Abstract
The objective of this study was to assess the relationship between river water quality and the distribution of benthic macroinvertebrate communities in the Haraz River in Iran. Using a surber net sampler, benthic macroinvertebrate communities along the stream was sampled in wet and dry seasons of 2015 at nine stations with three replications. The physicochemical water quality parameters were measured in the field by water checker. Hilsenhoff biotic Indices, Shannon Wiener Diversity Indices, Average Score per Taxon (ASPT) Index and Pielou Evenness Index were applied to carry out a biological assessment of water quality. A total of 3781 (spring 769, summer 1092, autumn 1095 and winter 825) benthic macroinvertebrate specimens belonging to 4 orders, 11 classes and 16 families were identified. The lowest number of taxa was recorded in spring while the highest was recorded in autumn. Station 9 had the lowest number of taxa while the highest number of taxa was recorded at station 3. The average values (±SD) of the water quality parameters were temperature 14.75 ± 4.38 °C, pH 7.93 ± 0.62, water flow 14.11 ± 9.04 m3 s−1, electric conductivity 532.75 ± 161.35 μmohs cm−1, total dissolved solids 296.61 ± 76.21 mg L−1, salinity 0.28 ± 0.07 mg L−1, turbidity 580.77 ± 149.92 NTU and dissolved oxygen 8.08 ± 0.75 mg L−1. The assessment of stations 1 to 6 indicated that water quality conditions were suitable. In addition, substantial level of organic pollution was observed in stations 7 and 8. In station 9 water quality was fairly poor, requiring a more favourable management based on the capacity of the self-purification of the Haraz River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The surface water quality is an important issue in aquatic organisms surviving and has become a major environmental concern worldwide. River ecosystems have been increasingly threatened by various anthropogenic activities over the past decades. Considering the importance of rivers, water quality monitoring is essential for assessing pollutants.
Water quality in river ecosystems are usually monitored by aquatic organisms as well as chemical and physical tests. Physical and chemical monitoring methods reflects immediate measurements, while aquatic organisms respond better to the environmental changes (Naumoski et al. 2009). Aquatic organisms, such as diatoms, macrophytes, benthic macroinvertebrates and fish, should be used as bioindicators (López-López and Sedeño-Díaz 2015), the reason is that they are the major indicators applied in the official bioassessment systems in the different countries (Ibáñez et al. 2010). Macroinvertebrate communities are very good bioindicator of water quality and play a key role in identifying the pollution load of surface water and food webs. Benthic macroinvertebrates are used as bioindicators of water quality due to their high diversity, known pollution tolerances, low displacement, different feeding strategies, and sensitivity to environmental disturbance.
Different methods based on the employing of macroinvertebrates for assessing river have been developed. The assessment methods of macroinvertebrates are mostly based on species diversity, density, biomass, tolerance value, and functional and trophic variables. The distribution, population density and diversity of the macroinvertebrates community are affected by seasonal alterations, although seasonal changes in macroinvertebrates community can be large in some systems while small in others. Seasonal patterns in climate, such as precipitation and temperature, result in within-year changes in aquatic ecosystems (Chi et al. 2017). Changes in temperature and precipitation present serious challenges to macroinvertebrates community in both wet and dry seasons.
A great many studies on benthic macroinvertebrate communities of rivers have been reported by several authors. As such (Rossaro et al. 2007; Resende et al. 2010; Mehari et al. 2014; Gebrehiwot et al. 2017; Wang and Tan 2017; Wright-Stow and Wilcock 2017). Meanwhile, Naderi Jolodar (2008) conducted a similar study on the upstream of the Haraz River with an emphasis on the effect of salmon farms on river macroinvertebrates. However, very few studies have been carried out on benthic macroinvertebrates in Iran. Varnosfaderany et al. (2010) have shown that macroinvertebrates diversity decreased from upstream to downstream in Zayanderud, the impact of fish farm effluents on water quality assessed in Tajan River (Namin et al. 2013). Shokri et al. (2014) estimated river ecology status by many biotic and abiotic indices and showed the relationships between macroinvertebrate genera and physicochemical parameters.
The major focus of this study was to appraise the relationship between river water quality and the distribution of benthic macroinvertebrate in the Haraz River, which are particularly, affected by pollutants.
Material and methods
Study area
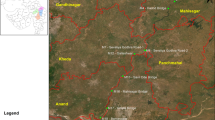
The Haraz River is located in the Mazandaran province in the north part of Iran. It lies between longitude of 52° 03′ 82˝E and 52° 26′ 60˝E, and latitude of 35° 50′ 01˝N and 36° 39′ 77˝N. The Haraz River originates from the Alborz Mountain, passing from Amol City, and then it enters the southern coast of the Caspian Sea (Fig. 1). The Haraz River is about 185 km long and has average width ranges between 50 to 500 m at different locations. Annual average precipitation is 888 mm, a large part is occurring in the cloudy seasons (Saremi et al. 2013; Banagar et al. 2008).
Water sampling
Applying Surber net samplers, we collected benthic macroinvertebrate’s samples along the stream in wet and dry seasons of 2015 at each of the nine stations with three replicates (30.5 cm × 30.5 cm). Latitude and longitude of sampling stations are reported in Table 1. All samples were preserved in the field in 4% formalin and brought to the laboratory. The macroinvertebrates were sorted, then they were identified up to the lowest possible taxonomic level and finally, they were counted under a Loupe. The water quality parameters such as temperature, pH, Electric Conductivity, Total Dissolved Solids, salinity, turbidity and Dissolved Oxygen were measured in the field by water checker (Hach Sension 5, USA and Aqualytic AL15, Germany).
Data analyses
Utilizing the PRIMER 5 software, macroinvertebrate metrics were calculated. The following indices were considered in particular: Hilsenhoff biotic Indices, Shannon Wiener Diversity Indices, and Average Score per Taxon (ASPT) and Pielou Evenness Index which were calculated by the equations below:
Hilsenhoff index
Where Xi is the number of specimens in each taxonomic group, ti is the pollution tolerance score for that taxonomic group, and n is the total number of organisms in sample. Macroinvertebrates are given with a numerical pollution tolerance score (ti) ranging from 0 to10 (Table 2).
Shannon–wiener index
Where Pi is the proportion of individuals found in the species i.
Average Score per Taxon (ASPT):
Where: BMWP = the value of Biological Monitoring Working Party; N = total number of families. The ASPT equals the average of the tolerance scores of all macroinvertebrate families that were found, and ranges from 0 to 10 (Hawkes 1998) (Table 3).
Pielou evenness index
Where H′max is the maximum possible value of Shannon diversity. The index oscillates from 0 to 1.
Data analyses were performed by utilizing the statistical packages Primer 5 and SPSS (version 24). The Kolmogorov–Smirnov test was accomplished in order to analyze the normality of data distribution. One way-ANOVA, followed by Tukey’s test (p < 0.05), was conducted so as to test the significant differences of biotic indices and physicochemical parameters among the sites.
Results
Table 4 shows the water quality analysis of the Haraz River. The average values of the water quality parameters were temperature 14.75 ± 4.38 °C, pH 7.93 ± 0.62, water flow14.11 ± 9.04 m3 s−1, electric conductivity (EC) 532.75 ± 161.35 μ-mhos cm−1, Total Dissolved Solids 296.61 ± 76.21 mg L−1, salinity 0.28 ± 0.07 mg L−1, turbidity 580.77 ± 149.92 NTU and Dissolved Oxygen 8.08 ± 0.75 mg L−1.
Maximum value of EC was 720 μ-mhos cm−1 at station 9 and the minimum amount was 334.25 μ-mhos cm−1 at station 3. The lowest turbidity was 491.25 NTU at station 4 and the highest value was 731.25 NTU at station 1. Maximum value of TDS was 372.5 at station 1 and the minimum TDS was 247.5 at station 4. Lowest and highest DO concentrations were recorded at station 9 (6.92 mg L−1) and station 1 (9.2 mg L−1), respectively. Maximum value of surface water salinity was 0.37 at stations 1 and 9 and the minimum value was 0.20 at station 3. The lowest pH was 6.88 at station 1 and the highest amount was 8.35 at station 4. The lowest surface water temperature was 9.92 °C at station 1 and the highest amount was 19.80 °C at station 9. Maximum water flow was 28.31 m3 s−1 at station 7 and the minimum value was 1.17 m3 s−1 at station 1.
The benthic macroinvertebrates families, identified in the investigated stations at the Haraz River, are shown in Table 5. A total of 3781 (spring 769, summer 1092, autumn 1095 and winter 825) benthic macroinvertebrate specimens belonging to four orders, 11 classes and 16 families were identified. The macroinvertebrates collected in all the 9 stations were included the following families, namely Hydropsychidae (30%), Heptageniidae (23%), Baetidae (20%), Chironomidae (14%), Simuliidae (9%), Lumbricidae (1%), in addition, Leuctridae, Coenagrionidae, Ichneumonidae, Tipulidae, Limoniidae, Culicidae, Elmidae, Gammaridae, and Lumbriculidae together (3%). Figure 2 shows the percentage composition of macroinvertebrate taxa in the nine stations. The lowest number of taxa was recorded in the spring while the highest amount was recorded in the autumn. Station 9 had the lowest number of taxa while the highest number of taxa was recorded at station 3.
Table 6 shows the seasonal variation in diversity indices and biomonitoring scores of the Haraz River. The highest BI value (8), was found in spring at station 9 and lowest value (3.66), was found in winter season at station 1. The distributions of the scores over the BI quality classes were the following: station 5, 6 and 7 (Very good), stations 1, 3, 4 and 8 (Good), station 2 (Fair), and station 9 (Very poor). The highest Shannon-Wiener Index (1.61) and Pielou Evenness Index (1) values were found in spring at station 1. The lowest values of Shannon-Wiener (0.22) and Pielou Evenness Index (0.20) were recorded in summer at station 7. The lowest and highest values of ASPT Index were recorded in winter at stations 9 and 6, respectively. At station 1, significant positive correlation was observed between turbidity and TDS (r = 0.996; p < 0.01), temperature and water flow (r = 0.990; p < 0.01) and water flow and abundance macroinvertebrates (r = 0.963; p < 0.05). At station 2, Pearson correlation coefficient matrix indicated a positive significant correlation between turbidity and TDS (r = 0.994; p < 0.01), TDS and temperature (r = 0.967; p < 0.05), DO and abundance macroinvertebrates (r = 0.983; p < 0.05) and pH and temperature (r = 0.964; p < 0.05). Significant negative correlation (r = −0.974; p < 0.05) was observed between the water flow and EC. At station 3, there were positive strong correlations between turbidity and TDS (r = 0.990; p < 0.01), TDS and DO (r = 0.987; p < 0.05) and DO and turbidity (r = 0.998; p < 0.01). In addition, there were significant negative correlation (p < 0.05) between EC and water flow (r = −0.954). At station 4, there was a positive strong relationship between turbidity and TDS (r = 0.972; p < 0.05) and DO and temperature (r = 0.981; p < 0.05). At station 5, significant negative correlation (r = −0.962; p < 0.05) was observed between EC and pH. At station 6, there were a positive strong correlation between turbidity and TDS (r = 0.985; p < 0.05), and temperature and water flow (r = 0.997; p < 0.01). In addition, there were strong negative correlation between EC and pH (r = −0.998; p < 0.01). At station 7, significant positive correlations obtained between EC and turbidity (r = 0.987; p < 0.05), EC and TDS (r = 0.985; p < 0.05), turbidity and TDS (r = 0.992; p < 0.01), pH and abundance of macroinvertebrates (r = 0.988; p < 0.05) and temperature and water flow (r = 0.988; p < 0.05). At station 8, Pearson correlation coefficient matrix indicated a positive significant correlation between EC and turbidity (r = 0.982; p < 0.05), EC and TDS (r = 1; p < 0.001) and turbidity and TDS (r = 0.980; p < 0.05). A strong negative correlation was observed between EC and pH (r = −0.975; p < 0.05), turbidity and temperature (r = −0.975; p < 0.05) and TDS and pH (r = −0.978; p < 0.05). At station 9, positive significant correlations were existed between EC and turbidity (r = 0.951; p < 0.05), EC and TDS (r = 0.971; p < 0.05) and turbidity and TDS (r = 0.985; p < 0.05).
The results of RDA analysis showed that for the spring campaign, the first and second axes accounted for 100% of the correlation between benthic families and the environmental parameters of water (Table 7). Considering the first axis as the basis for analysis, the families of Chironomidae and Culicidae had a positive and close relationship with the amount of EC, TDS and Turbidity, while the frequency of Limoniidae and Gammaridae showed an inverse relation with these environmental parameters. The highest frequency of Chironomidae and Culicidae was observed at stations 9 with the highest level of EC, TDS and Turbidity. The water flow and dissolved oxygen variables were well loaded in the second axis, and RDA analysis has shown that dissolved oxygen had a direct effect on the frequency of the Lumbriculidae, but has an inverse relationship with water temperature. The direction and lengths of vectors of EC, TDS and Turbidity in the first place, and pH and salinity in the second place were the most influential environmental variables responsible of the benthic macroinvertebrates variation (Fig. 3).
In summer the results of RDA analysis showed that the first and second axes, with 99.6% and 98%, respectively, justified the relationship between environmental parameters and abundance of benthic macroinvertebrates (Table 7). According to the first axis, the family Tipulidae, despite the low frequency, had a close and positive relationship with water flow, temperature and EC. These parameters had a negative relationship with the frequency of Simuliidae and Heptageniidae. The salinity, turbidity and TDS variables were well loaded in the second axis, and RDA analysis has shown that they had a direct relationship with Unionidae and an inverse relationship with Limoniidae and Elmidae. RDA showed that temperature in the first place and parameters such as salinity and water flow in the second place the most influential variables that played a role in the frequency variation of benthic macroinvertebrates (Fig. 4).
The results of direct classification of the redundancy analysis showed that for the environmental parameters measured in autumn, the first and second axes could justify 100% of the correlation between benthic families and environmental parameters of water (Table 7). Considering the first axis as the basis for relation analysis, Simuliidae, Tipulidae, Gammaridae, Baetidae and Heptageniidae had a positive and close relationship with dissolved oxygen, and a negative relationship with temperature, salinity, pH and water flow. The highest frequency of these families was observed at stations 1–4 with the highest level of dissolved oxygen. The turbidity was well loaded in the second axis, and RDA analysis has shown that it had a direct relationship with Unionidae and has an inverse relationship with Limoniidae and Hydropsychidae. The direction and lengths of vectors of each of the environmental variables, temperature in the first place, and parameters such as DO and water flow in the second place were the most influential variables that contributed to variations of benthic frequencies (Fig. 5).
In winter the results of direct classification of the redundancy analysis showed that for the measured environmental parameters, the first and second axes justified 100% of the correlation between benthic families and environmental parameters of water (Table 7). Unlike the previous seasons, based on the RDA graph, dissolved oxygen and water flow parameters were loaded at the second axis. Dissolved oxygen had positive and close relationship with the frequency of Limoniidae and Chironomidae, and a negative relationship with Tipulidae. Investigating the length and direction of the vectors showed that the water flow was the most influential parameters in relation to the abundance of benthic macroinvertebrates (Fig. 6).
Discussion
A similar study has been carried out about the impact of fish farm effluents on benthic macroinvertebrates (Naderi Jolodar 2008), 24 families were identified in different seasons ranging from spring to winter in 10 stations positioned on a 23 km long line along the Haraz River. In the present study, we considered 4 stations in the same area and 8 families in common with the study of Naderi Jolodar (2008).
In this study, almost all physicochemical parameters of water, except dissolved oxygen, increased from upstream to downstream.
The macroinvertebrate abundance experienced a sharp increase from station 1 to station 3, and then saw a decrease to the last station, which apparently depends on the flow rate.
There are specific groups of macroinvertebrates in the first two stations that can be related to the environmental conditions such as temperature, dissolved oxygen and nutrient deficiencies. The density of macroinvertebrates was on fall after the third station to the river estuary, due to the fact that substrates changed from cobbles to sand and mud; in agreement with changes of other environmental parameters and anthropogenic stressors such as fish farms, urbanization, agriculture and gravel mining and dam.
In this study, five families of Diptera were identified as Chironomidae, Simuliidae, Tipulidae, Limoniidae and Culicidae. This was the highest number of families compared to other orders. The Chironomidae family was the only family observed at all stations, with the highest frequency at stations 2 and 3, and with the lowest frequencies at stations 5 and 6. Families of Hydropsychidae, Heptageniidae and Baetidae were present in all stations, except at station 9. Fish farms effluent is one of the key factors that negatively affect the structure and variation of benthic invertebrate populations, increasing the number of tolerant groups and decreasing the number of susceptible groups (Naderi Jolodar et al. 2010). The highest frequency of large benthic macroinvertebrates species was observed in stations 3 and 4 that was related to the Hydropsychidae family. The reason for raising this issue could be the entry of nutrients into the river via the effluent of rainbow trout farms before and after these stations. The Culicidae family had the lowest frequency which observed at station 9, followed by the Coenagrionidae family at stations 1 and 4, as well as the Unionidae family only at station 9. Jafari et al. (2011) investigated the population structure of benthic macroinvertebrates in the Casellian River of Mazandaran. In this study, 31 families of benthic macroinvertebrates were identified. Individuals from the three orders of Diptera, Ephemeroptera and Trichoptera dominated all stations. Their study showed that Chironomidae family abundance was significantly increased due to the introduction of urban pollution in two stations. The results also showed that the Trichoptera order of Hydropsychidae family had the highest frequency. Pazira et al. (2009) investigated the effect of some environmental factors on biodiversity of macrobenthos of Daleki and Hella rivers of Bushehr for 14 months at seven stations. The highest biodiversity was in the warm months of the year (July and August). The highest relative frequency was Ephemeroptera and Diptera. In the study of the possibility of the influence of benthic population on the Taleghan River quality of water that was carried out by Mahdavi et al. (2010), a total of 13 families belonging to six orders were identified, all of which included insect larvae. Ephemeroptera, Trichoptera and Diptera orders were prevalent everywhere. The survey showed the healthy status of water in the Taleghan River that was not at high risk.
Based on the BMWP Index, the status of all stations positioned around the Haraz River, with the exception of stations 2 and 6, was favorable and the ASPT index corroborated this result. At stations 2 and 9, the organic loading was more than the other stations. The reason was the river pollution caused by villages and areas of tourism and services at station 2, and the intensive agriculture, aquaculture and urbanization at station 9. Moreover, the rate of water flow at these stations was too low. The results show that, despite severe human activities along the river, the bioindicators were less affected. The reason lay in the fact that the river flow and the river self-purification were very high during the year.
Based on the Hilsenhoff bioindicator, the mean water quality in the four seasons was very good at stations 5, 6 and 7, was good at stations 1, 3, 4 and 8, was fair at station 2 and was very poor at station 9. The results indicate that, on average, the minimum amount of bioindicator, observed at station 1 in winter, was 3.66 and its maximum value, detected at station in 9 in spring, was 8. According to Hilsenhoff qualitative classification, the score of 0 to 3.75 locates in the area of unpolluted water (excellent). Thus, considering the score of 3.66 for station 1 in the winter, this station belongs to this class. The remarkable point is that the average of none of the nine stations during the four seasons was not in the range of unpolluted water (excellent).
Based on the ASPT biological index, the mean water quality in the four seasons was good at stations 5 and 6, was moderate at stations 1, 3, 4, 7, and 8, was poor at station 2, and was very poor at station 9. On the basis of the results, on average, the minimum amount of biological indicator was 1.4 at station 9 in winter and its maximum value was 8.37 at station 6 in the winter. The qualitative classifications of ASPT indicate that a score greater than seven locates in the clean waters (very good class). Station 1 with a score of 7.16, station 5 with a score of 8.25 and station 6 with a score of 8.37, all in winter, and station 7 with a score of 7.1 and station 6 with a score of 7.17 in the spring were in this class. It is noticeable that the average of none of the nine stations during the four seasons was not within the clean waters (very good). The calculation of the results from the ASPT and Hilsenhoff index showed that water quality conditions in stations 5 and 6 were more appropriate than the other stations. In addition, the water quality at station 9 was very poor in comparison to the other stations. In other words, stations 5 and 6 that were located tens of kilometers from fish farming pools had returned to a good condition. The reason was occurring the self-purification along the river. However, when the river enters into the plain area, it was contaminated by entering various types of industrial, domestic and agricultural wastes, particularly at station 9. The Shannon-Wiener Diversity Index in the considered stations of the Haraz River reached its highest value at station 1 and it saw its lowest amount at station 7. The lowest value of Shannon-Weiner Index was 0.22 at station 7 in summer, and its highest value was 1.61 at station 1 in the spring. When the frequency distribution of all individuals of different species in the sample is uniform, one can predict that the Distribution Similarity Index approaches the maximal value, and if the distribution and the relative frequency of individuals are non-uniform, then the numerical value of this index will tend to zero (Barbour et al. 1992; Ludwig and Reynolds 1988). The study of the seasonal mean of Pielou uniformity Index in the study stations of the Haraz River showed that the lowest level of uniformity of the Pielou index was equal to 0.20 at station 7 in the summer, and its highest level was 1 at station 1 in the spring. The more uniform the distribution of the species is, the more stability and sustainability (Barnes et al. 1997) the river ecosystem will achieve, and as a result the greater the biodiversity will be.
Conclusion
According to the quality assessment of the Haraz River in this study, stations 1 to 6 have suitable conditions and those stations indicate the ability of self-purification of the river (particularly stations 5 and 6). However, the assessment of stations 7 and 8, especially station 9, were under the stressful conditions and human activities, and demonstrate the adverse conditions of the river at these stations, that require a more favorable management based on the capacity of the self-purification of the Haraz River.
References
Banagar GH, Kiabi BH, Homayoonnezhad I, Piri I, Amirian P (2008) Biodiversity of fish species in the Haraz River. World Appl Sci J 5(1):5–11
Barbour MT, Plafkin JL, Bradley BP, Graves CG, Wisseman RW (1992) Evaluation of rapid bioassessment metrics: metric redundancy and variability among reference stream sites. Environ Toxicol Chem 2:437–449 https://doi.org/10.1002/etc.5620110401
Barnes BV, Zak DR, Denton SR, Spurr SH (1997) Forest ecology, 4th edn. John Wiley & Sons, Inc., New York
Chi S, Li S, Chen S, Chen M, Zheng J, Hu J (2017) Temporal variations in macroinvertebrate communities from the tributaries in the three gorges reservoir catchment, China. Rev Chil Hist Nat 90:6 https://doi.org/10.1186/s40693-017-0069-y
Gebrehiwot M, Awoke A, Beyene A, Kifle D, Triest L (2017) Macroinvertebrate community structure and feeding interactions along a pollution gradient in Gilgel gibe watershed, Ethiopia: implications for biomonitoring. Limnologica 62:68–76. https://doi.org/10.1016/j.limno.2016.11.003
Hawkes HA (1998) Origin and development of the biological monitoring working party score system. Water Res 32(3):964–968 https://doi.org/10.1016/S0043-1354(97)00275-3
Hilsenhoff WL (1988) Rapid field assessment of organic pollution with a family-level biotic index. J North Am Benth Soc 7(1):65–68 https://doi.org/10.2307/1467832
Ibáñez C, Caiola N, Sharpe PJ, Trobajo P (2010) Application of ecological indicators to assess the health for river ecosystems. In: Jørgensen SV, Xu L, Costanza R (eds) Handbook of ecological indicators for assessment of ecosystem health. CRC Press, Taylor & Francis Group, Boca Raton, pp 447–464
Jafari A, Karami M, Abdoli A, Esmaili Sari A, Mortezaii G (2011) Macroinvertebrate population structure of the Keselian River, Mazandaran Province. J Fisheries 5(2):101–112 (In Persian)
López-López E, Sedeño-Díaz JE (2015) Biological indicators of water quality: the role of fish and macroinvertebrates as indicators of water quality. In: Armon RH, Hänninen O (eds) Environmental indicators. Springer, Dordrecht Heidelberg, pp 643–661
Ludwig J, Reynolds JF (1988) Statistical ecology: a primer on methods and computing, 1st edn. Wiley- Interscience Publication, New York
Mahdavi M, Bazrafshan O, Javanshir A, Mousavi Nodushani R, Babapour M (2010) Study of the effect of benthic community structure of Taleghan River on the determination of water quality. J Nat Env 63(1):75–91 (In Persian)
Mehari AK, Wondie A, Mingist M, Vijverberg J (2014) Spatial and seasonal variation in the macro-invertebrates and physico-chemical parameters of the Enfranz River, Lake Tana sub-basin (Ethiopia). Ecohydrol Hydrobiol 14:304–312
Naderi Jolodar M. 2008. Effect of salmon farms on the diversity and density of nutrition groups of macroinvertebrates in the Haraz River. Ph.D. thesis, Tarbiat Modarres University. 121 pp (In Persian)
Naderi Jolodar M, Abdoli A, Mirzakhani MK, Sharifi Jolodar R (2010) Benthic macroinvertebrates response in the Haraz River to the trout farms effluent. J Fisheries 2(64):163–175 (In Persian)
Namin JI, Sharifinia M, Makrani AB (2013) Assessment of fish farm effluents on macroinvertebrates based on biological indices in Tajan River (North Iran). Casp J Env Sci 11(1):29–39
Naumoski A, Kocev D, Atanasova N, Mitreski K, Krstić S, Džeroski S (2009) Predicting chemical parameters of the water from diatom abundance in Lake Prespa and its tributaries. In: Information Technologies in Environmental Engineering, ITEE. Springer, Berlin, Heidelberg, Thessaloniki, pp 264–277. https://doi.org/10.1007/978-3-540-88351-7
Pazira A, Emami SM, Kouhgardi E, Vatandoost S, Akrami R (2009) The effect of the environmental parameters on biodiversity of macrobenthos in Dalaki and Helle rivers of Boushehr. J Fisheries 2(4):65–70 (In Persian)
Resende PC, Resende P, Pardal M, Almeida S, Azeiteiro U (2010) Use of biological indicators to assess water quality of the Ul River (Portugal). Environ Monit Assess 170:535–544 https://doi.org/10.1007/s10661-009-1255-4
Rossaro B, Marziali L, Cardoso AC, Solimini A, Free G, Giacchini R (2007) A biotic index using benthic macroinvertebrates for Italian lakes. Ecol Indic 7:412–429. https://doi.org/10.1016/j.ecolind.2006.04.002
Saremi A, Saremi K, Saremi A, Sadeghi M, Sedghi H (2013) The effect of aquaculture effluents on water quality parameters of Haraz River, Iran. J Fish Sci 12(2):445–453 (In Persian)
Shokri M, Rossaro B, Rahmani H (2014) Response of macroinvertebrate communities to anthropogenic pressures in Tajan River (Iran). Biologia 69(10):1395–1409 https://doi.org/10.2478/s11756-014-0448-7
Varnosfaderany MN, Ebrahimi E, Mirghaffary N, Safyanian A (2010) Biological assessment of the Zayandeh Rud River, Iran, using benthic macroinvertebrates. Limnologica 40(3):226–232. https://doi.org/10.1016/j.limno.2009.10.002
Wang X, Tan X (2017) Macroinvertebrate community in relation to water quality and riparian land use in a subtropical mountain stream. China Env Sci Pollut Res Int 24(17):14682–14689. https://doi.org/10.1007/s11356-017-9042-1
Wright-Stow AE, Wilcock RJ (2017) Responses of stream macroinvertebrate communities and water quality of five dairy farming streams following adoption of mitigation practices. New Zeal J Mar Fresh 51:127–145. https://doi.org/10.1080/0028
Acknowledgments
This research was supported by the Islamic Azad University, Science and Research Branch, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Banagar, G., Riazi, B., Rahmani, H. et al. Monitoring and assessment of water quality in the Haraz River of Iran, using benthic macroinvertebrates indices. Biologia 73, 965–975 (2018). https://doi.org/10.2478/s11756-018-0107-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0107-5