Abstract
The main objective of the present study was to investigate the diversity and abundance of macrobenthos in the Dehbar, Zoshk and Kang rivers in Iran. Sampling was carried out at 10 stations for three seasons with 45 days intervals using a Surber sampler. Simpson, Shannon-Weiner and Margalef diversity indices, as well as biological indices for Biological Monitoring Working Party (BMWP), Average Score Per Taxon (ASPT), Hilsenhoff Family Biotic Index (HFBI), and Ephemeroptera, Plecoptera and Trichoptera (EPT) were calculated at all stations. In this study, a total of 21 families of macrobenthos in seven orders were identified. Organisms in the Baetidae family were dominant at stations 1D, 2D and 6D in the Dehbar River and at stations 1Z and 1 K in the Zoshk and Kang rivers, respectively. The greatest diversity of macrobenthos was observed in the spring. There was significant correlation between the three biological indices. In general, water quality of the rivers at the upstream stations was not good. The diversity indices decreased from upstream to downstream, and the biological indices showed a similar reduction in water quality from upstream to downstream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are many areas in the world where surface water is the only source of water for domestic, agriculture and industrial uses, but despite water shortages, misuse of water is widespread (Karr 1998; Sharma and Chowdhary 2011; Shokri et al. 2014). Therefore, water quality assessment is of particular importance (Asheghmolla et al. 2016). The study and identification of river quality is insufficient using only common measurement methods such as physical and chemical parameters of water, because they provide information restricted to the sampling time; while bioassessment is a low-cost and rapid field method requiring little laboratory equipment and provides acceptable results compared to physical and chemical evaluation (Esmaili Sari 2003; Aazami et al. 2015; MacEdo et al. 2016). One bioassessment method uses macroinvertebrates, which have different levels of resistance to pollution, and the difference is clearer for some species. Some of these species are only able to survive in clean water, free of any contamination, and some can survive in highly contaminated waters, so that the presence or absence of these aquatic organisms and their sensitivity to contaminations indicates water quality (Cooper and Knight 1991; Bere et al. 2016; Raburu et al. 2017). Consequently, biological studies to determine water quality have been considered.
The health status of a river can be determined by comparing biological indices (Stephens and Farris 2004). The basis of all these indices is the sensitivity of different species to changes in water quality. The Biological Monitoring Working Party (BMWP) is the most common biological index proposed for the assessment of water quality in water bodies (Pescador et al. 1995). In the BMWP index, organisms are identified to family level and each family is assigned a score based on the degree of resistance to pollution and, finally, with the addition of scores, water quality can be calculated (Wally and Hawkes 1997). Another index is the Average Score Per Taxon (ASPT). In water quality assessment, the ASPT index is more reliable than the BMWP index (Esmaili Sari 2003) because, unlike the BMWP index, the ASPT index is independent of factors such as seasonal variation and variation in biodiversity, so this index is a better measure of water quality (McCafferty 1983). Another indicator is the Hilsenhoff Family Biotic Index (HFBI), which allows a faster evaluation than other biological indices. The HFBI index is based on the identification of benthic invertebrates at the family level and their tolerance to pollution (Hilsenhoff 1988). A fourth index is the Ephemeroptera Plecoptera Trichoptera (EPT) index, which measures organism sensitivity to environmental stresses and is based on the number of organisms in these three orders (Fries and Bowles 2002).
Biodiversity includes genetic diversity, species and ecosystem diversity, and hence encompasses the entire diversity of the world. It is important to identify, preserve and protect biodiversity globally and in each region. The number of species and their abundance are two important principles descriptors of in any biotic community, and species diversity represents the stability of an ecosystem, community and is related to patterns and processes in an ecosystem (Ejtehadi et al. 2012). The rivers are habitats of many species of aquatic animals, with each of them having a distinct flora and fauna.
Measuring diversity can be done in a variety of ways. The most widely used methods include the Shannon-Weiner, Simpson and Margalef indices. The Shannon-Wiener Index is based on the relative frequency of species and assumes that individuals are randomly sampled from a very large community (Shannon and Wiener 1949). The Simpson Diversity Index emphasizes the most abundant species and has limited sensitivity to species richness. In this index it is assumed that all species in the community are present in the collected samples and that the samples collected are a good representation of the target community. Another index used in this study is the Margalef Richness Index, which is based on the total number of species (S) and the total number of individuals of all species (N) (Ejtehadi et al. 2012).
The Dehbar River is one of the most important permanent rivers in Khorasan Razavi Province, Iran. The water quality of this river is important because the water is used for agricultural purposes in gardens and farms along the river and it is also stored in Golestan Dam and is used in Mashhad city. The Zoshk and Kang rivers, besides supplying drinking water to local population around the rivers, are also used for farming and fish breeding. Therefore, the present study pursued the following goals: (i) to identify and investigate the diversity of benthic macroinvertebrate communities; and (ii) to use of macroinvertebrate as a bioindicator to assess water quality in the Dehbar, Kang, and Zoshk rivers.
Materials and methods
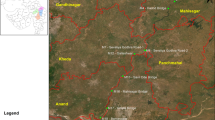
The Dehbar River is located in Khorasan Razavi Province, Iran. It has a longitude of 59°24′ E and a latitude of 36°18′ N in the Qar-e-Ghom watershed and eventually flows into the Golestan Dam. The Zoshk River is located 30 km northwest of Mashhad, Shandiz, Zoshk village. This river is one of the high-discharge rivers in Mashhad. It originates from the highlands of the Binaloud Mountain Range and finally it flows into the Kashafrod River. The Kang River also originates in the highlands of the Binaloud Mountain Range, is 17 km long and an average slope of 7% (Table 1) (Fig. 1).
Sampling was carried out between November 2016 (autumn) and August 2017 (autumn), and was repeated at intervals of 45 days. Sampling did not take place in the winter because of snowfall and blocked roads, so data were collected from three seasons: spring, summer and autumn. Six stations on the Dehbar River, two stations on the Zoshk River and two stations on the Kang River were selected. The sampling of the benthic macroinvertebrates was carried out by a Surber sampler (with 500 μm net and with a sampling surface of 12 × 12 in.), with three replications at each station Samples were transferred to plastic containers after initial washing with river water and fixed with 4% formalin. After preparing the samples in the laboratory, the samples were identified under a stereomicroscope using a valid identification key (Tachet et al. 2000).
Diversity indices
Shannon-Wiener diversity index (H)
The Shannon-Wiener Diversity Index (H) is calculated from the following formula:
where N is the total population size of all species, Ni is the proportion of individuals found in the species i, and S is the total number of species.
To evaluate the effects of organic contamination on the demographic structure of aquatic ecosystem organisms, the classification proposed by Wilhm and Dorris (1968) was used. Based on this classification, a Shannon-Wiener diversity index less than 1 represents a highly contaminated ecosystem, between 1 and 3 indicates a relatively contaminated ecosystem and more than 3 represents a non-contaminated ecosystem.
Margalef’s richness index (R)
Margalef’s Richness Index (R) is derived from the following formula:
where S is the number of species and N is the total number of individuals in the sample.
Simpson’s diversity index (D)
The Simpson Diversity Index (D) was presented by Simpson in 1949. In 1972 Krebs showed how to calculate it with the following formula (Krebs 1972),where ni is the total number of organisms of a particular species, N is the total number of organisms of all species and S is the total number of species.
Biological indices
Hilsenhoff index (HFBI)
This Index provides a measure of the tolerance of families to contamination, with scores between 0 and 10. Zero score indicates no resistance of a benthic macroinvertebrate family to contamination and a score of 10 indicates high resistance of the family to the contamination (Hilsenhoff 1988). The index is calculated based on the following formula:
where TVi is the score for each family, ni is the number of individuals per family and N is the total number of specimen per sample. After calculating the Hillsenhof index scores, the water quality class is determined based on the interpretation in Table 2.
Biological monitoring working party (BMWP)
The scoring in this system is based on the resistance of each family to organic contamination. The highest and lowest scores are assigned to families with the highest and lowest resistance to contamination, respectively. Finally, the scores of the families are gathered together and the BMWP score for each station is obtained. The BMWP index is calculated according to the formula:
where N is the number individuals of families present at each station and B is the score of the BMWP index (Hawkes 1998). The water quality classification based on the BMWP index is shown in Table 3.
Average score per taxon (ASPT)
This index is calculated based on the following formula, where ASPT is the ratio of BMWP and N is the total number of taxa at the station. The water quality classification based on the ASPT index is shown in Table 3.
EPT index
The EPT index is based on three orders, Ephemeroptera, Plecoptera, and Trichoptera, as they are considered most susceptible to contamination. The value of the index increases with decreasing degree of contamination. Calculation of this index is based on the sum of families in these three orders in the sample (Mandaville 2002). The water quality classification based on this index is shown in Table 3.
Statistical analysis
Diversity indices were calculated using PAST software. The normality of the data was assessed using the Kolmogorov-Smirnov test. The analysis of variance (two-way ANOVA) test was used to determine the significant difference between the biological indices and diversity indices between seasons and rivers. The analysis of variance (one-way ANOVA) was also used to determine the significant difference between the mentioned indices and to determine the effect of other environmental variables among the upstream stations. The Spearman test was used to determine the correlation between biological indices. Data were analyzed using SPSS software and graphs with Excel software.
Results
Abundance of benthic macroinvertebrates in different seasons
A total of 21 families from seven orders of benthic macroinvertebrates were identified during ten months of sampling (from November 2016 to August 2017) from the Dehbar, Zoshk and Kang rivers (Table 4). In these three rivers the most frequently recorded macroinvertebrates were observed in the autumn season. In the Dehbar River, the most frequent taxa belonged to the Trichoptera, Ephemeroptera and Oligochaeta, and the lowest frequencies were in Coleoptera and Gastropoda. In the Zoshk and Kang rivers, the highest frequencies were found in Diptera and Ephemeroptera and the lowest frequencies were observed in Coleoptera, Plecoptera, and Gastropoda.
Diversity indices
The Simpson, Shannon-Weiner and Margalef Diversity Indices in the Dehbar River are shown in Table 5. In the Dehbar River, the Simpson Index range was between 0.61 and 0.79, in the 3D and 6D stations respectively. The highest and lowest values of Simpson, Shannon-Weiner, and Margalef Indices were observed in spring and autumn, respectively (Table 5). Diversity indices studied in the Zoshk and Kang rivers indicated that the highest and lowest values of the Simpson Index were at the 1 K and 2Z stations in spring, respectively. The mean range of Shannon-Weiner’s Iindex in different seasons in the Dehbar River was between 1.16 and 1.24, in the Zoshk River between 1.49 and 0.59, and in the Kang River between 1.74 and 1.21. The highest value of the Shannon-Weiner index was obtained at the 1Z station in summer and the lowest value at the 2Z station in spring. The highest value of the Margalef Index was observed at the 1 K station in autumn and the lowest value at the 2 K station in summer (Table 5).
Biological indices
The value of the BMWP Index in the Dehbar River increased from spring to autumn, with the 1D, 2D, 3D, 4D and 5D stations in the “very good” quality class and the 6D station in the “bad” quality class (Table 3). Results for the ASPT index showed that stations 1D, 2D and 4D were in the “moderate” quality class and stations 3D, 5D, and 6D were in the “highly polluted” quality class (Table 3). Using the HFBI index, stations 1D, 2D and 4D were in the “good” quality class, station 3D station was “fair” quality, and stations 5D and 6D were in the “poor” quality class (Table 2). The results of the EPT index placed stations 1D, 2D, 3D and 4D in the “clean” quality class and stations 5D and 6D was in the “polluted” class (Fig. 2; Table 3).
In the Zoshk and Kang rivers, the highest values of the BMWP, ASPT and EPT indices were observed at the upstream stations and the lowest values were observed at the downstream stations. In contrast, the highest value of the HFBI index was recorded at the downstream stations and the lowest value at the upstream stations of the two rivers (Fig. 2). There was significant correlation between the BMWP, ASPT and EPT indices (p < 0.05).
Discussion
The abundance of benthic macroinvertebrates
The study of the structure of benthic macroinvertebrates communities in aquatic ecosystems is of particular importance in ecological studies. The importance of benthic macroinvertebrates is not only due to their role in the food chain, but also due to the presence or absence of particular species indicating water quality in terms of possible contamination (Griba et al. 2017; Ejlali Khanghah et al. 2017). In this study, 21 families of benthic macroinvertebrates were identified in the Dehbar, Zoshk, and Kang rivers. At the upstream stations (stations 1D, 2D, and 3D), the most frequent orders belonged to Ephemeroptera, Trichoptera and Plecoptera and the least frequent to the orders Diptera and Oligochaeta. At the downstream stations (stations 5D and 6D), the most frequent orders belonged to Oligochaeta and the least frequent to Ephemeroptera, Trichoptera, and Plecoptera. Individuals of EPT (Ephemeroptera, Trichoptera, and Plecoptera) are species susceptible to contaminations (Masese et al. 2009; Kalyoncu and Zeybek 2011; Rosero-López et al. 2019). From the upstream to downstream stations, the frequencies of EPT orders were reduced and the frequencies of species resistant to contamination, such as the family of Chironomidae (Diptera) and Lumbricidae (Oligochaeta), increased. Oligochaeta was the most frequent and abundant order (92%) in terms of numbers of individuals at the downstream station (6D), possibly due to the uncontrolled wastewater discharge from villages and cities.
Similar results were observed in the Zoshk and Kang rivers. At the upstream stations on both of these rivers, individuals of orders which were sensitive to contamination (Ephemeroptera, Trichoptera, and Plecoptera) had a high abundance in the macrobenthos, while at the downstream stations, contamination-resistant macrobenthos (Diptera and Oligochaeta) had the highest percentages. Ephemeroptera, Trichoptera and Plecoptera are sensitive to contamination and usually indicate the health status of the water (Perkins 1983). In general, species of the family Baetidae (Ephemeroptera) and species of Hydropsychidae (Trichoptera) were found abundantly in all three studied rivers, which indicated their good water quality status. The results of a study conducted in Singye Chhu River, Bhutan, showed that Trichoptera, Ephemeroptera and Plecoptera (pollution-sensitive benthic macroinvertebrates) were the dominant orders at upstream stations, whereas Basommatophora and Diptera (pollution-tolerant benthic macroinvertebrates) were the dominant orders at downstream stations. The results from the Osumi and Devolli rivers in Albania (Duka et al. 2017) were similar to those obtained in the present study.
In the present study, the highest frequency of macrobenthos was observed in the 1D, 1Z and 1 K stations, which could be due to the pristine nature of these areas and the absence of environmental stress such as household wastewater. In most studies on rivers, from upstream to downstream, the abundance, variety and density of the macrobenthos are reduced. In a study on the Zaremroud River in Iran the upstream station (station 1) had better quality water than the downstream stations and its macrobenthos diversity and density were higher (Azimi et al. 2015). Similar results were obtained in a study on the Singye Chhu River in Bhutan, where upstream stations (stations 1 and 2) had a higher abundance of benthic macroinvertebrates compared to other stations (stations 3 and 6). So that the numbers of the organisms were reduced from the upstream to downstream of the Chhu river (Giri and Singh 2012). Verberk et al. (2002) investigated benthic invertebrate diversity and abundance in the Nen River in Amsterdam and found that the frequencies of benthic macroinvertebrates were significantly different among stations. Also, from upstream to downstream, stations had reduced organism variety and density (Verberk et al. 2002). Giorgio et al. (2016) reviewed the quality of the Picentino River in Italy and found that the frequency of macrobenthos communities decreased from the upstream to downstream (Giorgio et al. 2016).
Diversity indices
The macrobenthic diversity index can be used to enhance understanding of biological health (Washington 2003). The Shannon-Weiner Index is one of the most popular and commonly used indices. It is an indicator of the species present and represents the richness of the species as a numerical quantity. Its use is based on the assumption that the structure of a macrobenthic community changes with environmental perturbations, because some species are more affected by environmental stress than others (Wilhm and Dorris 1968).
Values of the Shannon-Wiener Index vary from 1 to 5, with lower values indicating higher pollution (Wilhm and Dorris 1968). There were fewer variations between downstream stations (6D, 2Z and 2 K) due to the lower level of this index than at the upstream stations. In all other stations, the index values decreased from the upstream to downstream, which was similar to the results obtained in the San Marcos River in USA (Fries and Bowles 2002). In a study carried out in the Tajan River in Iran, the highest value of this index was observed at the upstream station (Station 1), and in other stations the index decreased from the upstream to downstream (Shokri et al. 2014). According to the Shannon-Weiner Index and Wilhm and Dorris’ classification (Wilhm and Dorris 1968; Shokri pour and Ashja Ardalan 2017), in this study the Dehbar River showed a moderate contamination and the Zoshk and Kang rivers showed a high contamination.
The Simpson Index indicates the degree of dominance and it is most often used to determine the dominance between species populations. From upstream to downstream, the results showed that the distribution of individuals between species was close and this index was close to zero. As a result, downstream stations had a more uniform distribution than the upstream stations, indicating less diversity in upstream stations than downstream stations. The range of the Simpson Index was between 0.19 and 0.66 in the Zoshk River, and between 0.31 and 0.75 on the Kang River. The results showed that the Zoshk River had a more uniform distribution than the Kang River. The results also showed that Simpson Index values were higher in the Kang River than the Zoshk River, and the families Baetidae and Lumbricidae were dominant at 1 K and 2 K stations, respectively, in the Kang River. In a study of macrobenthos conducted on the Beishi, Tonghou, Nanshi and Xindian streams in Taiwan by Narangarvuu et al. (2014), the Simpson Index values at the reference stations (XD3 and XD4) were higher than at the downstream stations (XD6 and XD7) (Narangarvuu et al. 2014). Similarly, in a study conducted in the Langat River in Malaysia, the Simpson index values were significantly higher upstream (Stations 1 to 4) than downstream (stations 5 to 8) (Azrina et al. 2006).
The Margalef Diversity Index represents diversity in biological populations. It is an ideal index for comparing macrobenthic communities, and determines rich and poor ecosystems in terms of species numbers. The greater its numerical value, the better the river’s quality (McCafferty 1983). The Margalef Index range was between 0.33 and 2.149 at stations 3D and 6D, respectively, in the Dehbar River. The highest and lowest value of the index was in spring and autumn. In the Zoshk and Kang rivers, the highest value of this index was recorded at station 1 K in autumn and the lowest index value was found at station 2 K in summer. In general, the Kang River had a greater richness than the Zoshk River at all seasons. In the present study, the values of the Margalef index at the upstream stations were higher than those at the downstream stations. Similar results were obtained in studies in other rivers. For example, Ogbeibu and Oribhabor (2002), in a study in the Tajan River in Iran, found that the highest Margalef index value was at an upstream station (station 1) and the lowest value was at a downstream station (station 3). Studies water quality in the Langat River in Malaysia (Azrina et al. 2006) and Shahrood River in Iran (Sharifinia et al. 2016) also showed that the value of this index decreased from upstream to downstream. In a study conducted in the Tajan River, the results showed that the index value decreased at the stations downstream of fish farms (stations 2 and 3), which indicates a decrease in macrobenthos diversity (Imanpour Namin et al. 2013).
Assessment of water quality using the BMWP, ASPT, HFBI and EPT biological indicators
According to the BMWP index results, the water quality of the Dehbar River was classified into two qualitative classes, good and bad; while the Zoshk and Kang rivers were classified into two qualitative classes of very good and good. The lowest index value at the 6D station was in summer and the highest one was at the 1D station in the Dehbar River in the autumn. In the Zoshk and Kang rivers, the lowest index values were recorded at the 2Z and 2 K stations and the highest index values were recorded at the 1 K and 2 K stations. In calculating the BMWPT index, families with a higher resistance to contamination received a lower score. Therefore, reduction in the index at the 6D station meant an increase in pollution-resistant groups. On the other hand, increase in the BMWP index at the 1D station was due to the higher relative abundance of species that are sensitive to pollution.
The quality of the Tajan River is classified using the BMWP index in three categories: good, moderate and bad. In this river, the downstream station showed the lowest value in summer. One of the reasons for this decline was the reduction in water flow and, consequently, the increased impact of industrial pollution on the river (Shokri et al. 2015). In another study, the water quality of the Langat River was classified according to the BMWP index into two classes: good and very poor. The values of this index were significantly higher in the upstream stations than in the downstream stations (Azrina et al. 2006).
The results of the ASPT Index indicated that the Dehbar, Zoshk and Kang rivers were subject to either medium or severe contamination. The lowest value of the ASPT index was recorded at stations 5D and 6D and the highest value at stations 1D and 2D in the Dehbar River. In general, the ASPT Index score decreased from the upstream to downstream, which could be due to an increase in pollution from fish farming, urban and rural wastewater and agricultural waste from localities adjacent to the river. In the Dohezar River, the ASPT Index decreased from upstream stations to downstream stations. One of the possible reasons was the increase in organic contamination caused by fish farms (Mesgaran Karimi et al. 2012). In another study on the Haraz River using this index, the river water quality was classified as moderately to severely contaminated (Banagar et al. 2018).
The results of the HFBI Index indicated that the stations studied in the Dehbar River belonged to four classes: good, fair, poor and very poor. In the Zoshk River the stations were allocated to fairly poor and very poor classes, and in the Kang River to two classes, good and fair. In the Dehbar River, the 1D, 2D and 4D stations were classified as good, the 3D station was fair, and the 5D and 6D stations were classified as relatively poor and very poor, respectively. At the 5D and 6D stations, compared to other stations in this river, the number and frequency of families of benthic macroinvertebrates resistant to contamination increased and the number of species of families sensitive to contamination was reduced. This was likely due to the input of agricultural and domestic waste waters, the 2Z and 2 K stations showed high values of the HFBI Iindex due to their location downstream from fish farms and tourist activities that caused the entry of wastewaters into the river. The water quality at 2Z and 2 K stations from the Zoshk and Kang rivers were similar to the 5D and 6 D stations from the Dehbar River. In general, the HFBI Index showed a decrease in all rivers studied in autumn, which could be due to increased rainfall and reduced pollution load.
Using the HFBI index, stations in the Haraz River were classified as very good to very poor (Banagar et al. 2018). The Beishi, Tonghou, Nanshi and Xindian streams in Taiwan were also classified as excellent to poor (Narangarvuu et al. 2014). In the Shahroud River in Iran, use of this Index also showed that the highest index values were at the stations downstream of fish farms (Dadgar et al. 2014). Water quality assessment of the Enfranz River in Ethiopia with macrobenthos and the HFBI Index showed that the water quality was lower in the downstream parts, which were severely affected by human activities (Mehari et al. 2014).
In the present study, the EPT orders represented a significant percentage of the composition of macroinvertebrates at the 1D, 2D, 3D, and 4D stations. Since these organisms belong to pollution-sensitive orders, this confirmed the good quality of the Dehbar River at these stations (Mehari et al. 2014; Archna et al. 2015). At stations 5D, 6D, 2Z and 2 K, the percentage of individuals in the EPT orders was reduced, while the percentages of Diptera and Oligochaeta were increased. Changes in the frequency of these orders at these stations could be due to the presence of stressors such as the entry of fish farms’ wastewater, agricultural and household wastewater, and other human activities.
In general, it can be stated that in contaminated areas, pollution-sensitive groups (including EPT) are reduced and, conversely, pollution-resistant groups are increased (Narangarvuu et al. 2014; Onana et al. 2019). Changes that occur in the composition and abundance of macroinvertebrates are in response to environmental factors and environmental stresses, and maintain ecological balance (Fore et al. 1996). Banagar et al. (2018) in a study in the Haraz River in Iran concluded that the effluents from fish farming could lead to the reduction of pollution-sensitive groups and an increase in pollution-resistant groups. Khosh Akhlagh et al. (2015) obtained similar results by studying the water quality of the Marber River in Iran. The results showed that the EPT index values were lower at the stations downstream of fish farms than in upstream stations, and the values of this index increased with distance from the farms. In the Ebrie Lagoon in Ivory Coast, the EPT index values at downstream stations were lower than at the reference stations, due to the presence of human activities (Yoboué et al. 2020).
By comparing the values of the BMWP, ASPT, HFBI and EPT indices in the Dehbar, Zoshk and Kang rivers in eastern Iran with data from other rivers in other parts of Iran, such as the Tajan River (Shokri et al. 2014), the Zaremroud River (Azimi et al. 2015), the Cheshmekile River (Abbaspour et al. 2013) and the Haraz River (Banagar et al. 2018), all in north Iran; the Dez River and Shahroud River in west Iran (Mohammadi Roozbahani et al. 2013; Dadgar et el. 2014); the Helleh River in south Iran (Tabatabaie et al. 2010), it can be stated that in almost all rivers, the trend of changes in indices from upstream to downstream shows a similar pattern, i.e. decrease of values of the BMWP, ASPT and EPT indices from upstream to downstream with decreasing water quality, and increase of the HFBI index values from upstream to downstream with decreasing water quality.
The results of analysis of variance showed that the biological indices (HFBI, BMWP, ASPT and EPT) and diversity indices (Shannon-Wiener, Margalef and Simpson) did not differ significantly between seasons and rivers (p > 0.05) (Table 6). Similar results were obtained by Xu et al. (2016) on the frequency and factors of season and region in the Yellow Sea in China. However, different results have been obtained by Shou et al. (2009) from the Zhoushan Sea in China, where there was a significant difference between the Shannon-Wiener Index among the seasons, although no significant difference was recorded between the regions.
The possible effect of environmental variables on the studied indices was evaluated using ANOVA. The analysis revealed significant differences between the values of biological indices (HFBI, BMWP, ASPT and EPT) and diversity index (Shannon-Wiener) among the upstream stations (p < 0.05). These results suggest that other factors are likely to affect the community’s composition and biotic indices in the stations studied. Therefore, it is necessary to examine other environmental variables in similar and future research.
Conclusion
The Shannon-Weiner, Simpson and Margalef Diversity Indices showed that the diversity of macrobenthos in the Dehbar River was higher than in the Zoshk and Kang rivers. Since the use of only one biological index cannot adequately reflect the health status (water quality) of a complex river ecosystem, in this study several diverse biological indices such as BMWP, ASPT, HFBI and EPT were used and the results were consistent. In general, it can be concluded that the water quality of the rivers at the 1D, 2D, 3D, 1Z and 1 K stations (upstream stations) was suitable, while the water quality at the 4D, 5D, 6D, 2Z, and 2 K stations (downstream stations) was unsuitable. Poor water quality at downstream stations can be due to various human activities such as aquaculture (fish farms), agriculture (use of pesticides and fertilizers and their entry into the river ecosystem) and other activities such as the entry of pollutants from human settlements, including sewage and wastewater into the rivers. Effective decision-making managers will hopefully take note of these results, particularly with regard to pollution caused by agricultural wastewater. The results generally indicate that biological indices can be a good tool for monitoring the quality of water in rivers. However, it should be noted that the results also pointed to other environmental factors affecting the composition of communities and biotic indicators.
References
Aazami J, Esmaili-Sari A, Abdoli A, Sohrabi H, Van den Brink PJ (2015) Monitoring and assessment of water health quality in the Tajan River, Iran using physicochemical, fish and macroinvertebrates indices. J Environ Heal Sci Eng 13:29. https://doi.org/10.1186/s40201-015-0186-y
Abbaspour R, Hedayati Fard M, Alizadeh Sabet HR, Hassanzadeh H, Masgaran Karimi J (2013) Biological assessment of Cheshmekile River of Tonekabon (Mazandaran) with use biological indicators, population structure and biomaas of large invertebrate macrobiotic. Environ Sci Eng 1:59–73
Archna A, Sharad S, Pratibha A (2015) Seasonal biological water quality assessment of river Kshipra using benthic macroinvertebrates. Soc Iss Environ Prob 3:1–7
Asheghmolla M, Malakmohammadi B, Torabian A (2016) Investigating the importance of rivers self- pollution capacity in developing a standard for discharge of wastewater. Environ Res 13:103–116
Azimi A, Amirnezhad R, Nasrollahzadeh H, Soleymanirodi A (2015) Quality classification of Zaremroud River (Sari-Mazandaran) using hilsenhoff family biotic index. J Wetl Ecobiol 7:39–48
Azrina M, Yap C, Ismail A, Ismail A, Tan S (2006) Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River, peninsular Malaysia. Ecotoxicol Environ Saf 64:337–347. https://doi.org/10.1016/j.ecoenv.2005.04.003
Banagar G, Riazi B, Rahmani H, Jolodar MN (2018) Monitoring and assessment of water quality in the Haraz River of Iran, using benthic macroinvertebrates indices. Biologia 73:965–975. https://doi.org/10.2478/s11756-018-0107-5
Bere T, Dalu T, Mwedzi T (2016) Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci Total Environ 572:147–156. https://doi.org/10.1016/j.scitotenv.2016.07.204
Cooper CM, Knight SS (1991) Water quality cycles in two hill land streams subjected tonatural, municipal, and non-point agricultural stresses in the Yazoo Basin of Mississippi, USA (1985-1987). Int Verein Theor Angew Limnol Verh 24:1654–1663. https://doi.org/10.1080/03680770.1989.11899044
Dadgar S, Chehrzad F, Razmi K (2014) Study of qualitative effects of rainbow trout farms on Shahroud River using Hilsenhoff macrobenthic rapid bioassessment index. J Anim Environ 6:143–152
Duka S, Pepa B, Keci E, Paparisto A, Lazo P (2017) Biomonitoring of water quality of the Osumi, Devolli, and Shkumbini rivers through benthic macroinvertebrates and chemical parameters. J Environ Sci Heal - Part A Toxic/Hazardous Subst Environ Eng 52:471–478. https://doi.org/10.1080/10934529.2016.1274167
Ejlali Khanghah K, Akbarzadeh G, Rashidi S, Mousavi SA (2017) Investigation of the effect of monsoon on diversity and density of macrobenthos in Iranian coast of Makran Sea (Oman Sea). J Oceanogr 8:87–101. https://doi.org/10.18869/acadpub.joc.8.29.87
Ejtehadi H, Sepehri A, Akafi H (2012) Methods of measuring biodiversity. Ferdowsi University Mashhad, Mashhad
Esmaili Sari A (2003) Pollution, health and environmental standards. Naghshe Mehr, Tehran
Fore LS, Karr JR, Wisseman RW (1996) Assessing invertebrate responses to human activities: evaluating alternative approaches. J North Am Benthol Soc 15:212–231
Fries LT, Bowles DE (2002) Water quality and macro invertebrate community structure associated with a sportfish hatchery outfall. J North Am J Aquacult 64:257–266. https://doi.org/10.1577/1548-8454(2002)064<0257:WQAMCS>2.0.CO;2
Giorgio A, De Bonis S, Guida M (2016) Macroinvertebrate and diatom communities as indicators for the biological assessment of river Picentino (Campania, Italy). Ecol Indic 64:85–91. https://doi.org/10.1016/j.ecolind.2015.12.001
Giri N, Singh OP (2012) Bio-monitoring of water quality using benthic macro-invertebrates of river Singye Chhu in Bhutan. J Basic Appl Biol 6:44–49
Griba J, Laadel N, El Idrissi H, Rhafouri R, Serghini A, Fekhaoui M (2017) Inventory of benthic macroinvertebrates as bio-indicators of Afenourirlake (Morocco). J Mater Environ Sci 8:3986–3992
Hawkes HA (1998) Origin and development of the biological monitoring working party score system. Water Res 32:964–968. https://doi.org/10.1016/S0043-1354(97)00275-3
Hilsenhoff WL (1988) Rapid field assessment of organic pollution with a family-level biotic index. J North Am Benthol Soc 7:65–68
Imanpour Namin J, Sharifinia M, Bozorgi Makrani A (2013) Assessment of fish farm effluents on macroinvertebrates based on biological indices in Tajan River (North Iran). Casp J Environ Sci 11:29–39
Kalyoncu H, Zeybek M (2011) An application of different biotic and diversity indices for assessing water quality: a case study in the Rivers Cukurca and Isparta (Turkey). African J Agric Res 6:19–27
Karr JR (1998) Rivers as sentinels: using the biology of rivers to guide landscape management. Springer, New York
Khosh Akhlagh M, Kamrani A, Ebrahimi Dorcheh A, Sourinejad I (2015) The effect of rainbow trout farms effluents on benthic macro-invertebrates of Marber River in Semirom. J Aquat Ecol 5:103–112
Krebs CJ (1972) The experimental analysis of distribution and abundance. Harper and Row, New York
MacEdo DR, Hughes RM, Ferreira WR, Firmiano KR, Silva DRO, Ligeiro R, Kaufmann PR, Callisto M (2016) Development of a benthic macroinvertebrate multimetric index (MMI) for Neotropical savanna headwater streams. Ecol Indic 64:132–141. https://doi.org/10.1016/j.ecolind.2015.12.019
Mandaville SM (2002) Benthic macroinvertebrates in freshwaters: taxa tolerance values, metrics, and protocols. Nova Scotia: Soil Water Conserv Soc Metro Halifax 128:1–48
Masese FO, Raburu PO, Muchiri M (2009) A preliminary benthic macroinvertebrate index of biotic integrity (B-IBI) for monitoring the Moiben River, Lake Victoria Basin, Kenya. African J Aquat Sci 34:1–14. https://doi.org/10.2989/AJAS.2009.34.1.1.726
McCafferty WP (1983) Aquatic entomology: the fishermen’s and ecologists' illustrated guide to insects and their relatives. Jones & Bartlett Learning
Mehari AK, Wondie A, Mingist M, Vijverberg J (2014) Spatial and seasonal variation in the macro-invertebrates and physico-chemical parameters of the Enfranz River, Lake Tana sub-basin (Ethiopia). Ecohydrol Hydrobiol 14:304–312. https://doi.org/10.1016/j.ecohyd.2014.07.004
Mesgaran Karimi J, Azari TG, Khara H, Abbaspour R (2012) Effluence of aquaculture on bioabundance of macroinvertebrates in Dohazar River in Tonekabon. J Anim Biol 5:37–49
Mohammadi Roozbahani M, Roghanizadehgan N, Dehghan Mediseh S (2013) Assessment of Dez River water quality by BMWP index. J Wetl Ecobiol 5:55–66
Narangarvuu D, Bin HC, Shieh SH, Wu FC, Yang PS (2014) Macroinvertebrate assemblage patterns as indicators of water quality in the Xindian watershed, Taiwan. J Asia Pac Entomol 17:505–513. https://doi.org/10.1016/j.aspen.2014.04.011
Onana FM, Togouet SHZ, Tamsa AA, Tchatcho NLN, Tchakonte S, Koji E, Yemeli AWY, Makong ANSM (2019) Comparing freshwater benthic macroinvertebrate communities in forest and urban streams of the coastal ecological region of Cameroon. Open J Ecol 09:521–537. https://doi.org/10.4236/oje.2019.912034
Perkins J (1983) Bioassay evaluation of diversity and community comparison indexes. J Water Pollut Control Fed 55:522–530
Pescador ML, Rasmussen AK, Harris SC (1995) Identification manual for the caddisfly (Trichoptera) larvae of Florida. Tallahassee, Florida
Raburu PO, Masese FO, Tonderski KS (2017) Use of macroinvertebrate assemblages for assessing performance of stabilization ponds treating effluents from sugarcane and molasses processing. Environ Monit Assess 189:79. https://doi.org/10.1007/s10661-017-5786-9
Rosero-López D, Walter MT, Flecker AS, Lloret P, De Bièvre B, González-Zeas D, Calvez R, Dangles O (2019) Streamlined eco-engineering approach helps define environmental flows for tropical Andean headwaters. Freshw Biol 64:1315–1325. https://doi.org/10.1111/fwb.13307
Shannon CE, Wiener W (1949) The mathematical theory of communication urban. University of Illinois Press, Urbana
Sharifinia M, Mahmoudifard A, Imanpour Namin J, Ramezanpour Z, Yap CK (2016) Pollution evaluation in the Shahrood River: do physico-chemical and macroinvertebrate-based indices indicate same responses to anthropogenic activities? Chemosphere 159:584–594. https://doi.org/10.1016/j.chemosphere.2016.06.064
Sharma KK, Chowdhary S (2011) Macroinvertebrate assemblages as biological indicators of pollution in a central Himalayan River, Tawi (JK). Int J Biodivers Conserv 3:167–174
Shokri pour Z, Ashja Ardalan A (2017) Identify and evaluate the diversity of macrobenthos in Karaj River. J Anim Res (Iranian J Biol) 29:442–453
Shokri M, Rossaro B, Rahmani H (2014) Response of macroinvertebrate communities to anthropogenic pressures in Tajan River (Iran). Biologia 69:1395–1409. https://doi.org/10.2478/s11756-014-0448-7
Shokri M, Ahmadi MR, Rahmani H, Kamrani E (2015) Investigation of Tajan River quality with using population structure of benthic invertebrates and BMWP index. J Anim Environ 6:221–230
Shou L, Huang Y, Zeng J, Gao A, Liao Y, Chen Q (2009) Seasonal changes of macrobenthos distribution and diversity in Zhoushan Sea area. Aquat Ecosyst Heal Manag 12:110–115. https://doi.org/10.1080/14634980802697902
Stephens WW, Farris JL (2004) Instream community assessment of aquaculture effluents. Aquaculture 231:149–162. https://doi.org/10.1016/j.aquaculture.2003.08.009
Tabatabaie T, Amiri F, Pazira A, Mombaini S (2010) Study the structure and diversity of the macrobenthic communities in Helleh River. J Mar Biol 2:37–46
Tachet H, Richoux P, Bournaud M (2000) Invertebrates d Eau Douce, systematique, biologie, ecologie. Centre national de la recherche scientifique, Paris
Verberk W, Brock AMT, Van Duinen GA, Van Es M, Kuper JT, Peeters TMJ, Smits MJA, Timan L, Esselink H (2002) Seasonal and spatial patterns in macroinvertebrate assemblage in a heterogeneous landscape. In: Proceedings of Experimental and Applied Entomology. Nederlandse Entomologische Vereniging, Leiden pp 35–43
Wally WJ, Hawkes HA (1997) A computer- based reappraisal of the biological monitoring working party scores using data from the 1990 river quality survey of England and wales. J Water Res 30:2086–2094. https://doi.org/10.1016/0043-1354(96)00013-9
Washington H (2003) Diversity,biotic and similarity indices: a review with special relevance to aquatic ecosystems. Water Res 18:94–653. https://doi.org/10.1016/0043-1354(84)90164-7
Wilhm JL, Dorris TC (1968) Biological parameters for water quality criteria. Bioscience 18:477–481
Xu Y, Li X, Wang H, Zhang B (2016) Seasonal and spatial variations of macrobenthic community structure and diversity in the South Yellow Sea. Aquat Ecosyst Heal Manag 19:92–100. https://doi.org/10.1080/14634988.2016.1139429
Yoboué KP, Ouattara NI, Berté S, Aboua BRD, Coulibaly JK, Kouamélan EP (2020) Structure of benthic macroinvertebrates population in an area of Mopoyem Bay (Ebrie lagoon, Côte d’Ivoire) exposed to the discharge of a fish farm effluents. Environ Monit Assess 192(203):1–9. https://doi.org/10.1007/s10661-020-8167-8
Acknowledgments
The author is grateful to Lashkari for their field assistance. Thanks are given to Professor James Menzies and Professor Chris Banks who improved the draft and added valuable comments to the manuscript. This paper is an extract from the author’s thesis presented to the Faculty of the Geography and Environmental Sciences of Hakim Sabzevari University, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malvandi, H., Moghanizade, R. & Abdoli, A. The use of biological indices and diversity indices to evaluate water quality of rivers in Mashhad, Iran. Biologia 76, 959–971 (2021). https://doi.org/10.2478/s11756-020-00618-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00618-4