Abstract
Wastewater discharge from sugarcane processing is a significant pollutant of tropical aquatic ecosystems. For most developing countries, monitoring of the level of pollutants is done mostly through chemical analysis, but this does not reflect potential impacts on aquatic assemblages. In addition, laboratory facilities for accurate concentration measurements are often not available for regular monitoring programs. In this study, we investigated the use of benthic macroinvertebrates for biological monitoring in western Kenya. Benthic macroinvertebrates were sampled in stabilization ponds treating wastewater from sugarcane- and molasses-based processing plants to assess their composition and abundance in relation to different concentrations of chemical variables. Optimum concentrations and tolerance values were identified for various taxa, and a biotic index was developed that combined tolerance values (ranked between 0 and 10) for the various macroinvertebrate taxa. A succession in composition and distribution of macroinvertebrate taxa was observed from the inlet to the outlet of the pond systems. Diptera dominated in the first ponds that had high concentrations of chemical oxygen demand (COD), biological oxygen demand (BOD5), and nutrients, while intolerant Ephemeroptera, Plecoptera, and Trichoptera (EPT) appeared as the concentrations dropped in subsequent ponds. The effluent quality was classified as “good,” “fair,” and “poor,” corresponding with biotic index value ranges 0–3.50, 3.51–6.50, and 6.51–10, respectively. During validation, the index grouped sites with respect to levels of measured environmental variables. The study revealed that the developed biotic index would help in monitoring the quality of sugarcane processing and molasses effluents before release into recipient aquatic ecosystems, replacing the need for costly chemical analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
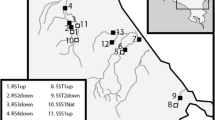
Wastewater from sugarcane processing is one of the major causes of pollution in many tropical or subtropical aquatic ecosystems (Hervé and Jean 2006; Gunkel et al. 2007; Kumar et al. 2007; Sahu and Chaudhari 2015). In the Lake Victoria Basin (LVB), cultivation of sugarcane and wastewater from its processing has had deteriorating effects on water quality (Bootsma and Hecky 1993; Calamari et al. 1995; Tonderski et al. 2007). At the ecosystem level, organic matter discharge from sugarcane processing has been found to have significant effects on carbon sources and its contribution to riverine food webs (Ojwang’ et al. 2007). In the LVB, the discharges have resulted in deteriorated water quality, algal blooms, increased biomass of aquatic macrophytes, depleted oxygen levels in water, fish kills and a reduced biodiversity (Lung’ayia et al. 2001). The major components of environmental concern in wastewater from sugarcane processing are organic matter (biological oxygen demand; BOD), total suspended solids (TSS), total dissolved solids (TDS), chemical oxygen demand (COD), dissolved oxygen, color, phosphorus, ammonium nitrogen (NH4-N), and temperature (Hosetti 1995; Calero et al. 2000; Hervé and Jean 2006; Sahu and Chaudhari 2015). Other pollutants and contaminants that have been linked to wastewater from sugarcane processing include organochlorides, herbicides such as atrazine, and heavy metals such as copper (Azevedo et al. 2004; Corbi et al. 2006; Silva et al. 2008), including trace metal accumulation in tissues of fishes in recipient streams and rivers (Ngelinkoto et al. 2014). Monitoring of levels of contaminants and pollutants in the final wastewater outflows from treatment systems is mostly done by chemical analysis of various variables. This is often expensive and, due to a lack of systematic quality control, also uncertain. The approach also lacks the capacity to integrate the effects on resident biota. Considering the ability of biotic assemblages to respond to changes in physico-chemical characteristics of their environment, development of a biological monitoring approach would be rapid and cost-effective in this part of the world (Fig. 1).
Location of the study sites (Chemelil, Mumias, and Spectre Factories) used for development and testing of the biotic index used to evaluate the performance of stabilization ponds treating wastewater from sugarcane and molasses processing in western Kenya. Map modified from Ojwang’ et al. (2007)
Macroinvertebrate assemblages have been useful as indicators for assessing effects of wastewater discharges in aquatic ecosystems (Dickens and Graham 1998; Buss et al. 2002; Buss et al. 2015). By utilizing their various tolerances to variations in environmental conditions, the composition and abundance of macroinvertebrate taxa can potentially also evaluate the effectiveness of wastewater treatment systems. Similar approaches have been used to develop rapid bioassessment protocols and indices for evaluating the effects of pollutants on water quality and macroinvertebrate communities in different regions around the globe (Barbour et al. 1999; Henne et al. 2002; Dickens and Graham 2002; Junqueira et al. 2010; Blakely et al. 2014; Aschalew and Moog 2015; Kaaya et al. 2015). However, knowledge about macroinvertebrate community composition and distribution in stabilization ponds is generally poor, particularly for ponds treating wastewater from sugarcane and molasses processing.
Studies focusing on the effect of wastewater discharges from sugarcane processing on water quality and macroinvertebrate assemblage characteristics in the recipient rivers have recorded a decrease in taxa richness caused by elimination of sensitive taxa, mostly represented by insect orders Ephemeroptera, Plecoptera, and Trichoptera, and a dominance of a few pollution-tolerant groups, such as midges and oligochaetes (Weigel et al. 2002; Buss et al. 2002). In the LVB, low taxa richness and dominance of Diptera downstream of the discharge points of wastewater from sugarcane processing are recorded (Raburu 2003; Raburu et al. 2009). In stabilization ponds at Chemelil Sugar Factory, Tonderski et al. (2007) recorded a sharp increase in the number of families when the factory was closed for maintenance, implying that in the absence of wastewater discharges, more sensitive macroinvertebrate taxa could also colonize the ponds. It was also observed that there was succession of taxa in the stabilization ponds as treatment progressed from one pond to the next (Tonderski et al. 2007). This successional pattern reflects how various tolerant macroinvertebrate taxa respond to changes in the levels and concentrations of water physico-chemistry and toxicants in wastewater. Subsequently, by examining the composition and abundance of these taxa, together with their various environmental optima in relation to measured environmental variables, inferences can be made on the level of pollutants without necessarily undertaking chemical analyses. Because most sugar factories have annexed alcohol distilleries without a separate wastewater treatment system, it is important to know the effects of the two sources of wastewater on biota so that an efficient monitoring tool can be developed.
The use of biomonitoring tools to monitor the status of water quality and ecosystem integrity of a riverine ecosystem is gaining ground in eastern Africa (Masese et al. 2009a, b; Masese et al. 2013; M’Erimba et al. 2014; Kilonzo et al. 2014; Kaaya et al. 2015; Aschalew and Moog 2015). While concerted efforts have been witnessed to help in efficient treatment of pollutant loads from sugar and molasses factories, such as the use of constructed wetlands (Bojcevska and Tonderskii 2007; Tonderski et al. 2007; Olguin et al. 2008), there is a need for a cost-effective way of assessing the performance of these treatment systems. This is especially important given the temporal fluctuations in the levels of pollutants in the final outflows (e.g., Tonderski et al. 2007), which may not be recorded if chemical analyses are infrequent. In such circumstances, biotic assemblages are important because they integrate temporal performance of the treatment system and thereby help monitor the quality of effluents continuously released into recipient aquatic systems.
In this study, we used benthic macroinvertebrate assemblages in stabilization ponds receiving wastewater from sugarcane and molasses processing in western Kenya to assess the treatment efficiency of stabilization ponds used to treat wastewater before release into the environment. Our objectives were to (1) investigate if the macroinvertebrate community composition in industrial sugarcane and molasses wastewater stabilization ponds correlates with the concentration and levels of a number of physico-chemical variables, (2) determine environmental concentration optima and tolerance values for various macroinvertebrate taxa in the stabilization ponds, and (3) develop a biotic index for monitoring the quality of the effluent from the ponds and receiving water.
Methodology
Study site
The study was conducted in Chemelil Sugar Factory (hereafter Chemelil) and Spectre International Molasses Factory (hereafter Spectre) in the LVB, western Kenya (Fig. 1). The region has a favorable climate for agriculture, with the activity covering more than 60% of the catchment. Sugarcane is the major cash crop especially in the middle and lower reaches of most river catchments. The annual rainfall displays a bimodal distribution with two distinct short and long rainy and dry seasons. The long rains occur during March to June with a peak in May. A second milder peak occurs during the short rainy season between September and November. The mean annual rainfall ranges from about 1100 to 1600 mm with a minimum and maximum mean monthly rainfall of 72 and 243 mm, respectively. The average annual temperature experienced in the region is 30 °C with a monthly minimum of 15–19 °C.
Chemelil is the second largest sugar factory in western Kenya. Both Chemelil and Spectre use stabilization ponds in treating their industrial effluents, which is a common form of wastewater treatment for many factories in the region. The ponds are constructed in series but differ in dimensions. There are 12 and 6 treatment ponds at Chemelil and Spectre, respectively. At Chemelil, the effluent from the factory (1800–2500 m3 day−1) passes a grease trap and is then discharged into the ponds, which cover 4.05 ha. The pH of the effluent is regulated at the inflow by liming. At the two factories, the ponds are more than 1 m deep in the middle but the edges are shallow allowing for the growth of aquatic plants (macrophytes). The edges are covered by sedges and grass, and this is where most invertebrate taxa attached themselves and were collected from. There was algal growth in the ponds at Spectre, Chemelil, and Mumias. The ponds were dominated by Chlorophyceae (mainly Botryococcus sp. and Selenastrum sp.), Cyanophyceae (Phormidium sp. and Microcystis sp.), Bacillariophyceae (mainly Nitzschia sp.), and Euglenaceae (Euglena sp. and Phacus sp.).
Sampling of macroinvertebrates and water quality were done in ponds 1 (CH1), 5 (CH2), 7 (CH3), 9 (CH4), and 12 (CH5) (Fig. 2a). At Spectre, the ponds cover 2.5 ha. The last five ponds (SP1 to SP5) were sampled (Fig. 2b). Water and macroinvertebrate samples were collected from the ponds on the same date. For Chemelil, samples were collected monthly from March to September 2002. At Spectre, samples were collected once during the dry season in July and August 2008.
Physico-chemical variables
Triplicate water column samples were collected from each stabilization pond (site) at the sugarcane and molasses factories in 1-L HDEP bottles and transported to the laboratory in a cooler at 4 °C. Total phosphorus (TP) and total nitrogen (TN) were analyzed using the ascorbic acid-molybdate and Kjeldahl methods, respectively. Total suspended solids (TSS) was measured gravimetrically and chemical oxygen demand (COD) using the HACH-Lange method with mercury as a catalyst (HACH 2002). Biochemical oxygen demand (BOD5) was determined according to APHA (1998). A Hydrolab multiprobe instrument was used to record in situ measurements of temperature, pH, specific conductance, and dissolved oxygen.
Macroinvertebrate sampling and identification
Benthic macroinvertebrate samples were collected using a D-frame dip net (mesh size 300 μm). Three 1-m2 replicate samples were collected from each pond. One additional sample was collected in the outflow channel of the sampled ponds (total = four replicate samples per pond). The outflow channel was specifically targeted because of the rheophilic taxa such as Simuliidae and Hydropsychidae that live only in flowing water. Where it was not possible to collect macroinvertebrate samples from the bottom sediments of the ponds because they could not support life, samples were collected from the shallow vegetated edges of the ponds. Four 1-min (total sampling time = 4 min) multihabitat samples were collected from the vegetated edges of ponds near the outlet (three samples) and in the outlet channel (one sample). Sampling consisted of vigorous kicking of the bottom and vegetated edges then sweeping through the water column to collect suspended invertebrates. The samples were immediately placed in plastic containers, preserved with 70% alcohol, and transported to the laboratory where they were sorted, identified, and counted. Identification was done to the lowest possible taxonomic level with the help of a series of guides (Day et al. 2002; de Moor et al. 2003a, b; Stals and de Moor 2007; Merritt et al. 2008).
Data analysis
Before any statistical tests, count data (macroinvertebrate abundances for each taxon) and physico-chemical data were log10 x + 1 transformed to meet the statistical criteria for normality (Zar 2001). Differences in physico-chemical parameters between the sugarcane (Chemelil) and molasses (Spectre) factories were investigated by two-way analysis of variance (ANOVA) using site and pond as factors. A one-way ANOVA was used to investigate differences between stabilization ponds in each factory. Post hoc Tukey’s HSD test was performed to compare means of the individual stabilization ponds. The relationships between taxa ordinations and physico-chemical conditions at the stabilization ponds were examined using canonical correspondence analysis (CCA) (ter Braak 1986). Pairwise Pearson’s correlations were performed on physico-chemical variables and all macroinvertebrate taxa to test for redundancy and collinearity so as to reduce the number used in CCA (Livingstone 2009). Physico-chemical variables and macroinvertebrate taxa were considered redundant if the correlation value was ≥0.85, in which case only one taxon from the redundant group was selected and used in the CCA. This also helped reduce the number of taxa used in the CCA so as to avoid overcrowding the diagram and also to ensure that the responses in macroinvertebrate ordination were constrained by physico-chemical variables. The unimodal response model in CCA closely approximates biological responses in which an organism’s abundance increases to a maximum and then declines as the environmental value increases. Maximum abundance of a taxon is achieved at a specific value of the environmental variable which reflects the taxon’s optimum (Jongman et al. 1987). Canonical correspondence analysis was used to analyze fauna-environment relationships in order to identify environmental factors potentially influencing macroinvertebrate assemblages. The environmental preferences of macroinvertebrates were inferred by projecting the species scores onto the axes representing environmental variables used in CCA (Jongman et al. 1987). Similar environmental preferences and optima values were awarded to macroinvertebrate taxa whose correlation value was ≥0.85. All tests, except CCA, were run in SPSS for Windows (Version 13.0, SPSS Inc. Chicago, IL). The CCA was run in a modified biplot display in Microsoft Excel (Lipkovich and Smith 2002).
Macroinvertebrate tolerance
The tolerance values for various macroinvertebrate taxa in the stabilization ponds were determined by converting the preferences observed for physico-chemical variables that had a strong correlation with the two axes of the CCA to a scale of 0 (least tolerant) to 10 (most tolerant) and then calculating an average tolerance value for all variables. Each index was scaled by setting the distance from the minimum to the maximum tolerance value equal to 10 units with the restriction that a preference of zero corresponded to a value of five units (Jongman et al. 1987). For each taxon, a mean tolerance value (absolute value) for all physico-chemical variables in the effluents was calculated and rounded to the nearest whole number to provide a single tolerance value. To get a combined tolerance value for each taxon to sugar and molasses wastewaters, an average of the two values (one for molasses and one for sugarcane wastewater) was used and rounded to a whole number. Similar tolerance values were assigned to macroinvertebrate taxa whose correlation value was ≥0.85.
Biotic index
The biotic index was developed using data collected during the dry season from July to September 2002 at Chemelil and July and August 2008 at Specter. The dry season was chosen because of the stability of environmental conditions within the ponds. By utilizing tolerance values for various taxa in this study, a biotic index was developed for evaluating the quality of outflows from stabilization ponds treating wastewater from sugar and molasses factories. The tolerance value assigned to each taxon is based on its susceptibility or resistance to pollution and perturbations caused by wastewater discharges. The lowest scores are assigned to the taxa that are intolerant to pollution and the highest score to those that are tolerant or resistant to pollution. Values of 0–3 are assigned to intolerant taxa to pollution, 4–6 to moderately tolerant taxa, and 7–10 to tolerant taxa.
A total of three indices are calculated for the biotic index: “Total Index Score,” “Number of Taxa,” and the “Site Index Score.” The Number of Taxa index is calculated by listing all taxa noted at a site irrespective of abundance. A “Total Index Score” value is determined by assigning respective tolerance values to all taxa and then summing them together. To get the Site Index Score, the Total Index Score is divided by the Number of Taxa. Of the three indices, the Site Index Score is the most relevant and reliable for assessing the quality of wastewater discharges. This is because it is not affected by the number of taxa present at a site, but by the tolerance values of the taxa present (Chutter 1998). In addition, the abundance data of the various taxa is not useful in the calculation of the biotic index proposed here because of the time it takes to process and count the individuals of a taxon. The biotic index we propose is, thus, intended to be rapid but also rigorous in terms of the tolerance values assigned to each taxon. The range of Site Index Score values, 0 to 10, was then subdivided into three category classes “good” (0–3.50), “fair” (3.51–6.50), and “poor” (6.51–10) corresponding to the quality of the wastewater.
To validate the biotic index, data used were from the outflow of the last stabilization pond at Mumias Sugar Factory (hereafter Mumias), which is the largest in western Kenya, from upstream and downstream sites in rivers receiving effluents from Chemelil and Mumias, from the Lake Victoria where Spectre discharges its wastewater, and from the last stabilization pond at Chemelil collected from March to June 2002, excluding May when the factory was under maintenance. These dates were chosen to cover both the wet and the dry seasons to evaluate the suitability of the index to assess performance of the stabilization ponds all year round. In total, six sites and eight macroinvertebrate samples were used for validation of the index.
Results
Physico-chemical water quality
There were no significant differences in TSS, COD, and TP between Chemelil and Spectre stabilization ponds (Table 1), but the TN was higher and the pH lower in the Chemelil ponds (p < 0.05). Significantly higher values were recorded for COD, TSS, and temperature in the first stabilization pond at the two factories, where DO was also significantly lower (p < 0.05). The last ponds at the two factories had the lowest concentrations for most of the physico-chemical variables (p < 0.05). The concentrations in the effluent discharge from the ponds were above WHO standards for wastewater discharges, set at 100 and 150 mg/L for COD and TSS, respectively.
Macroinvertebrate assemblages
The stabilization ponds were dominated by the orders Diptera, Hemiptera, Coleoptera, and Crustacea (Table 2; Fig. 3). Ephemeroptera, Plecoptera, and Trichoptera occurred in Chemelil ponds CH4 and CH5. Common families and genera in the stabilization ponds at the two factories included Dytiscidae, Gyrinidae, Chironomidae (Chironomus sp.), Culicidae (Aedes sp.), Ephydridae (Ephydra sp.), Tabanidae (Tabanus sp.), Syrphidae (Eristalis sp.), Tipulidae (Tipula sp.,), Belostomatidae (Belostoma sp.), and Noteridae (Noterus sp.). Belostoma sp. was the only genus that occurred in all stabilization ponds.
Physico-chemical variables and macroinvertebrates
Because most of the macroinvertebrate taxa were redundant with each other, i.e., their distributions in the ponds displayed a correlation value of ≥0.85, only nine and eight macroinvertebrate taxa from sugar and molasses factories, respectively, were used in the CCA. Similarly, only five environmental variables (TP, temperature, pH, DO, and COD) were superimposed on the CCA biplot of macroinvertebrate taxa and sites. The concentration of TN was redundant with TP, and TSS, BOD, and specific conductivity with COD hence eliminated from the CCA. For Spectre, pH, TSS, and COD were depicted by environmental axes that were approximately parallel and in close proximity to one another. This indicated that biota displayed similar optima and tolerances (projection of species scores onto the environmental axis) to the variables.
The ordination biplots (Fig. 4) depict the main patterns of variation in community composition as constrained by the physico-chemical and nutrient variables. Species-environment correlations were strong (>0.70) for axes 1 and 2 in the two constrained ordinations (Table 3). This indicates that the environmental variables account for majority of the variation in taxa distribution, which was accounted for by the first ordination axis (Chemelil, 46%; Spectre, 45%). The second ordination axes accounted for less of the variability in the distribution, 31 and 26% for Chemelil and Spectre, respectively (Table 3). The first ordination axis separated stabilization ponds into two groups (Fig. 4a) that were defined by differences in pH, temperature, and COD associated with ponds CH1 and CH2 while ponds CH3–CH5 were associated with high TP. Ephydra sp. and Belostoma sp. were associated with the first group of ponds while the second group was associated with Baetis sp. and Afrocaenis sp. The same parameters separated ponds in the second axis whereby Hedria sp., Limnocoris sp., and Laccophilus sp. were associated with low pH. For Spectre, the first ordination axis separated the ponds into two groups (Fig. 4b) that were defined by differences in DO which was associated with pond SP5, pH, and COD associated with SP1, SP3, and SP4. Pond SP2 was associated with high TP. Aedes sp. and Tabanus sp. were associated with low DO concentration while Ephydra sp., Eristalis sp., and Corixa sp. were associated with high pH and COD levels. The second axis was defined by DO with which Micronecta sp. was negatively correlated.
Macroinvertebrate tolerance to environmental conditions
Tolerance of macroinvertebrate taxa to wastewater was determined by converting the preferences observed for COD, TP, pH, temperature, and DO to a scale of 0 (least tolerant) to 10 (most tolerant) (Table 4). Mean tolerance values determined for the various taxa and that for COD are presented in Table 4. Optima values have been multiplied by 10 and rounded to the nearest whole number. Large positive values indicate that the taxa optima are associated with high levels of the environmental variable while large negative values indicate that the optima are associated with low levels.
The biotic index
The correlation tests for redundancy on the distribution of macroinvertebrate taxa in the Chemelil stabilization ponds grouped Baetis sp., Simulium sp., Neoperla sp., Protoneura sp., and Cheumatopsyche sp.; Ectopria sp., Afronurus sp., Potamonautes sp., Limnophila sp., and Metrobates sp.; Lara sp. and Philopotamus sp.; and Laccophilus sp. and Limnocoris sp. Taxa in each group were assigned similar tolerance values corresponding to the ones developed for Chemelil macroinvertebrate taxa (Table 4). A combined tolerance value to industrial sugar and molasses wastewaters for all taxa is given in Table 5. Single tolerance values for taxa that did not occur in both sugar and molasses wastewater stabilization ponds were adopted as the tolerance value for wastewater from the two factories. The common tolerance values were then used to calculate the Total Index Score and the Site Index Score for final outflows from the three factories (Table 6).
Sampling sites used for validating the biotic index displayed different levels of pollutants arising from discharge of wastewater from sugarcane and molasses processing (Table 7). This was also reflected in the diversity and composition of macroinvertebrate assemblages (Fig. 5). Validation results grouped sites with respect to levels of measured environmental variables. The site index score for upstream and downstream sites at Chemelil and Mumias were 3.47 and 4.17 and 5.8 and 5.2, respectively. The site index score for the last stabilization pond at Mumias was 6.00 while scores for the last stabilization pond at Chemelil during March, April, and June 2004 were 6.20, 5.38, and 6.29, respectively. Site index score of “good” category was only recorded for Mumias upstream. The rest of the sites and ponds were of the “fair” category. The site index score of good quality was defined by low COD and TSS values, which met WHO standards. The “poor” and fair categories had values above WHO standards for the two parameters.
Relative abundance of macroinvertebrate taxonomic groups at different sampling stations used in validating the biotic index developed in this study: MU Mumias upstream, MD Mumias downstream, MP Mumias last stabilization pond, LW Lake Water—site sampled at the discharge point of wastewater from Spectre into Lake Victoria, CU Chemelil upstream, CD Chemelil downstream, CHE1, CHE 2, and CHE 3 Chemelil last stabilization pond in March, April, and June 2002, respectively
Discussion
Effluent from the two factories displayed similar results for TSS, COD, and temperature among the environmental variables assessed. The differences in pH and TN can be attributed to treatment efficiency especially considering the factory breakdown in Chemelil that led to overloading of the treatment system, thus causing a decline in the quality of wastewater in the final outflow. Although more variables were considered in this study, TSS and COD were used as critical indicators of effluent quality because of their known environmental concern (e.g., Hervé and Jean 2006; Sahu and Chaudhari 2015).
Macroinvertebrate assemblages in sugar and molasses stabilization ponds were dominated by Diptera—Ephydra sp., Eristalis sp., Hedria sp. and Antocha sp.; Hemiptera—Belostoma sp. and Corixa sp.; Coleoptera—Gyrinidae and Dityscidae; and Gastropoda—Planorbis sp. These groups dominated the raw wastewater in the first stabilization ponds at the inflow where pollutants had not been resorbed from wastewater, indicating that they were the most tolerant to wastewater from sugar and molasses factories. Notably, family Syrphidae, represented here by Eristalis sp., has been noted to thrive in waters receiving effluent from sugarcane processing (Takougang et al. 2008). However, as water treatment progressed and pollutant levels reduced, tolerant groups were replaced by relatively intolerant EPT taxa such as Baetis sp., Afrocaenis sp., Cheumatopsyche sp., Afronurus sp., and Elmidae (Coleoptera). This change in macroinvertebrate composition, which corresponded with measured levels of pollutants in the final outflows, supports their use in an index for evaluating treatment efficiency of wastewater from sugarcane-based industries.
The biotic index developed was able to register poor effluent quality released from stabilization ponds at the two factories. This means that the use of stabilization ponds for wastewater treatment was not effective in keeping the environmental variables below the set regulatory standards. For instance, the means for TSS and COD were 63.6 ± 7.8 and 602 ± 171.3 and 151.3 ± 14 and 580.5 ± 51.9 at the last ponds at Chemelil and Spectre, respectively, which are above the required standards set at 30 mg L−1 for TSS and 50 mg L−1 COD, respectively, in Kenya. These values were also above WHO standards, except for the low TSS value for Chemelil. The discharges also affected water quality in the recipient rivers downstream where low index scores were recorded, compared to upstream of the discharge points. However, the site index score belonging to the fair category upstream of the Mumias discharge point was due to the presence of sensitive taxa which were not found in the stabilization ponds and thus not included in the scoring because their tolerance values could be not developed.
The validation results confirmed the utility of the index in assessing the quality of the wastewater when applied to Mumias discharges and on Chemelil data collected during the rainy and dry seasons. The final outflow from Mumias stabilization ponds recorded low pH (4.2) and DO (0.8 mg/l) and higher temperature (26.7 °C) and conductivity (1260 μS/cm). Consequently, the macroinvertebrate samples were dominated by Eristalis sp. and Ephydra sp. with an index site score of 7.52 belonging to the poor category. The two taxa also exclusively occurred at the first stabilization pond at Chemelil and together with Aedes sp. and Micronecta sp. at the first stabilization pond at Spectre. The occurrence of these genera, together with Pedicia sp., Gyrinus sp., Psychoda sp., Antocha sp., Tabanus sp., Tipula sp., Hedria sp., and Planorbis sp. in wastewaters low in pH and DO and high in TSS and COD, indicate that they can be used to assess low treatment efficiency of wastewater from sugarcane and molasses processing factories. Low variability of the index for the last stabilization pond at Chemelil Sugar Factory indicates that it was stable during both the dry and rainy seasons. This implies that the index is applicable throughout the year. With respect to index values developed in this study, effluents in poor and fair categories do not meet WHO standards set for wastewater discharges. Only the good category met these standards for TSS and COD.
Conclusions
Sugar processing at Chemelil and Mumias and molasses processing at Spectre produce effluents of similar characteristics, high in TSS, COD, and conductivity and low in DO. This similarity was reflected in the composition and distribution of macroinvertebrate assemblages in the stabilization ponds. The biotic index developed in this study revealed that macroinvertebrate assemblages were useful for evaluating treatment efficiency of wastewater from sugar- and molasses-based processing factories. The index was stable during dry and rainy seasons and reflected effects of wastewater on downstream aquatic assemblages. The index also proved useful in evaluating the integrity of the recipient rivers. Therefore, we recommend that this index should be adopted for use in monitoring treatment efficiency of wastewater from sugarcane-based industries in the region as this will not only cut down on monitoring costs but will also help to maintain the integrity of recipient waterbodies. Using a similar approach, the index can be adopted and modified for use in assessing the treatment efficiency of wastewater from sugarcane and molasses processing in different regions around the world.
References
APHA (American Public Health Association). (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington, D.C.: American Public Health Association, American Water Works Association, and Water Pollution Control Federation.
Aschalew, L., & Moog, O. (2015). Benthic macroinvertebrates based new biotic score “ETHbios” for assessing ecological conditions of highland streams and rivers in Ethiopia. Limnologica - Ecology and Management of Inland Waters, 52, 11–19.
Azevedo, D. A., Gerchon, E., & Reis, E. O. (2004). Monitoring of pesticides and polycyclic aromatic hydrocarbons in water from Paraı’ba do Sul River, Brazil. Journal of the Brazilian Chemical Society, 15, 292–299.
Barbour MT, Gerritsen J, Snyder BD, Stribling JB. 1999. Rapid bioassessment protocols for use in wadeable streams and rivers: periphyton, benthic invertebrates, and fish (2nd edn). Washington DC: US Environmental Protection Agency, Office of Water. Report EPA 841-B-99–002.
Blakely, T. J., Eikaas, H. S., & Harding, J. S. (2014). The SingScore: a macroinvertebrate biotic index for assessing the health of Singapore’s streams and canals. Raffles Bulletin of Zoology, 62, 540.
Bojcevska, H., & Tonderskii, K. (2007). Impact of loads, season, and plant species on the performance of a tropical constructed wetland polishing effluent from sugar factory stabilization ponds. Ecological Engineering, 29, 66–76.
Bootsma, H. A., & Hecky, R. E. (1993). Conservation of the African great lakes: a limnological perspective. Conservation Biology, 7, 644–656.
Buss, D. F., Baptista, D. F., Silveira, M. P., Nessimian, J. L., & Dorville, L. F. M. (2002). Influence of water chemistry and environmental degradation on macroinvertebrate assemblages in a river basin in south-east Brazil. Hydrobiologia, 481, 125–136.
Buss, D. F., Carlisle, D. M., Chon, T. S., Culp, J., Harding, J. S., Keizer-Vlek, H. E., Robinson, W. A., Strachan, S., Thirion, C., & Hughes, R. M. (2015). Stream biomonitoring using macroinvertebrates around the globe: a comparison of large-scale programs. Environmental Monitoring and Assessment, 187, 1–21.
Calamari, D., Aketch, M. O., & Ochumba, P. B. O. (1995). Pollution of Winam Gulf, Lake Victoria, Kenya. A case study for preliminary risk assessment. Lakes and Reservoirs: Research Management, 1, 89–106.
Calero, C. X., Mara, D. D., & Pena, M. R. (2000). Anoxic ponds in the sugar cane industry: a case from Colombia. Water Science and Technology, 42(10–11), 67–74.
Chutter FM (1998). Research on the rapid biological assessment of water quality impacts in streams and rivers. Report to the water research commission, Pretoria. WRC Report No. 422/1/98.
Corbi, J. J., Strixino, S. T., Santos, A., & Del Grande, M. (2006). Diagnóstico ambiental de metais e organoclorados em córregos adjacentes a áreas de cultivo de cana-de-açúcar (Estado de São Paulo, Brasil). Química Nova, 29, 61–65.
de Moor, I.J., Day, J.A., de Moor, F.C. (2003a). Guides to the freshwater invertebrates of Southern Africa, Vol. 7, Insecta I: Ephemeroptera, Odonata and Plecoptera, WRC Report No. TT 207/03, South Africa.
de Moor, I.J., Day, J.A., de Moor, F.C. (2003b). Guides to the freshwater invertebrates of Southern Africa. Vol. 8, Insecta II: Hemiptera, Megaloptera, Neuroptera, Trichoptera and Lepidoptera, WRC Report No. TT 214/03, South Africa.
Day, J.A., Harrison, A.D., de Moor, I.J. (2002). Guides to the freshwater invertebrates of Southern Africa, Vol. 9: Diptera, WRC Report No. TT 201/02, South Africa.
Dickens, C. W. S., & Graham, P. M. (1998). Biomonitoring for effective management of wastewater discharges and the health of the river environment. Aquatic Ecosystem Health and Management, 1, 199–217.
Dickens, C. W. S., & Graham, P. M. (2002). The South African Scoring System (SASS) version 5 rapid bioassessment method for rivers. African Journal of Aquatic Science, 27, 1–10.
Gunkel, G., Kosmol, J., Sobral, M., Hendryk, R., Montenegro, S., & Aureliano, J. (2007). Sugar-cane industry as a source of water pollution—case study on the situation in Ipojuca River, Pernambuco, Brazil. Water Air & Soil Pollution, 180(1–4), 261–269.
HACH, 2002. Procedures Manual. HACH Company, Henne, L. J., Schneider, D. W., & Martinez, L. M. (2002). Rapid assessment of organic pollution in a west-central Mexican river using a family-level biotic index. Journal of Environmental Planning and Management, 45(5), 613–632.
Henne, L. J., Schneider, D. W., Martinez, L. M. (2002). Rapid assessment of organic pollution in a West-central Mexican river using a family-level biotic index. Journal of Environmental Planning and Management, 45(5), 613–632.
Hervé, M., & Jean, L. M. (2006). Overview of the biological processes available for the treatment of sugarcane mill wastewater. International Sugar Journal, 108(1), 431–439.
Hosetti, B. (1995). Treatment of sugar industry effluents by ponds and lagoons. Journal of Environmental Biology, 16(2), 143–149.
Jongman, R. H. G., ter Braak, C. J. F., & van Tongeren, O. F. R. (1987). Data analysis in community and landscape ecology. Den Haag: Pudoc–Wageningen 299 pp.
Junqueira, M. V., Friedrich, G., & Pereira de Araujo, P. R. (2010). A saprobic index for biological assessment of river water quality in Brazil (Minas Gerais and Rio de Janeiro states). Environmental Monitoring and Assessment, 163(1–4), 545–554.
Kaaya, L. T., Day, J. A., & Dallas, H. F. (2015). Tanzania River Scoring System (TARISS): a macroinvertebrate based biotic index for rapid bioassessment of rivers. African Journal of Aquatic Science, 40(2), 109–117. doi:10.2989/16085914.2015.1051941.
Kilonzo, F., Masese, F. O., van Griensven, A., Bauwens, W., Obando, J., & Lens, P. (2014). Spatial-temporal variability in water quality and macroinvertebrate assemblages in the upper Mara River basin, Kenya. Physics and Chemistry of the Earth, 67–69, 93–104.
Kumar, G. S., Gupta, S. K., & Singh, G. (2007). Biodegradation of distillery spent wash in anaerobic hybrid reactor. Water Research, 41(4), 721–730.
Lipkovich, I., & Smith, E. P. (2002). Biplot and singular value decomposition macros for excel. Journal of Statistical Software, 7, 1–15.
Livingstone, D. (2009). A practical guide to scientific data analysis. Chichester: John Wiley and Sons 341 pp.
Lung’ayia, H., Sitoki, L., & Kenyanya, M. (2001). The nutrient enrichment of Lake Victoria (Kenyan waters). Hydrobiologia, 458, 75–82.
M’Erimba, C. M., Mathooko, J. M., Karanja, H. T., & Mbaka, J. G. (2014). Monitoring water and habitat quality in six rivers draining the Mt. Kenya and Aberdare catchments using macroinvertebrates and qualitative habitat scoring. Egerton Journal of Science & Technology, 14, 81–104.
Masese, F. O., Muchiri, M., & Raburu, P. O. (2009a). Macroinvertebrate assemblages as biological indicators of water quality in the Moiben River, Kenya. African Journal of Aquatic Science, 34, 15–26.
Masese, F. O., Raburu, P. O., & Muchiri, M. (2009b). A preliminary benthic macroinvertebrate index of biotic integrity (B-IBI) for monitoring the Moiben River, Lake Victoria Basin, Kenya. African Journal of Aquatic Science, 34, 1–14.
Masese, F. O., Omukoto, J. O., & Kobingi Nyakeya, K. (2013). Biomonitoring as a pre-requisite for sustainable water resources: a review of current status and challenges in East Africa. Ecohydrology & Hydrobiology, 13, 173–191.
Merritt, R. W., Cummins, K. W., & Berg, M. B. (Eds.). (2008). An introduction to the aquatic insects of North America. Dubuque: Kendall/Hunt Publishing Company.
Ngelinkoto, P., Thevenon, F., Devarajan, N., Birane, N., Maliani, J., Buluku, A., Musibono, D., Mubedi, J. I., & Poté, J. (2014). Trace metal pollution in aquatic sediments and some fish species from the Kwilu-Ngongo River, Democratic Republic of Congo (Bas-Congo). Toxicological & Environmental Chemistry, 96(1), 48–57.
Ojwang’, W. O., Kaufman, L., Soule, E., & Asila, A. A. (2007). Evidence of stenotopy and anthropogenic influence on carbon source for two major riverine fishes of the Lake Victoria watershed. Journal of Fish Biology, 70, 1430–1446.
Olguin, E. J., Sanchez-Galvan, G., Gonzalez-Portela, R. E., & Lopez-Vela, M. (2008). Constructed wetland mesocosms for the treatment of diluted sugarcane molasses stillage from ethanol production using Pontederia Sagittata. Water Research, 42, 3659–3666.
Raburu PO. (2003). Water quality and the status of aquatic macroinvertebrates and ichthyofauna in River Nyando, Kenya. PhD thesis, Moi University, Kenya.
Raburu, P. O., Okeyo-Owuor, J. B., & Masese, F. O. (2009). Macroinvertebrate-based index of biotic integrity (M-IBI) for monitoring the Nyando River, Lake Victoria Basin, Kenya. Scientific Research and Essays, 4, 1468–1477.
Sahu, O. P., Chaudhari, P. K. (2015). The characteristics, effects, and treatment of wastewater in sugarcane industry. Water Quality, Exposure and Health, 7(3), 435–444.
Silva, D. M. L., P. B. de Camargo, L. A. Martinelli, F. M. Lanas, J. S. S. Pinto, and W. E. P. Avelar (2008). Organochlorine pesticides in the Piracicaba river basin (S São Paulo-Brazil): a survey of sediment, bivalves, and fish Química Nov. 31:214–219.
Stals, R., de Moor, I.J. (2007). Guides to the freshwater invertebrates of Southern Africa, Vol. 10: Coleoptera, WRC Report No. TT 320/07, South Africa.
ter Braak, C. J. F. (1986). Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology, 67, 1167–1179.
Takougang, I., Barbazan, P., Tchounwou, P. B., & Noumi, E. (2008). The value of the freshwater snail dip scoop sampling method in macroinvertebrates Bioassessment of sugar mill wastewater pollution in Mbandjock, Cameroon. International Journal of Environmental Research and Public Health, 5, 68–75.
Tonderski, K. S., Gronlund, E., Billgren, C., & Raburu, P. (2007). Management of sugar effluent in Lake Victoria region. Ecohydrology & Hydrobiology, 7, 345–351.
Weigel, B. M., Henne, L. J., & Martinez-Rivera, L. M. (2002). Macroinvertebrate-based index of biotic integrity for protection of streams in west-central Mexico. Journal of the North American Benthological Society, 21(4), 686–700.
Zar, J. H. (2001). Biostatistical analysis. Englewood Cliff: Prentice Hall 718 pp.
Acknowledgements
We are grateful to the management of the three factories for allowing us to conduct this study in their premises. We are also grateful to SIDA-SAREC and Kenya National Council for Science and Technology which provided funding for studies in Chemelil and Mumias Sugarcane Factories, respectively. We are grateful to the comments of two anonymous reviewers whose comments helped improve earlier drafts of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raburu, P.O., Masese, F.O. & Tonderski, K.S. Use of macroinvertebrate assemblages for assessing performance of stabilization ponds treating effluents from sugarcane and molasses processing. Environ Monit Assess 189, 79 (2017). https://doi.org/10.1007/s10661-017-5786-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5786-9