride (that was weighted by a balance, AB 204-Smettler, Toledo, USA) were prepared from dilution of stock solutions in 100 mL of doubled distilled water. Real samples were obtained from two Qantas located in Alborz, Iran.
Equipment. The determination of the compounds was carried out by a gas chromatography-flame ionization detection system (GC-FID, Agilent, 7890 N, Agilent Technologies, Palo Alto, CA, USA) with the following conditions: pure nitrogen as a carrier gas (99.999% purity) at a constant flow rate (1 mL/min), the split/splitless injector with splitless mode at the temperature of 200°C, DB-5 fused silica capillary column (30 m length, 0.25 mm i.d. and 0.25 µm film thickness), the oven temperature program was as follows: initially held at 30°C for 6 min, then increased to 75°C at a rate of 2°C/min. 0.5 µL of the sample was injected by a 1-µL microsyringe (Hamilton, Reno, NV, Australia). Flame ionization detector was applied (200°C).
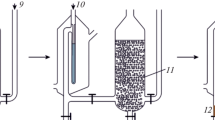
Experimental set-up and sample preparation procedure. Different parts of the special hand-made organic gas steam-liquid extraction (OGS-LE) cell are presented in Fig. 1. The set-up consisted of an extracting organic solvent tube (1), a heated chamber (2), a N2 evaporating gas inlet (3), a sample column (4) and a water tube (5).
A 0.3-mL aliquot of n-hexane was injected to the organic solvent tube, the heated chamber warmed up to 70°C by circulating thermostated water/ethylene glycol using a Polyscience 9505 circulator (USA). 20 mL of the aqueous saline solution was injected to the sample tube. Using N2 stream (flow rate of 20 mL/min) at the bottom of the organic solvent tube, the gas steam of the organic solvent was transferred to the sample column and moved longitudinal from bottom to top. The organic steam dissolved in the sample solution and was gathered as a thin layer on the top of the aqueous phase after supersaturation. Solvation and supersaturation are dynamic phenomena in which the organic solvent enters the aqueous sample and after being saturated goes out continuously. Time of the extraction process, i.e., the interval between the beginning of N2 flotation and the end of n-hexane evaporation from organic solvent tube, is 5 min. At the final stage, a small amount of double distilled water was added from the side tube (part 5 in Fig. 1) to increase the level of aqueous phase up to the conical part, where the organic sample (15 µL) could be collected by using a microsyringe for the subsequent analysis by GC-FID.
Optimization strategy. The experimental response data were analyzed by a regression procedure based on the response surface methodology (RSM) [17]. The response surface methodology including face central composite design (FCCD) approach was utilized to find the optimum values of the effective parameters. The model that can be fitted to a composite design is an empirical function determined from the statistical correlation suitability of the observed responses and the experimental factors. For this purpose, the conventional second order polynomial model equation was used [18, 19]. Several factors may affect the extraction efficiency of target compounds such as the organic solvent volume, ionic strength, heated chamber temperature and flow rate of evaporating gas. To find the optimum conditions and interrelationship between variables, the FCCD method was applied by the Minitab software. The design was made of four factors at 3 levels with 6 center points (Table 1). A total of 30 experiments were designed and performed (Table 2). The ratio of analyte to organic solvent peak areas on the chromatogram was employed as the analytical signal.
RESULTS AND DISCUSSION
Regardless of the applied extraction method, one of the critical steps in such techniques is the selection of an appropriate solvent. In this study, the main criteria for choosing a proper solvent are its water immiscibility and lower density compared to water. The latter criterion allows the organic solvent to be floated on top of water. Moreover, the boiling point of the solvent should be either higher or lower than that of analytes to prevent overlapping of the solvent peaks with the analytes’ peaks on the chromatogram. Thus, in this project, n-hexane was chosen as an appropriate solvent.
Optimization. Regardless of the selected response (peak area) for the analyte (benzene, toluene, ethylbenzene and xylene), the FCCD approach showed similar results to find the optimum values of effective parameters involved in the extraction system. Therefore, only the results of benzene were reported as the representative example of analyte response (Table 2).
The coefficients of the empirical model and their statistical characteristics were evaluated for benzene results. According to the obtained results, the effects of the main factors on the response were in the following order: organic solvent volume > ionic strength > N2 evaporating gas flow rate > heated chamber temperature. Flow rate of N2 evaporating gas and heated chamber temperature parameters had positive effects on extraction efficiency. In contrast, the salt concentration and the organic solvent volume had negative effects [20]. It can be seen that by increasing the N2 evaporating gas flow rate and the heated chamber temperature, the evaporation of the organic solvent from the top of the aqueous solution increased, which results in the increase in the preconcentration factor. However, with the increase in the solvent volume and ionic strength, this factor diminished due to the increase in the volume of collected organic solvent and thus a decrease in analyte concentration.
If the model is a good predictor of the experimental data, the calculated F-value would be lower than the tabular F-value. The P-value can be used as a tool to check the significance of the regression coefficients. The linear, interaction and quadratic coefficients show the linear effect of each variable, interactive effect between the variables and quadratic effects, respectively.
The smaller the magnitude of the P-value, the more significant is the corresponding coefficient. The P-values have revealed that square terms (volume2, salt2, temperature2, flow rate2) and some of the interaction terms (volume × flow rate, salt × temperature and salt × flow rate) in the predicted model at the 95% confidence level are not significant (P-value > 0.05). Regarding this matter, these terms could be ignored in the predicted model. Table 3 shows the results of analysis of variance (ANOVA). The coefficients of the empirical model and their statistical characteristics are given in Table 4. Tables 3 and 4 show that the model obtained by excluding insignificant terms fits 91.3% (coefficient of determination, R2) of the experimental raw data.
From the table of coefficients (Table 4), the following empirical equation is obtained under optimal conditions:
where X1, X2, X3 and X4 are the predicted response, volume, salt, temperature and flaw rate, respectively.
The optimal experimental conditions for achieving a maximum response by the model can visually be obtained from Fig. 2 as follows: volume of n-hexane—0.3 mL, NaCl concentration—0 M, heated chamber temperature—70°C, N2 evaporating gas flow rate—20 mL/min.
Analytical evaluation. Under the optimized conditions, regression equation, coefficient of determination (r2), dynamic linear range (DLR), limit of detection (LOD) and preconcentration factor (PF) for each analyte were calculated and summarized in Table 5. The LODs were calculated as the analyte concentrations equal to three times the standard deviation of the blank signal divided by the slope of the calibration curve. The practical applicability of the developed OGS-LE method was confirmed by the determination of BTEX in real samples obtained from two separate Qanats located in Alborz, Iran. 17.55 g of NaCl and 50 µL of ethanol were dissolved in 100 mL of the sample for matrix matching of real samples and standards. All samples were analyzed three times under the optimized conditions. According to the results, no BTEX compounds were found in the real samples. To find recoveries of the method, the real samples were spiked with 100 µg/L BTEX. The chromatogram of the extracted BTEX from spiked sample is shown in Fig. 3.
The results shown in Table 6 indicate a good agreement confirming practical suitability of the method. A comparison between the proposed method and other related methods is presented in Table 7. Clearly, the DLR in OGS-LE/GC-FID is comparable with the other methods. The relative standard deviation (RSD, %) of the proposed method is comparable with those of both SPME/GC-MS and headspace/GC-MS methods.
CONCLUSIONS
In this present study, the organic gas stream-liquid extraction with GC-FID detection was used for the monitoring of BTEX pollutants in water samples. The parameters affecting the process were optimized by the response surface methodology. The method was successfully applied for the determination of BTEX compounds in the spiked water samples. The simplicity, rapidity, less consumption of organic solvent, capability of operating with low-density solvents and eliminating the centrifugation step are the main advantages of the presented method.
REFERENCES
Mehlman, M.A., Environ. Res., 1992, vol. 59, no. 1, p. 238. https://doi.org/10.1016/s0013-9351(05)80243-9
Bowlen, G.F. and Kosson, D.S., in Microbial Transformations and Degradation of Toxic Organic Chemicals, Young, L.Y. and Cerniglia, C.E., Eds., New York: Wiley, 1995.
Saeed, T. and Al-Mutairi, M., Environ. Int., 1999, vol. 25, no. 1, p. 117.
Srijata, M. and Pranab, R., J. Environ., 2011, vol. 5, p. 394. https://doi.org/10.3923/rjes.2011.394.398
Górna-Binkul, A., Keymeulen, R., Langenhove, H.V., and Buszewski, B., J. Chromatogr. A, 1996, vol. 734, p. 297.
Prosen, H. and Zupanciicikralj, L., TrAC, Trends Anal. Chem., 1999, vol. 18, p. 272.
Psillakis, E. and Kalogerakis, N., TrAC, Trends Anal. Chem., 2003, vol. 22, p. 563.
Arambrri, I., Lasa, M., Garcia, R., and Millan, E., J. Chromatogr. A, 2004, vol. 1033, p. 193. https://doi.org/10.1016/j.chroma.2004.01.046
Palit, M., Pardasni, O., Gupta., A.K., and Dubey, D.K., Anal. Chem., 2005, vol. 77, p. 711.
Ii, J., Deng, C., Shen, W., and Zheng, X., Talanta, 2006, vol. 69, p. 894.
Gaujac, A., Emidio, E.S., Navickiene, S., Ferreira, S.L.C., and Dorea, H.S., J. Chromatogr. A, 2008, vol. 1203, p. 99.
Bahri, M. and Driss, M.R., Desalination, 2010, vol. 250, p. 414.
Li, Q., Ma, X., Yuan, D., and Chen, J., J. Chromatogr. A, 2010, vol. 1217, p. 2191.
Bianchin, J.N., Nardin, G., Merib, J., Dias, A.N., Martendal, E., and Carasek, E., J. Chromatogr. A, 2012, vol. 1233, p. 22.
Khajeh, M. and Musavizadeh, F., Bull. Environ. Contam. Toxicol., 2019, vol. 89, p. 38.
Gilbert-Lopez, B., Robles-Molina, J., Garcia-Reyes, J.F, and Molina-Diaz, A., Talanta, 2010, vol. 83, p. 391.
Montgomery, D.C., Design and Analysis of Experiments, New York: Wiley, 2001, 5th ed.
Deyhimi, F., Arabieh, M., and Parvin, L., Biocatal. Biotransform., 2006, vol. 24, p. 263.
Deyhimi, F., Ahangari, R.S., Arabie, M., and Parvin, L., Int. J. Environ. Anal. Chem., 2006, vol. 86, p. 1151.
Hasseine, A., Meniai, A.H., and Korichi, M., Desalination, 2009, vol. 242, p. 264.
Sarafraz-Yazdia, A., Amiri, A.H., and Es’haghi, Z., Talanta, 2009, vol. 7, p. 936.
Assadi, Y., Ahmadi, F., and Hossieni, M.R.M., Chromatographia, 2010, vol. 71, p. 1137.
Farajzadeh, M.A., and Matin, A.A., Chromatographia, 2008, vol. 68, p. 443.
Sarafraz-Yazdi, A., Amiri, A.H., and Es’haghi, Z., Chemosphere, 2008, vol. 71, p. 671.
ACKNOWLEDGMENTS
The authors would like to express their deep gratitude to Nuclear Fuel cycle Research School for support of research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharifkhani, S., Yaftian, M.R., Hosseini, M.H. et al. Introducing Organic Gas Steam-Liquid Extraction as a New Preconcentration Method for Benzene, Toluene, Ethylbenzene and Xylene Determination in Water Samples by Gas Chromatography-Flame Ionization Detection. J Anal Chem 77, 505–512 (2022). https://doi.org/10.1134/S1061934822040098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822040098