Abstract
Emergence of antimicrobial resistance (AMR) in cultured fishes is one of the major challenges faced in aquaculture. The high prevalence of bacterial infections in fishes leads to frequent use of antibiotics and thus their persistence in the aquatic environment, which in turn results in the proliferation of antibiotic resistant bacteria. The AMR in aquaculture can be transferred to clinically important strains of natural environment through horizontal gene transfer, thereby affecting the whole ecosystem. Most of the cultured fishes, including ornamental possess diverse pathogens exhibiting multiple antibiotic resistance. A thorough understanding of the gene transfer systems such as plasmids, transposons, integrons and gene cassettes can unravel the complexity of antimicrobial resistance in aquaculture. Continuous monitoring programmes, timely detections of the resistant bacteria and implementation of proper regulations are necessary to curb the dissemination of AMR in aquaculture. The present review summarises the antimicrobial use and AMR in cultured fishes, genetic mechanisms involved in the development of resistance, and the management strategies to restrict the spread of AMR in aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aquaculture, several bacterial diseases routinely encountered, which affect successful production, are mainly due to Gram negative organisms such as Aeromonas hydrophila, A. salmonicida, Vibrio anguillarum, V. harveyi, Flavobacterium psychrophilum, Edwardsiellatarda, Citrobacter freundii, Pseudomonas fluorescens, and Yersinia ruckeri; rarely by Gram positive ones such as Streptococcus and Staphylococcus; and also by acid fast Mycobacterium sp. (Lewbart 2001,Sørum 2006). Among these, the most prevalent reported bacterial pathogen in freshwater aquaculture is Aeromonas hydrophila (Igbinosa et al. 2012). The consumption of such infected cultured fishes poses public health concerns including humans (Huss et al. 2000). This incidence enforces the farmers to use antibiotics frequently in the aquaculture system. Concomitant with the rise in antibiotic administration in aquaculture as a part of therapy and prophylaxis, there has been an emergence of antimicrobial resistance (AMR) among the bacterial fish pathogens (FAO 2005). Many cultured fishes such as carp, salmon, tilapia, catfish and crustaceans like shrimps worldwide have been reported to possess antimicrobial resistant pathogens (Watts et al. 2017). Multiple antimicrobial resistance has evident among the bacteria associated with ornamental fishes also and several of them are zoonotic that may infect the fish handlers (Weir et al. 2012). Continuous use of antibiotics for alleviating bacterial diseases in aquaculture has led to “pseudo-durability” and their omnipresence in the environment, which has caused the development of selective pressure on the microbial community (Gao et al. 2012). Antimicrobial resistant bacteria formed under the selective pressure can develop to an environmental reservoir of antibiotic resistant genes. Aquaculture systems and fish farms have been observed as the ‘hotspots for AMR genes’ and hence the assessment of resistome, the AMR gene collection in aquaculture, is an important topic of research worldwide (Watts et al. 2017). Recently, Brunton et al. (2019) performed a detailed survey on the identification of hotspots for antimicrobial resistance, emergence and selection in aquaculture systems through system thinking approach. The transfer of antimicrobial resistant pathogens from aquaculture environment to natural aquatic environment could leads to the emergence of antimicrobial resistance in wild fishes and the related food products. This has been considered with due seriousness as it impacts human health due to their direct consumption and near impossible management measures (Cizek et al. 2010). Rhodes et al. (2000) suggested that aquaculture and hospital environments could act as the single interactive environment because of the transfer of resistance gene carrying plasmids between fish and human pathogens. This wider dissemination of determinants from aquatic farms to human should be the main concern during the technological crossover between different kinds of fish farms (Cizek et al. 2010). The flow of AMR genes from aquatic pathogens to humans may not be continuous, but some intermediaries such as environmental bacteria might be involved (Buschmann et al. 2012). Since most of the antimicrobials used in aquaculture are those used in human medicines, the application of antimicrobials in aquaculture severely impacts the development of AMR in other ecological niches mainly human environment (Heuer et al. 2009). Around 51 antibiotics which are recommended in aquaculture are of important to human medicine and about six classes of those antibiotics are recorded as critically important antimicrobials in World Health Organisation’s (WHO) list (Done et al. 2015). Increased frequency of severe infections and treatment failures had reported in humans due to the consequences of transfer of antimicrobial resistance from aquaculture to human through the consumption of aquaculture products (Kruse and Sørum 1994; Akinbowale et al. 2006).
Mobile genetic elements (MGE) such as plasmids, transposons and integrons with gene cassettes play crucial roles in the spreading of resistance determinants among bacteria (Gao et al. 2012). Horizontal gene transfer through these MGEs and clonal selection due to antimicrobial selective pressure are the main factors behind the antimicrobial resistance in bacterial populations (Schmidt et al. 2001a). The antibiogram profile and the associated resistance genes of fish pathogens vary with the particular aquatic environment (Piotrowska and Popowska 2014). In this context, various advanced molecular tools can be exploited to unravel the genetic complexity of antimicrobial resistant determinants associated with the pathogens.
Considering the impact of antimicrobial resistance to the global consumer health, previous reviews were oriented towards the survey of antibiotic uses in aquaculture and AMR reports on various fish farms, ornamental fishes and fish products from different countries (Alderman and Hastings 1998; Cabello 2006; Weir et al. 2012; Romero et al. 2012; Cabello et al. 2013; Santos and Ramos 2018), concern of AMR in global shrimp industry (Thornber et al. 2019), molecular mechanisms responsible for the antibiotic resistance (Stalder et al. 2012; Gao et al. 2012) and the alternatives to antibiotics for the prevention/treatment of bacterial diseases in fishes (Defoirdt et al. 2011), feed additives as immunostimulants in aquaculture (Dawood et al. 2017). Rico et al. (2017) undertook a challenging effort to study the risks of developing antimicrobial resistance in intensive aquaculture production through probabilistic approach. Though reviews are available on the selected aspects, there is a dearth of exhaustive analysis on this issue of paramount importance. The present review is a comprehensive and an elaborative study addressing different aspects of antimicrobial resistance starting from the antimicrobial uses in aquaculture, emergence of antimicrobial resistance in cultured and ornamental fishes, diversity of the associated zoonotic pathogens, genetic mechanisms involved in the antibiotic resistance to the alternative strategies such as the vaccination, application of natural pharmaceuticals, probiotics, phage therapy etc. to overcome the menace. In the present scenario, this review stands separately addressing multiple affairs that may shed light on the complexity of the particular concern for future studies.
Application of antimicrobials and sources of antimicrobial resistance in aquaculture
Around 73% of the major aquaculture producing countries were reported to use oxytetracycline, florfenicol and sulphadiazine and 55% applied erythromycin, amoxicillin, sulphadimethoxine, and enrofloxacin as reported by Lulijwa et al. (2019). They have also noticed that China and Vietnam are major consumers of antibiotics users while India, Korea, Bangladesh, Philippines and Thailand are infrequent consumers. Recently, application of huge quantities of veterinary antibiotics and the antibiotic contamination followed by the emergence of antibiotic resistance in China’s aquaculture industry was discussed in detail by Mo et al. (2017). Defoirdt et al. (2011) stated that around 600 metric tons of antibiotics were applied in shrimp aquaculture farms in Thailand, which remind the possibility of rapid development of AMR. Oxytetracycline, florfenicol, sulphonamides, erythromycin and sarafloxacin are some of the authorized antibiotics for use in aquaculture and chloramphenicol, enrofloxacin, spectinomycin, and rifampin are the banned antibiotics for animals intended for food production (FAO 2005). Later it is reported that tetracyclines, sulphonamides and quinolones are the most popular antibiotics, although betalactams and macrolides are occasionally used, in aquaculture due to the economic gains (FAO/OIE/WHO 2006). While florfenicol, oxytetracycline and Sulfadimethoxine/ormetoprim are the FDA approved antibiotics in aquaculture (https://www.fda.gov/animalveterinary/developmentapprovalprocess/aquaculture/ucm132954.htm). Although not allowed in aquaculture because of its adverse effects to human health, evidence of chloramphenicol residues in fishery products from South East Asia (Sørum 2006) and chloramphenicol resistance among the aeromonads (Weir et al. 2012) rise major threat to the aquatic ecosystem. Lulijwa et al. (2019) commented that there is an overall increase in the usage of antibiotics in Asian aquaculture production.
In Italy, the current legislation permits the use of various antibiotics such as, tetracycline, oxytetracycline, amoxycillin, flumequine, sulfadiazide in combination with trimethoprim etc. in fish farms (Labella et al. 2013). But there is a substantial reduction in the application of antibiotics in Japanese and Norwegian aquaculture industry (Lulijwa et al. 2019). Norway and Chile allowed the use of oxytetracycline, florfenicol and quinolones in salmon aquaculture (Lozano et al. 2018; Cabello et al. 2013). In addition, Chile also permited the aquacultural use of erythromycin, amoxicillin, furazolidone, gentamycin and chloramphenicol (Cabello et al. 2013) and later the authorities announced a list of around 13 antibiotics (Liu et al. 2017). Very recently the current status of antimicrobial resistance development as a result of intensive use of antibiotics in Chilean Salmon fish farms has been updated, which forced the investigation of link between the bacteria harbouring the farms and human and fish pathogens (Miranda et al. 2018). Developed countries such as USA and Canada authorized the use of limited drugs such as oxytetracycline, florfenicol and Sulfa/trimethoprim for specific fishes such as catfish and salmonids and could be broadly used to treat infections in aquaculture (Dawood et al. 2017; Chuah et al. 2016). Of these, oxytetracycline is the most commonly used antibiotic in USA and also in Denmark (Singh et al. 2009). While oxytetracycline, oxolinic acid, amoxicillin and co-trimazine were earlier reported as the licenced antimicrobial drugs for treating fish diseases in UK (Alderman and Hastings 1998). Several countries like South Africa and Australia have no legistlation or standardised guidelines to follow the usage of antimicrobials in aquaculture (Akinbowale et al. 2006; Jacobs and Chenia 2007). Since most of the recommended antimicrobials such as oxytetracycline and amoxicillin used in aquaculture are equally important for humans, there exists a significant link between terrestrial and aquatic resistomes (Watts et al. 2017). It is also recently repoted by Topp et al. (2018) that the excess use of antibiotics in USA, Japan and European aquaculture systems had been strictly regulated and restricted to minimum approved therapeutics. A detailed review on the current status of antibiotic use in aquaculture of different countries, their policies and regulation, environmental health concerns were discussed by Lulijwa et al. (2019). Various antibiotics approved for use in aquaculture in different countries are listed in Table. 1.
The unrestricted prophylactic use of antibiotics as growth promoters and to treat bacterial infections leads to the persistence of antibiotics in aquatic environment. The administration of antimicrobials in aquaculture is mainly medicated through feed or immersion and by direct application in the water (Heuer et al. 2009). Uningested food and fish feces could retain the antimicrobial residues depending on the biodegradability, initial concentration and physical and chemical characteristics (Burridge et al. 2010). This results in their selective pressure for prolonged periods and thereby emerging antimicrobial resistant determinants and the fluctuating antibiotic environment forces the bacteria to adapt and thus get selected for survival through multiple mechanisms (Baquero et al. 1998). The selection leads to the alterations of biodiversity of aquatic environment by substituting resistant communities in place of susceptible ones or the genetic fluctuations in susceptible ones which give rise to the antimicrobial resistant types (Cabello et al. 2013). The major disadvantage of the selection pressure is that, once resistance got acquired, the determinants could be retained within the community even in the absence of responsible antibiotics (Chiew et al. 1998). This enhances the risk of wider dissemination of resistance determinants (Jacobs and Chenia 2007). Reconnaissance of significant quantities of around 47 antibiotic residues among tilapia, shrimp, trout and salmon farms from around 11 countries such as China, US, Mexico, Canada, Scotland, Thailand etc. indicated the possibility of rapid emergence of antibiotic resistance worldwide (Done and Halden 2015). Zhu et al. (2017) noticed the positive correlation of concentration of tetracycline and macrolides with the abundance of total AMR genes in an estuarine sediment. Meanwhile even in the absence of selection pressure, AMR genes like tetracycline resistant genes persist in aquaculture farms, thereby highlighting the genetic transfer of AMR genes from the other niches (Tamminen et al. 2011).
In addition to the direct application of antibiotics in the aquaculture systems, integrated fish farming systems also play a major role in the transfer of AMR globally. Watts et al. (2017) reported the spread of AMR through such integrated farming systems mainly in Asia and Africa. In Thailand, a sudden rise of AMR from 5% to 100% was noticed among Acinetobacter spp. towards oxytetracycline and sulfamethoxazole in integrated chicken-fish farms (Petersen et al. 2002). Integrated fish farming increased the risks of developing multiple antibiotic resistant bacteria such as Enterococcus spp. and Aeromonas in the intestine of cultured fishes (Petersen and Dalsgaard 2003). Massive catfish industries mainly contributed the development of AMR associated with catfish production (Sarter et al. 2007). Various other studies also confirmed the prevalence of AMR genes in integrated agriculture/aquaculture systems (Neela et al. 2014).
Application of antibiotics in the cultured open systems such as pond increases the risk of persistence and distribution of AMR genes in the system due to the absence of frequent water exchange and extended time for bacterial adaptation (Neela et al. 2014). Flumequine treatment in pond water and rainbow trout farms showed that aeromonad pathogens on the gut and skin of treated fishes and biofilms became multi drug resistant against streptomycin, sulfamethoxazole, quinolones and fluoroquinolones, oxytetracycline, florfenicol, chloramphenicol, and trimethoprim, which increases the transfer of relevant genes to wider aquatic environments during harvest time (Naviner et al. 2011). The distribution of AMR bacteria in closed systems such as RASs is very little known and the presence of AMR pathogens could be described by using infected fish stocks (Saavedra et al. 2010). This necessitates the continuous monitoring and surveillance of unique antibiotic resistant determinants of fish species belonging to different aquaculture systems.
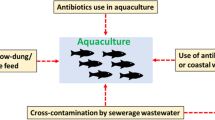
In addition to the development of antimicrobial resistance in aquaculture, natural aquatic environment also acts as reservoir of antibiotic resistant pathogens. The physico-chemical parameters of water also influences the increased antibiotic insusceptibilty among the aquatic bacteria (Pathak et al. 1993). Unlike the direct application in the cultured systems, the selection of antibiotic resistant organisms in the natural aquatic environment is mainly due to the natural production of antibiotics within the system, runoff waste products of antibiotic treated animals/human, terrestrial land run-off, entry of animal feeds and crops, effluent discharges and from the domestic sewage containing antibiotics (Witte 1998; Kumar et al. 2017). Sewage effluents act as the major contributor of antibiotic resistant enteric pathogens among the marine wild life species, which was confirmed by comparing the fishes from polluted and unpolluted sites by Al-Bahry et al. (2009). Thus the natural production and lateral entry of these antibiotics could exert a selective pressure that promote the emergence and spread of antibiotic resistance in natural aquatic environment. Wild freshwater species from higher and lower anthropogenic activities involving reservoirs were compared for the presence of antibiotic resistant genes recently by Marti et al. (2018). They detected sulphonamide, quinolone and erythromycin resistance genes in brown trout and ebro barbel from reservoirs with higher anthropogenic activities while only beta lactam group resistance genes in common carp from the reservoirs with lower anthropogenic activities thereby highlighting the influence of recreational activities on the establishment of AMR genes in the natural aquatic environment. This implies that the antibiotic resistant wild fishes can be taken as the bio-indicator of pollution in natural marine and freshwater environment. As a part of management, the run of wastes like domestic sewages and agricultural and industrial effluents should undergo treatments before the disposal so as to minimize the entry of AMR pathogens to the systems and reduce the extent of transfer of resistant genes through horizontal transfer to other aquatic environment. A map showing the reported percentage of occurrence of antimicrobial resistance in various countries are given in Fig. 1.
Antimicrobial resistance in gram negative bacteria
Among the Gram negative fish pathogens, aeromonads could be considered as the indicator bacteria for characterizing the occurrence and establishment of antimicrobial resistance in fish farms (Cizek et al. 2010). They are well known to cause infections in humans also and hence play as a significant zoonotic pathogen in aquaculture (Ko et al. 2000). The first reported fish pathogen which showed antimicrobial resistance was Aeromonas salmonicida, against sulphathiazol and tetracycline (Snieszko and Bullock 1957). Schmidt et al. (2001b) detected the multi drug resistant phenotypes of aeromonads from Danish rainbow trouts. Aeromonas salmonicida and A. hydrophila isolates associated with tilapia, trout and koi from South African aquaculture systems exhibited higher resistance level to different kinds of antimicrobial agents as compared to A. encheleia, A.popoffii, A. veronii, A. media and A. ichtiosoma isolates (Jacobs and Chenia 2007). It is not surprising because in the earlier period onwards, huge amounts of antibiotics such as oxytetracycline, trimethoprim and quinolones have been reportedly used to treat furunculosis (A. salmonicida causing disease) infected salmonids as in Norwegian fish farming (Grave et al. 1996).
Another zoonotic fish pathogen, Vibrio has also been reported to exhibit multiple antibiotic resistance (Labella et al. 2013). During an outbreak of vibriosis in freshwater fish ayu, in 1973, majority of Vibrio anguillarum isolates were found to possess transferrable R plasmids showing resistance to sulphonamides, chloramphenicol, tetracycline and streptomycin (Aoki et al. 1974). Vibrio and Aeromonas are the most reported antimicrobial resistant organisms identified in various fishes such as catfish, tilapia and koi carp (Ashiru et al. 2011) and most of the antibiotic resistance studies were carried out on these pathogens due to their unusual biofilm formation and antibiotic resistance (Odeyemi and Ahmad 2017). The virulent pathogens such as V.harveyi and V. aestuarianus associated with gilthead sea bream of Italian mariculture were insusceptible to around 10 antibiotics (Scarano et al. 2014).
Edwardsiellosis is one of the severe diseases infecting fishes which is caused by Edwardsiella tarda, which is having zoonotic importance (Novotny et al. 2004). E. tarda from Japanese flounder possessed multiple antibiotic resistance genes that resist tetracycline, streptomycin and kanamycin, which are maintained within potential virulence genes of a single large pCK41 plasmid (Yu et al. 2012). Another highly significant zoonotic fish pathogen and the causative agent of mycobacteriosis in marine and freshwater fishes, Mycobacterium showed significant resistance against the multiple antibiotics. For example, Mycobacterium peregrinum recovered from ornamental fishes were found to be completely insusceptible to the majority of tested antibiotics such as antituberculosis drug, isoniazid, rifampicin, co-trimoxazol, clofazimine, streptomycin and erythromycin (Guz et al. 2013), highlighting the potential of this zoonotic pathogen as a threat to the public. Sing et al. (2016) recovered multiple drug resistant zoonotic pathogen, Salmonella with higher multiple antibiotic resistance (MAR) index from African catfishes belonging to different fish farms, which highlighted the exposure of cultured catfishes to several antibiotics.
Flavobacterium columnare, causative agent of popular columnaris disease, isolated from ornamental fish and wild catfish exhibited multidrug resistance towards chloramphenicol, nitrofuran, ampicillin, oxytetracycline, flumequine, enrofloxacin and oxolinic acid, which highlighted the unprudent use of antimicrobials among ornamental fishes and the extend of spread of AMR to the natural aquatic environment (Declercq et al. 2013). Bacterial cold water disease (BCWD) causing F. psychrophilum isolated from salmonids of Ontario fish farming industry was found to show decreased susceptibility towards florfenicol, oxytetracycline, trimethoprim–sulfamethoxazole and ormetoprim–sulfadimethoxine (Hesami et al. 2010). Huang et al. (2014) suggested that enteric red-mouth disease causing Yersinia ruckeri was also capable of developing mutations so as to acquire antibiotic resistance. Highly invasive and cytotoxic Pseudomonas aeruginosa from diseased cultured gilthead sea bream was found to exhibit multiple antibiotic resistance against tetracycline, ampicillin and erythromycin (Lamari et al. 2017). Recently, Ruzauskas et al. (2018) recovered multidrug resistant gram negative isolates including Pseudomonas, Chryseobacterium, Aeromonas and Enterobacteriaceae prevalent in cultured fishes.
Antimicrobial resistance in gram positive bacteria
Gram positive organisms such as Streptococcus and Staphylococcus are reported as zoonotically significant fish pathogens (Novotny et al. 2004). Multiple antibiotic resistant Staphylococcus aureus was found to be associated with Australian aquaculture farms eventhough there were no antibiotics registered for use in Australian aquaculture (Akinbowale et al. 2006). Generally it is known that tetracycline and macrolide resistance is the worldwide threat among Streptococci (Botrel et al. 2010). Nguyen et al. (2017) recently reported the antibiotic resistance in Gram positive fish pathogen, Streptococcus dysgalactiae against the very common tetracycline and macrolide drugs in cobia, mullet and loach from Taiwan and Japan aquaculture farms.
Several non-pathogens and beneficial bacteria associated with commensal bacteria in fishes were also found to possess AMR genes, revealed through Real Time qPCR (Duran and Marshall 2005). Hence there is possibility of transfer of resistant genes from non pathogens to pathogens through conjugation, thereby spreading the antibiotic resistance worldwide. Diverse fishes, associated pathogens and their resistant antibiotics are listed in Table 2 and a pie chart showing the percentage of antimicrobial resistance exhibited by different pathogens are shown in Fig. 2.
Incidence of antimicrobial resistance in cultured fin fishes and shell fishes
Compared to the fishes in the natural ecosystem, antimicrobial resistance studies are mainly focused on the cultured fishes, as the right knowledge about the antibiotic resistance of pathogens in the cultured system will facilitates the formulation of a proper preventive strategy. For example, an increased incidence of antimicrobial resistant Aeromonas hydrophila and Plesiomonas shigelloides has been observed in the cultured catfish than the riverine catfishes, indicated a higher level of resistant bacteria in the cultured system (McPhearson et al. 1991).
Freshwater aquaculture
Farm raised freshwater fishes like Ctenopharyngodon idella, Labeo rohita and Catla catla were found to carry intestine associated aeomonads which exhibited antibiotic resistance towards ampicillin, oxytetracycline, amoxicillin and novobiocin (Hatha et al. 2005). Most of the efflux pumps involved in elevating the multiple resistance to ampicillin, tetracycline, nalidixic acid, chloramphenicol, rifampicin, quinolones and chloramphenicol were characterised in Gram negative bacteria from the farmed catfishes (Sarter et al. 2007). Singh et al. (2009) recovered the highly virulent oxytetracycline resistant Enterobacteriaceae and Flavobacterium with increased minimum inhibitory concentration (MIC) from freshwater carp aquaculture system.
All the above studies pointed out the excess use of antibiotics resulting in severe antimicrobial resistance in aquaculture systems. In addition, it is also suggested that increased levels of nutrients in the system could lead to the higher frequency of antibiotic resistance (Vaughan et al. 1996). As an exception, multi-drug resistant enteric pathogens were reported in European eel farms devoid of frequent drug therapy and disease outbreaks (Alcaide et al. 2005). This might be due to the usage of organic manures from the commercial poultry farms, where tetracyclines and sulphonamide antibiotics were extensively used (Turkson 2008). Like wise, multiple antibiotic resistance (MAR) was noticed in freshwater fishes belonging to ponds with negligible therapeutic applications (Shah et al. 2012). Similarly, MAR pathogens such as Salmonella, Shigella, Pseudomonas and E.coli were recovered from freshwater fishes such as catfishes and tilapia, which were not treated with any antibiotics (Agoba et al. 2017). The authors suggested the occurrence of already acquired resistance genes persisted in the cultured environment and not because of any direct application of antibiotics.
Mariculture
The reports on antimicrobial resistance in fishes of mariculture systems are found to be limited. Vibrio splendidus and Pseudoalteromonas act as the major reservoir of multidrug resistance determinants in abalone and turbots mariculture farms, China (Dang et al. 2007). Edwardsiella tarda, responsible for the major outbreak of septicaemia in trout, Scophthalmus maximus from mariculture farm, exhibited strong resistance to chloramphenicol due to the presence of resistant determinant, cat III (Xiao et al. 2009). Fish feed could also act as a major reservoir of antimicrobial resistant genes in mariculture environment (Han et al. 2017). Oxytetracycline resistant isolates were recovered from fish feeds used in carp farms, indicating the role of fish feeds in the introduction of resistant bacterial pathogens into the farming system (Singh et al. 2009).
Ornamental fish culture
Bacterial infections in ornamental fishes lead to major die-offs and fish destructions over the past decade contributing to extensive economic losses (Lewbart 2001). Very earlier period onwards multi drug resistant aeromonads were detected in ornamental fishes such as domestic goldfish and koi carp (Dixon and Issvoran 1993). Preena et al. (2019a, 2019b) very recently detected multi drug resistant pathogens such as Aeromonas, Pseudomonas, Acinetobacter etc. associated with infected guppy fishes and Edwardsiella tarda, Lactococcus, Aeromonas, Comamonas, Pseudomonas etc. with koi carp and gold fish. Multiple antibiotic resistant Serratia marcescens was isolated from guppy for the first time by Dharmaratnam et al. (2017) and a multi drug resistant Proteus hauseri from infected ornamental fish, Koi carp by Kumar et al. (2015). A commercially valuable pet fish, cichlid oscar, was found to be infected with multi drug resistant A.veronii, which was reported for the first time by Sreedharan et al. (2011). Dias et al. (2012) noticed higher level of AMR (80%) in ornamental fish associated Aeromonas hydrophila, A.veronii, A.caviae, A.media, A.aquariorum, A.jandaei and A.culicicola towards around 30 tested antibiotics (Dias et al. 2012). Higher resistance of aeromonads (A.caviae, A.sobria and A.hydrophila) observed in cultured ornamental fishes such as gold fish, carp, red sword tail, oscar and sucker to nalidixic acid highlighted the excess use of those antibiotics in th farm (John and Hatha 2012). The occurrence of highly virulent antimicrobial resistant gene (ARG) carrying aeromonads among the ornamental fishes in freshwater culture system pose higher threats to the humans who are in direct contact with the fishes (Sreedharan et al. 2012). Further significant tetracycline and erythromycin resistant diverse aeromonads including A.dhakensis were identified in freshwater ornamental fishes (Jagoda et al. 2014). Multi drug resistant aeromonads infected goldfish was found to be susceptible to herpes virus infection, which implies the proneness of antimicrobial resistant pathogen bearing fishes to viral infections (Sahoo et al. 2016).
All these reports of antibiotic resistance in ornamental fishes indicated the indiscriminate use of antibiotics in the aquarium. Although there is a dearth of information regarding the MAR bacteria in the aquariums, their close associations during handling and transportation with human could pose great health concerns. This could be exemplified by the spread of Salmonella Java infections from the tropical ornamental fish aquarium to human infants (Musto et al. 2006). In addition, AMR bacteria can transmit their corresponding ARG through HGT to other aquatic microbes. Verner-Jeffreys et al. (2009) observed that ornamental fish and its carriage warm water serve as the major container of multi resistant genes. This might be due to the release of those resistant gene carrying isolates in to the surrounding water through fish excreta, which may further transfer their genes to other bacteria (Agoba et al. 2017). Hence the ornamental fish industry should also take appropriate measures to curb the dissemination of resistance in the system.
Shellfish culture
There are early reports on the occurrence of antimicrobial resistant bacteria among the shellfishes, especially in the shrimps, where Abraham et al. (1997) reported that Vibrio harveyi from diseased cultured shrimp exhibited a broad range of multiple antibiotic resistance against erythromycin, gentamycin, ampicillin, polymyxin B, oxytetracycline, rifampicin, chlorotetracycline, streptomycin, ciprofloxacin, furazolidone, nalidixic acid and neomycin. Vibrio harveyi isolated from pond cultured shrimps also exhibited multiple antibiotic resistance towards oxytetracycline, oxolinic acid, chloramphenicol and furazolidone, indicating the abuse of antibiotics in open shrimp culture systems (Tendencia and Pena 2001). Ripabelli et al. (2003) identified the multi drug resistant V.vulnificus and V.alginolyticus from the mussels, Mytilus galloprovincialis and Kang et al. (2016) recovered multi drig resistant V.alginolyticus from oysters. High incidence of resistance to ampicillin and tetracycline classes was exhibited by cultivated marine shrimp (Litopenaeus vannamei) associated Vibrio isolates in Brazil (Reboucas et al. 2011). The distribution of tetracycline and ampicillin resistant Aeromonas veronii and A. aquariorum was observed in shrimps even at low salinity ponds (Yano et al. 2015). All the observed data indicates the establishment of AMR in freshwater, brackish and marine cultured shrimps, highlighting the role of crustaceans in dissemination of antibiotic resistance besides the fin fishes.
Molecular mechanism of antimicrobial resistance and the associated genes
Antimicrobial resistance mechanism differ in various ways among bacteria, which include antibiotic detoxification, inhibition of antibiotic deposition inside the cells, target protection and substitution (Bennett 2008). Bacterial conjugative plasmids, transposable elements and integron systems are the three significant gene transfer systems responsible for gene acquisition and thereby spreading the antibiotic resistance determinants (Stokes and Gillings 2011). Antibiotic resistance genes such as tet, sul, qnr, ere etc. are assembled on the major scaffold of bacterial plasmid, by means of transposition (transposable elements) and site-specific recombination events (integron and gene cassettes) (Bennett 2008). There are several reports of plasmid mediated and integron associated antibiotic resistance genes in fish pathogens, and their dissemination in aquaculture had been documented by various researchers.
Mobile genetic elements in spread of antimicrobial resistance associated with fishes
Plasmid mediated resistance
Plasmids, the extrachromosomal mobile genetic element constitute various genes which confer resistance to various antibiotics and toxic heavy metals (Partridge et al. 2009). Early reports are available on the plasmid mediated antibiotic resistance among various fish pathogens (Dixon et al. 1990). Vibrio anguillarum, Pseudomonas fluorescens, Aeromonas hydrophila, A. salmonicida, Pasteurella piscicida, Yersinia ruckeri, Edwardsiella tarda are the well known bacterial fish pathogens carrying transferrable R plasmids and thereby exhibiting plasmid mediated antimicrobial resistance (Alderman and Hastings 1998). Incompatible plasmids like IncA/C carrying antibiotic resistance genes gained more attention in North American aquaculture pathogens (Pan et al. 2008). Verner-Jeffreys et al. (2009) observed the blaTEM21, dfr,sul1 qacE2, tetA, tetD, tetE, floR genes along with various aminoglycoside resistant genes embedded within the incompatible IncA/C host range plasmids of cultured ornamental fishes.
Tetracycline resistance
It was demonstrated in many studies that the aquatic fish farming environment act as the reservoir of tranferable tetracycline genes (Jacobs and Chenia 2007). Excessive tetracycline resistance genes have evolved due to the coexistence of tetracycline producing microflora in the environmental niche (Schmidt et al. 2001b). For example, tetracycline resistance genes tetA-G have been detected in fish pathogens belonging to fish species of different geographical locations (Miranda et al. 2003). Various tetracycline resistance genes such as tetC, tetM and tetA involved in efflux mechanisms of resistance and ribosomal protection were also detected among the pathogens associated with rainbow trout farms (Ndi and Barton 2011).
Oxytetracycline is the most frequently given antibiotic during the disease outbreaks in aquaculture (Jacobs and Chenia 2007). A novel oxytetracycline resistance gene, tet 34 was identified in a Vibrio strain from cultured yellowtail by Nonaka and Suzuki (2002). Acinetobacter strain resolved from freshwater trout farms was found to possess another novel tetracycline resistance determinant, tet39, located on transferable plasmid (Agerso and Guardabassi 2005). Nonaka et al. (2007) and Agersø et al. (2007) detected tetM harbouring Vibrio strains and tet E carrying Aeromonas strains respectively from various fish farms, all of which can be transferred through HGT. Several studies reported the coexistence of tetracycline and sulphonamide resistance genes that complicated the treatments. For example, tet39 together with sul2 genes located on the plasmids of Acinetobacter were observed in the Thailand fish farms (Agersø and Petersen 2007). Later Su et al. (2011) noticed the co-occurrence of tetA and tetC with sul2 among the majority strains of Enterobacteriaceae from Chinese fish farms.
Quinolone resistance
Han et al. (2012) described qnrs5, a qnrs variant for the first time and analysed QRDR derived from a motile aeromonad associated with diseased fish and water. Aeromonas acts as the major vehicle for transmitting those mobile genetic elements through HGT in aquaculture (Poirel et al. 2012). The basic mechanism behind the quinolone resistance is the incidence of mutations in DNA gyrase and DNA topoisomerase along with the plasmid mediated resistance and that of fluoroquinolone resistance due to the QRDR (quinolone resistance-determining region) based mutation (Hooper and Jacoby 2015). Recently, Chenia (2016) detected a high incidence of plasmid borne qnr alleles, responsible for high level of fluoroquinone resistance, in Aeromonas isolates of South African freshwater fish.
While Cattoir and Nordmann (2009) reported that water borne Vibrionaceae could also act as reservoir of qnr determinants. Lunn et al. (2010) commented that plasmid-mediated transferable quinolone resistance (PMQR) determinants (qnrA, qnrB and qnrS) were mainly found in Enterobacteriaceae as compared to Aeromonadaceae and Vibrionaceae. Simultaneous occurrence of various protective mechanisms responsible for resistance were observed among the strains derived from Chile fish farms (Buschmann et al. 2012). Such mechanisms include the production of qnr proteins (topoisomerase protection) and aminoglycoside transferases and QepA mediated efflux mechanisms (Miranda et al. 2013).
Li (2005) stated that most of the qnr plasmids are integron associated and capable of showing multiple resistance to other antimicrobials like aminoglycosides and beta lactams. Prolonged use of quinolones in fish farms resulted in the elevated mutations of gyrA gene in fish pathogens like Flavobacterium psychrophilum, Yersinia ruckeri and V. anguillarum, which leads to hyper drug resistance (Izumi et al. 2007; Shah et al. 2012). Because of the high frequency of multiple plasmid mediated qnr genes in marine bacteria, it was hypothesised that some of the PMQR genes were originated from aquatic source (Jacoby and Hooper 2013). The occurrence of a single mutation in the gyrA gene of fish pathogens such as Aeromonas salmonicida, Photobacterium damselae and Edwardsiella tarda resulted in resistance to oxolinic acid (Miranda et al. 2013). Another point to be noted is the abundance of quinolone resistance genes in the aquaculture system as compared to non aquaculture sites (Tomova et al. 2015).
Beta lactam resistance
It is reported that beta lactam antibiotic resistance is mainly associated with the production of beta lactamases (Alderman and Hastings 1998). Vibrio cholerae, V.vulnificus and V.aestuarianus are the major aquaculture pathogens that possess β-lactam resistant genes (Wang et al. 2006). The increased expression of chromosomal ampC beta lactamase gene results in strong resistance to β-lactam antibiotics such as amoxicillin, cefoxitin, ampicillin and a low level resistance to cephalosporins (Slama et al. 2010). Even the fecal matter of Gilthead seabream was found to be enriched with beta lactamase resistance genes (blaSHV-12 and blaTEM-52) along with sul1–3, cmlA, aadA and tetA (Sousa et al. 2011). The extended spectrum beta-lactamases (ESBL) are widely distributed among the aeromonads, Alderman and Hastings (1998) reported the presence of such three beta-lactamases in A. salmonicida. The expression of ESBL denoted the resistance capability of fish pathogens to even third and fourth generation cephalosporin groups that make it difficult to control (Verner-Jeffreys et al. 2009). Extended spectrum beta lactamase genes such as blaTEM, blaSHV, blaCphA and blaOXA-B were noticed in Aeromonas isolates from cultured rainbow trouts by Vega-Sanchez et al. (2014) and this indicates the severity of the emergence of new generation antibiotic resistant fish pathogens.
Macrolide resistance
Macrolide resistance is also a serious problem in aquatic fish farms, where target ribosome-site modification, production of macrolide inhibiting enzymes and synthesis of drug efflux proteins are the major mechanisms behind the phenomenon (Nguyen et al. 2017). Erythromycin, a class of macrolide, along with gentamicin, kanamycin, chloramphenicol, ampicillin and oxytetracycline resistance were detected in both Gram positive and Gram negative pathogens associated with crustaceans, farmed fish and crabs (Akinbowale et al. 2006).
Phenicol resistance
The use of phenicols such as chloramphenicol is limited in aquaculture in mid 1990s due to its toxicity, however a fluorinated derivative, florfenicol became popular in aquaculture due to its effectiveness against wide fish pathogens (Michel et al. 2003). Vibrio damsela is the first reported florfenicol resistant fish pathogen in Japanese aquaculture (Kim et al. 1993). Chloramphenicol resistance mainly occur due to the drug inhibition mediated by chloramphenicol acetyl transferases (cat). Those protein encoding cat genes exist in limited sources, but spread among aquatic organisms even in the absence of high selection pressure (Yoo et al. 2003). Hence, elucidating the structural arrangements of cat genes in multiple drug resistant fish pathogens would be valuable so as to determine the origin of those genes. Efflux pump mechanism and production of enzymes such as RNA methyl transferases (cfr gene coding) and specific hydrolases are the other reasons behind the resistance towards the licenced phenicol drugs (Tao et al. 2012). The resistance capability of Chryseobacterium isolates from diseased fishes and aquatic habitats, against phenicols, was inhibited in the presence of efflux pump inhibitor, phenyl-arginin-β-naphthylamide, thereby highlighting the involvement of efflux mechanism in their resistance (Michel et al. 2005).
Transposon mediated resistance
Most of the antibiotic resistance genes on R plasmids reside on transposons, help in the rapid spread of genetic determinants (Alekshun and Levy 2007). Resistance transposons could be generally defined as the jumping gene system since these elements can be transferred intra or inter molecularly and various resistance genes in association with IS elements could be incorporated within the element (Bennett 2005). The major fish pathogen, A. salmonicida was found to possess transposon, Tn5393 on a conjugative R plasmid, carrying various streptomycin, sulphonamide and tetracycline resistance genes (Labee-Lund and Sorum 2001). Transposon bearing tetracycline and kanamycin resistance determinants were observed earlier on a fish pathogen Pasteurella piscicida by Kim and Aoki (1993). Transposons, Tn 1721 and Tn5706 in Acinetobacter and Moraxella strains play a vital role in the wider dissemination of tetA genes and tetH genes in salmon fish farms (Chenia and Vietze 2012). Transposon-like elements in clinical pathogens, Salmonella and other Enterobacteriaceae recovered from United States and Asia were found to be identical to the gene cassettes possessing sugE, blaCMY − 2 and blc found in Aeromonas salmonicida of Atlantic salmon farms (Huang et al. 2015). This again confirms the incidence of intensive spreading of antimicrobial resitance determinants across the world.
Integrons and gene cassettes associated resistance
Among the Gram negative bacteria, integrons play a significant role in the wider dissemination of antimicrobial resistance in aquaculture (Gao et al. 2012). This phylogenetically diverse integron/gene cassette system is meant for adaptation rather than simply confined to antibiotic resistance (Labbate et al. 2009). The relative abundance of these integrons usually increased with the exposure of excess antibiotics and other environmental stresses such as heavy metal contamination (Rosewarne et al. 2010). The genetic structure of integron has a peculiar characteristics to acquire, excise and express genes embedded within the gene cassettes (GCs), and are commonly harboured on mobile genetic elements such as plasmids and transposons, which facilitates the spread of determinants through horizontal gene transfer and transposition within the community (Stalder et al. 2012).
Integrons mainly are of two types; chromosomal and mobile. Chromosomal integrons (CI), otherwise super integrons, could carry around 200 cassettes, possessing various proteins of unknown functions (Stalder et al. 2012). The resistant genes present on the chromosomal integrons could also be transferred to the mobile ones, which may lead to the evolution and a wider spread (Rahube and Yost 2010). Mobile integrons (MIs) also known as resistant integrons (RIs)/multidrug resistance integrons (MRIs), located on mobile genetic elements carry lesser GCs, which mainly encode antibiotic resistance determinants and are responsible for wider dissemination (Stalder et al. 2012). Mobile integrons are of a major concern as they are the responsible entities for the gene transfer. Of these MIs, class 1, 2 and 3 integrons are the commonly detected ones and class 1 integrons are usually associated with transposons, which encompass mainly 5′ conserved (int 1 gene, promoters, attI and attC), 3′ conserved (qacED1 and sul1 genes) and variable regions (gene cassettes) (Gao et al. 2012).
Transposon, Tn7 associated class 2 integrons are the second popular integrons, usually consisting GC arrays such as dfrA1, sat2, aadA1 and orfX, which were reported to confer resistance to trimethoprim, streptothrycin, streptomycin and spectinomycin (Hansson et al. 2002). Jacobs and Chenia (2007) successfully amplified the class 2 integrons coding gene from Aeromonas isolate of South African aquaculture system, but failed to detect the associated resistance gene cassettes. However, the prevalence of class 2 integrons in aquatic ecosystems is found to be very low (Luo et al. 2010). Only a few class 3 integrons were described so far, while the aquatic ecosystem constitutes major pool of these systems (Stalder et al. 2012).
Gene cassettes (GCs), the non replicative mobile elements are usually inserted within the integron through recombination and could express genes responsible for antibiotic resistance using the Pc promoter (Stalder et al. 2012). Labee-Lund and Sorum (2001) stated the possibility of occurrence of single gene cassette only on integrons from pathogens of aquaculture origin, which was corroborated with the studies of Ndi and Barton (2011). Later, Partridge et al. (2009) reported more than 130 gene cassettes that conferred resistance to antimicrobials such as trimethoprim (dfr), beta lactams(blaCARB-2, blaOXA, blaP1), chloramphenicol (catB), macrolides, aminoglycosides (aad, aac), erythromycin (ereA) foscomycin, quinolones, rifampicin (arr), lincosamides and quarternary ammonium compounds (qac). This information reveals the implication of another major route of resistance gene transfer besides the plasmid mediated resistance genes. The major molecular mechanism behind the antibiotic resistance is the SOS response (a global regulatory network) which gets activated on exposure to antimicrobials and other environmental stresses and further induces integrase expression thereby leading to GCs recombination events (Cambray et al. 2011).
The class 1 integrons and their gene cassette arrays can be considered as the important targets to characterise the antimicrobial determinants and to study the wider dissemination of AR factors in aquaculture. Presence of integron associated antibiotic resistance genes can be detected through the PCR amplification and sequencing of conserved and variable regions of mobile integrons (Schmidt et al. 2001a). Several studies had been undertaken to analyse the distribution of integrons in fish farming environments. Tilapia, koi and trout cultures derived Aeromonas isolates from South Africa were found to possess class 1integrons with gene cassettes dhfr1, oxa2a, ant(3”)Ia, aac(6’)Ia (Jacobs and Chenia 2007). Around 50% of the pathogenic isolates recovered from freshwater fish farms possessed antibiotic resistance bearing class 1 integrons (Schmidt et al. 2001a; Verner-Jeffreys et al. 2009). Genetic analysis of Aeromonas isolates from various fishes, ornamental fish and shrimps of Chinese aquaculture revealed multi drug resistant genes, such as blaCTX-M-3,blaTEM-1, drfA12-orfF-aadA2, drfA12-orfF, dfrA17, aac(6′)-II-blaOXA-21-cat3, tetA, catB3 and arr-3 carrying gene cassettes (Deng et al. 2016).
As already discussed, in addition to gene cassette associated resistance, MIs containing plasmids/transposons also harbour additional resistance genes (Li et al. 2010). Thus the coselection of plasmid/transposon associated resistance and gene cassette bearing MIs leads to the development of multidrug resistant phenotypes. The potential of multi drug resistant bacteria to distribute the resistance genes in the aquaculture systems and to the human pathogens through the mobile genetic elements are very high (Smith et al. 1994). The multi drug resistance could be designated as ‘superbug’ phenomenon, elevates the overall resistance power and unfortunately this combinatorial resistance acquired through mobile genetic elements are difficult to get eliminated from the system (Enne et al. 2001). Plasmid mediated quinolone and aminoglycoside resistant genes in addition to integrons were observed on Aeromonas isolates from tropical ornamental fish and cold water koi carps (Dobiasova et al. 2014). However, it is not always necessary for the integrons to carry antibiotic resistance encoding gene cassettes and those without gene cassettes could be denoted as “empty integrons” (Schmidt et al. 2001a). Hence the presence of specific antibiotic resistance genes (plasmid encoded) needs to be analysed besides identifying the integrase genes alone.
Management measures and alternative strategies
As recommended by Food and Agriculture Organization (FAO) and World Health Organization (WHO), it is highly essential to develop and implement valid measurement methods at national and international levels to evaluate and detect the resistant pathogens and associated genes so as to control the spread of AMR in aquaculture (WHO 2006). According to FAO action plan on AMR 2016–2020, implementation of effective policies such as proper regulations and enforcement, acquiring information on the field of disease diagnosis, risk assessment, disease control and management, capacity building on every aspect of aquaculture production chain, prudent use of antimicrobials for the prevention of disease outbreaks and effective biosecurity practices are necessary (www.fao.org/cofi/3079806222458cbe49b16e15c7743d3b642c04). Accordingly, it is imperative to keep the aquatic system clean, safe and disease free so as to prevent the development of bacterial infections, minimise antibiotic uses and furtherance of resistance. A proper multistage monitoring of antibiotic resistance is inevitable during every outbreak of diseases. Through understanding the details behind the resistance mechanism, effective modifications and appropriate selection and application of antibiotics in the respective fish farms can be executed. The antibiotic residues persisted in aquatic environment due to their over dosage and excess feed, can be removed by appropriate adsorption methods, filtration, biological processes, sedimentation and flocculation (Homem and Santos 2011).
Effective alternative strategies have to be put forward in the aquaculture industry thereby regulating the dependence on antibiotics and the emergence of antibiotic resistance. Preventive measures such as vaccination are being used in aquaculture for controlling the disease onset. Oral fish vaccines are effective against many aquatic diseases through the production of humoral antibodies (Newaj-Fyzul and Austin 2015). The oral vaccine developed using the porin gene of V. anguillarum was found to protect sea bass (Kumar et al. 2008). Chitosan nano particles incorporated DNA vaccine using OMP K gene of Vibrio parahaemolyticus was effective for black sea bream (Li et al. 2013). However, vaccines corresponding to all kinds of fish diseases are not available worldwide. For example, vast amounts of quinolones were applied to treat those infections in Chile since because of the absence of effective vaccines against Piscirickettsia salmonis, (Cabello 2004). This indicates the perpetual use of antibiotics under special circumstances.
In addition to vaccines, probiotics are also increasingly used in the control of aquatic diseases by conferring health benefits. Probiotics have the evident potential to antagonize the pathogens such as Vibrio harveyi, a major threat in aquaculture, via attaching the intestinal mucus (Chabrillon et al. 2005). Bacillus amyloliquefaciens, B. coagulans, Brevibacillus brevi are some of the other reported probiotic bacteria effective against fish pathogens such as A. hydrophila, Edwardsiella tarda, V. harveyi V. parahaemolyticus and V. anguillarum (Newaj-Fyzul and Austin 2015). Bacteriocins like antimicrobial peptides are found to be another promising natural alternative to antibiotics (Marshall and Arenas 2003). Marine bacteriocins such as divercins and pisciocins produced by Carnobacterium associated with fish intestine and those produced by lactic acid bacteria and autochthonous bacteria were effective for treating bacterial infections in aquaculture (Desriac et al. 2010).
Immunostimulants like β-1,3 glucans are the other components effective against various aquatic diseases like vibriosis, enteric redmouth, aeromonadiasis, pasteurellosis and Hitra disease (Ngamkala et al. 2010). Another immunostimulant, LPS also has been reported to increase the bactericidal activities in common carp and to reduce mortality of aeromonad infected rainbow trout (Nya and Austin 2010; Kadowaki et al. 2013). Broad-host range phages can also be applied in aquaculture to counteract bacterial infections in fishes. Phages were successfully applied in aquaculture, because of the unavailability of appropriate vaccines, to protect salmonids from rainbow trout fry syndrome (RTFS) causing Flavobacterium psychrophilum (Castillo et al. 2012). However, bacteriophage resistance mechanisms were also noticed in fish pathogens such as F.psychrophilum (Castillo et al. 2015).
Immunomodulation, protection from bacterial diseases, inhibition of infections are offered by traditional medicinal plant products also. Seaweeds such as Ceramium rubrum, Gracilaria cornea and Asparagopsis armata also act as antimicrobial compounds against fish pathogens such as Vibrio anguillarum and Pseudomonas anguilliseptica (Bansemir et al. 2006). The immunity of spotted snakeheads towards Aeromonas hydrophila infections was found to be increased and mortality rate reduced through the application of Solanum nigrum (Rajendiran et al. 2008). Harikrishnan et al. (2012) reported that when kudzu vine fed to Epinephelus bruneus, an enhanced protection was observed against Vibrio harveyi. Another Chinese medicinal herb, Ku Shen was found to be effective in Tilapia when challenged with Streptococcus agalactia (Wu et al. 2013). Extracts of mango, peppermint, turmeric, jasmine, neem etc. are also the other promising alternatives to treat bacterial infections caused by aeromonads and vibrios in aquatic species (Newaj-Fyzul and Austin 2015). Photodynamic therapy ia an alternative to be applied to inactivate microbial actions in fish farming plants (Almeida et al. 2009). Quorom sensing inhibition compounds such as halogenated furanones, from marine algae, can be administered for disrupting biofilm forming aquaculture pathogens such as Vibrio anguillarum, Aeromonas hydrophila, A. salmonicida, Edwardsiella tarda and Yersinia ruckeri (Defoirdt et al. 2011). Metal based nanoparticles having both antibacterial and antifungal activities were also successfully applied against the fish pathogens in aquaculture (Swain et al. 2014). Recently a combined thiamphenicol/florfenicol was successfully applied against aeromoniosis without lowering their efficacy (Assane et al. 2019). Thus the combination of different antimicrobials could reduce the application of antibiotics without reducing their therapeutic effect; this could combat the high intense antimicrobial resistance.
Larsson et al. (2018) reported that further researches are immediately needed to recognize the dimensions of AMR in various environmental systems and the four major areas seriously quoted were: the source of antibiotics and the resistant bacteria; the role of environmental system in the evolution of resistance; consequences of exposure of antibiotic resistant bacteria to the global health system; and the effectiveness of various technologies to alleviate the AMR issue. Hence, as discussed in the present review, future studies are highly inevitable in the aquaculture system also for the sustainable ecosystem. Thrust areas should be the source of antimicrobials (direct /indirect application); role of different aquaculture/non aquaculture systems in spreading AMR; molecular mechanisms of AMR bacteria to combat the antibiotic effect; and various management measures and substitute methods to control the threats. One health approach could be taken as one of the serious remedies to overcome the everlasting AMR menace. It is interestingly stated by Cavalli et al. (2015) that aquaculture could be maintained under “one health umbrella” through one health concept. For the great success of this concept, it is inevitable to break the interdisciplinary barriers that separates animal and human medicines from the evolutionary, ecological and environmental sciences (Destoumieux-Garzón et al. 2018). Thus the initiative of “one health” implies a global strategy which indicates the development of transdisciplinary and holistic approach, where integrated concepts can be taken up for the good health of human, animals and the whole ecosystem.
Conclusion
Effective aquaculture operations, safe management practices, appropriate stocking programmes and proper hygienic conditions can control the introduction of bacterial pathogens, incidence of bacterial infections and thereby the use of antimicrobials. Continuous monitoring programmes following proper guidelines, and effective policies have to be implemented to overcome the threat of antimicrobial resistance. Assessment offish mortality, evaluation of antibiotic residues, identification of responsible pathogens and determination of their antimicrobial susceptibilities should be performed frequently as part of the monitoring programme. Detection of antimicrobial resistant genes encompassing the gene transfer systems, such as integrons and gene cassettes, through molecular methods is crucial in the treatment improvisation thus preventing the wider spread of antibiotic resistance. Hindrance in the emergence of antimicrobial resistance in aquaculture can restrict the flow of AMR genes to the natural environment and its progress as a public health hazard.
Abbreviations
- AMR:
-
Antimicrobial Resistance
- ARG:
-
Antimicrobial Resistant Gene
- MGE:
-
Mobile genetic elements
- HGT:
-
Horizontal gene transfer
- MIC:
-
Minimum inhibitory concentration
- MAR:
-
Multiple Antibiotic Resistance
- ESBL:
-
Extended spectrum beta lactamases
- CI:
-
Chromosomal integrons
- MI:
-
Mobile integrons
- MRI:
-
Multidrug resistance integrons
- GC:
-
Gene cassettes
References
Abraham TJ, Manley R, Palaniappan R, Dhevendaran K (1997) Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J Aquacult Trop 121:1–8
Agerso Y, Guardabassi L (2005) Identification of Tet 39, a novel class of tetracycline resistance determinant in Acinetobacter spp. of environmental and clinical origin. J Antimicrob Chemother 55:566–569. https://doi.org/10.1093/jac/dki051
Agersø Y, Petersen A (2007) The tetracycline resistance determinant Tet 39 and the sulphonamide resistance gene sulII are common among resistant Acinetobacter spp. isolated from integrated fish farms in Thailand. J Antimicrob Chemother 59:23–27. https://doi.org/10.1093/jac/dkl419
Agersø Y, Bruun MS, Dalsgaard I, Larsen JL (2007) The tetracycline resistance gene tet(E) is frequently occurring and present on large horizontally transferable plasmids in Aeromonas spp. from fish farms. Aquaculture 266:47–52. https://doi.org/10.1016/j.aquaculture.2007.01.012
Agoba EE, Agyare AFC, Boamah VE, Boakye YD (2017) Antibiotic resistance patterns of bacterial isolates from hatcheries and selected fish farms in the Ashanti region of Ghana. J Microbiol Antimicrob 9:35–46. https://doi.org/10.5897/JMA2017.0387
Akinbowale OL, Peng H, Barton MD (2006) Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. J Appl Microbiol 100:1103–1113. https://doi.org/10.1111/j.1365-2672.2006.02812.x
Akinbowale OL, Peng H, Grant P, Barton MD (2007) Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int J Antimicrob Agents 30:177–182. https://doi.org/10.1016/j.ijantimicag.2007.03.012
Al-Bahry SN, Mahmoud IY, Al-Belushi KI, Elshafie AE, Al-Harthy A, Bakheit CK (2009) Coastal sewage discharge and its impact on fish withreference to antibiotic resistant enteric bacteria and enteric pathogens as bio-indicators of pollution. Chemosphere 77:1534–1539. https://doi.org/10.1016/j.chemosphere.2009.09.052
Alcaide E, Blasco M-D, Esteve C (2005) Occurrence of drug-resistant bacteria in two European eel farms. Appl Environ Microbiol 71:3348–3350. https://doi.org/10.1128/AEM.71.6.3348-3350.2005
Alderman DJ, Hastings TS (1998) Antibiotic use in aquaculture: development of antibiotic resistance – potential for consumer health risks. Int J Food Sci Technol 33:139–155. https://doi.org/10.1046/j.1365-2621.1998.3320139.x
Alekshun MN, Levy SB (2007) Molecular mechanisms of antibacterial multidrug resistance. Cell 128:1037–1050. https://doi.org/10.1016/j.cell.2007.03.004
Almeida A, Cunha A, Gomes NCM, Alves E, Costa L, Faustino MAF (2009) Phage therapy and photodynamic therapy: low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar drugs 7:268–313. https://doi.org/10.3390/md7030268
Aoki T, Egusa S, Arai T (1974) Detection of R factors in naturally occurring Vibrio anguillarum strains. Antimicrob Agents Chemother 6:534–538. https://doi.org/10.1128/aac.6.5.534
Ashiru A, Uaboi-Egbeni P, Oguntowo J, Idika C (2011) Isolation and antibiotic profile of Aeromonas species from tilapia fish (Tilapia nilotica) and catfish (Clariasbetrachus). Pak J Nutr 10:982–986. https://doi.org/10.3923/pjn.2011.982.986
Assane IM, Gozi KS, Valladao GMR, Pilarski F (2019) Combination of antimicrobials as an approach to reduce their application in aquaculture: emphasis on the use of thiamphenicol/florfenicol against Aeromonas hydrophila. Aquaculture 507:238–245. https://doi.org/10.1016/j.aquaculture.2019.04.021
Bansemir A, Blume M, Schröder S, Lindequist U (2006) Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 252:79–84. https://doi.org/10.1016/j.aquaculture.2005.11.051
Baquero F, Negri M-C, Morosini M-I, Blazquez J (1998) Antibiotic-selective environments. Clin Infect Dis 27(Suppl. 1):S5–S11. https://doi.org/10.1086/514916
Bennett PM (2005) Genome plasticity. In: Woodford N, Johnson A (eds) methods in molecular biology, Vol 266. Genomics, proteomics and clinical bacteriology. Humana press Inc., Totowa, pp 71–113
Bennett PM (2008) Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol 153(Suppl 1):S347–S357. https://doi.org/10.1038/sj.bjp.0707607
Botrel MA, Haenni M, Morignat E, Sulpice P, Madec JY, Calavas D (2010) Distribution and antimicrobial resistance of clinical and subclinical mastitis pathogens in dairy cows in Rhône-Alpes, France. Foodborne Pathog Dis 7:479–487. https://doi.org/10.1089/fpd.2009.0425
Brunton LA, Desbois AP, Garza M, Wieland B, Mohan CV, Häsler B, Tam CC, Le PNT, Nguyen TP, Van PT et al (2019) Identifying hotspots for antibiotic resistance emergence and selection, and elucidating pathways to human exposure: application of a systems-thinking approach to aquaculture systems. Sci Total Environ 687:1344–1356. https://doi.org/10.1016/j.scitotenv.2019.06.134
Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306:7–23. https://doi.org/10.1016/j.aquaculture.2010.05.020
Buschmann AH, Tomova A, Lopez A, Maldonado MA, Henriquez LA, Ivanova L, Moy F, Godfrey HP, Cabello FC (2012) Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS One 7:e42724. https://doi.org/10.1371/journal.pone.0042724
Cabello FC (2004) Antibiotics and aquaculture in Chile: implications for human and animal health. Rev Med Chil 132:1001–1006. https://doi.org/10.4067/s0034-98872004000800014
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144. https://doi.org/10.1111/j.1462-2920.2006.01054.x
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Do¨lz H, Millanao A, Buschmann AH (2013) Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol 15:1917–1942. https://doi.org/10.1111/1462-2920.12134
Cambray G, Sanchez-Alberola N, Campoy S, Guerin E, Da Re S, Gonzalez-Zorn B, Ploy M-C, Barbe J, Mazel D, Erill I (2011) Prevalence of SOS-mediated control of integron integrase expression as an adaptive trait of chromosomal and mobile integrons. Mob DNA 2:6. https://doi.org/10.1186/1759-8753-2-6
Castillo D, Higuera G, Villa M, Middelboe M, Dalsgaard I, Madsen L, Espejo RT (2012) Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J Fish Dis 35:193–201. https://doi.org/10.1111/j.1365-2761.2011.01336.x
Castillo D, Christiansen RH, Dalsgaard I, Madsen L, Middelboe M (2015) Bacteriophage resistance mechanisms in the fish pathogen Flavobacterium psychrophilum: linking genomic mutations to changes in bacterial virulence factors. Appl Environ Microbiol 81:1157–1167. https://doi.org/10.1128/AEM.03699-14
Cattoir V, Nordmann P (2009) Plasmid-mediated quinoloneb resistance in gram-negative bacterial species: an update. Curr Med Chem 16:1028–1046. https://doi.org/10.2174/092986709787581879
Cavalli LS, Brito KCT, Brito BG (2015). One health, one aquaculture: aquaculture under One Health umbrella. J Mar Biol Aquacult 2015:1:1–8.
Chabrillon M, Rico RM, Arijo S, Diaz-Rosales P, Balebona MC, Morinigo MA (2005) Interactions of microorganisms isolated from gilthead sea bream, Sparus aurata L., on Vibrio harveyi, a pathogen of farmed Senegalese sole, Solea senegalensis (Kaup). J Fish Dis 28:531–537. https://doi.org/10.1111/j.1365-2761.2005.00657.x
Chenia HY (2016) Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from south African freshwater fish. Int J Food Microbiol 231:26–32. https://doi.org/10.1016/j.ijfoodmicro.2016.04.030
Chenia HY, Vietze C (2012) Tetracycline resistance determinants of heterotrophic bacteria isolated from a south African tilapia aquaculture system. Afr J Microbiol Res 6:6761–6768. https://doi.org/10.5897/AJMR10.840
Chiew Y-F, Yeo S-F, Hall LMC, Livermore DM (1998) Can susceptibility to an antimicrobial be restored by halting its use? The case of streptomycine versus Enterobacteriaceae. J Antimicrob Chemother 41:247–251. https://doi.org/10.1093/jac/41.2.247
Chuah L, Effarizah ME, Goni AM, Rusul G (2016) Antibiotic application and emergence of multiple antibiotic resistance (MAR) in global catfish aquaculture. Curr Environ Health Rep 3:118–127. https://doi.org/10.1007/s40572-016-0091-2
Cizek A, Dolejska M, Sochorova R, Strachotova K, Piackova V, Vesely T (2010) Antimicrobial resistance and its genetic determinants in aeromonads isolated in ornamental (koi) carp (Cyprinus carpio koi) and common carp (Cyprinus carpio). Vet Microbiol 142:435–439. https://doi.org/10.1016/j.vetmic.2009.10.001
Dang HY, Zhang XX, Song LS, Chang YQ, Yang GP (2007) Molecular determination of oxytetracycline-resistant bacteria and their resistance genes from mariculture environments of China. J Appl Microbiol 103:2580–2592. https://doi.org/10.1111/j.1365-2672.2007.03494.x
Dawood MAO, Koshio S, Esteban MA (2017) Beneficial roles of feed additives as immunostimulants in aquaculture: areview. Rev Aquacult 10:950–974. https://doi.org/10.1111/raq.12209
Declercq AM, Boyen F, Van den Broeck W, Bossier P, Karsi A, Haesebrouck F, Decostere A (2013) Antimicrobial susceptibility pattern of Flavobacterium columnare isolates collected worldwide from 17 fish species. J Fish Dis 36:45–55. https://doi.org/10.1111/j.1365-2761.2012.01410.x
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258. https://doi.org/10.1016/j.mib.2011.03.004
Deng W, Li N, Zheng H, Lin H (2016) Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotox Environ Safe 125:121–127. https://doi.org/10.1016/j.ecoenv.2015.12.002
Desriac F, Defer D, Bourgougnon N, Brillet B, Chevalier PL, Fleury Y (2010) Bacteriocin as weapons in the marine animal-associated bacteria warfare: inventory and potential applications as an aquaculture probiotic. Mar Drugs 8:1153–1177. https://doi.org/10.3390/md8041153
Destoumieux-Garzón D, Mavingui P, Boetsch G, Boissier J, Darriet F, Duboz P, et al. (2018) The one health concept: 10 years old and a long road ahead. Front Vet Sci 5:1–12. https://doi.org/10.3389/fvets.2018.00014
Dharmaratnam A, Kumar R, Basheer VS, Sood N, Swaminathan TR, Jena JK (2017) Isolation and characterisation of virulent Serratia marcescens associated with a disease outbreak in farmed ornamental fish, Poecilia reticulata in Kerala, India. Indian J fish 64:71–79. https://doi.org/10.21077/ijf.2017.64.4.71261-10
Dias C, Mota V, Martinez-Murcia A, Saavedra MJ (2012) Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamental fish. J Aquac res Dev 3: 3. https://doi.org/10.4172/2155-9546.1000131
Dixon BA, Issvoran G (1993) Antibacterial drug resistance in Aeromonas spp. isolated from domestic goldfish and koi from California. J World Aquacult Soc 24:102–104. https://doi.org/10.1111/j.1749-7345.1993.tb00155.x
Dixon BA, Yamashita J, Evelyn F (1990) Antibiotic resistance of Aeromonas spp. isolated from tropical fish imported from Singapore. J Aquat Anim Health 2:295–297. https://doi.org/10.1577/1548-8667(1990)002<0295:AROASI>2.3.CO;2
Dobiasova H, Kutilova I, Piackova V, Vesely T, Cizek A, Dolejska M (2014) Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Vet Microbiol 171:413–421. https://doi.org/10.1016/j.vetmic.2014.02.011
Done HY, Halden RU (2015) Reconnaissance of 47 antibiotics and associated microbial risks in seafood sold in the United States. J Hazard Mater 282:10–17. https://doi.org/10.1016/j.jhazmat.2014.08.075
Done HY, Venkatesan AK, Halden RU (2015) Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J 17:513–524. https://doi.org/10.1208/s12248-015-9722-z
Duran GM, Marshall DL (2005) Ready-to-eat shrimp as an international vehicle of antibiotic-resistant bacteria. J Food Prot 68:2395–2401. https://doi.org/10.4315/0362-028X-68.11.2395
Enne VI, Livermore DM, Stephens P, Hall LM (2001) Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 357:1325–1328. https://doi.org/10.1016/S0140-6736(00)04519-0
FAO (Food and Agriculture Organization of the United Nations) 2005. Responsible use of antibiotics in Aquaculture. In: Serrano PH (eds) Available: http://www.fao.org/3/a-a0282e.pdf
FAO/OIE/WHO (Food and Agriculture Organization of the United Nations/World Organization for Animal Health/ World Health Organization) 2006. Antimicrobial use in aquaculture and antimicrobial Resistance. Available: http://www.who.int/topics/foodborne_diseases/aquaculture_rep_13_16june2006.pdf. [accessed 18 April 2013]
Furushita M, Shiba T, Maeda T, Yahata M, Kaneoka A, Takahashi Y, Torii K, Hasegawa T, Ohta M (2003) Similarity of tetracycline resistance genes isolated from fish farm bacteria to those from clinical isolates. Appl Environ Microbiol 69:5336–5342. https://doi.org/10.1128/AEM.69.9.5336-5342.2003
Gao P, Mao D, Luo Y, Wang L, Xu B, Xu L (2012) Occurrence of sulfonamide and tetracycline-resistant bacteria and resistant genes in aquaculture environment. Water Res 46:2355–2364. https://doi.org/10.1016/j.watres.2012.02.004
Grave K, Markestad A, Bangen M (1996) Comparison in prescribing patterns of antibacterial drugs in salmonid farming in Norway during the periods 1980–1988 and 1989–1994. J Vet Pharmacol Ther 19:184–191. https://doi.org/10.1111/j.1365-2885.1996.tb00037.x
Guz L, Grazdzki Z, Krajewska M, Lipiec M, Zabost A, Augustynowicz-Kopec E, Zwolska Z, Szulowski K (2013) Occurrence and antimicrobial susceptibility of Mycobacterium peregrinum in ornamental fish. B. Vet I Pulawy 57:489–492. https://doi.org/10.2478/bvip-2013-0085
Han JE, Kim JH, Cheresca CH, Shin SP, Jun JW, Chai JY, Han SY, Park SC (2012) First description of the qnrS-like (qnrS5) gene and analysis of quinolone resistance-determining regions in motile Aeromonas spp. from diseased fish and water. Res Microbiol 163:73–79. https://doi.org/10.1016/j.resmic.2011.09.001
Han Y, Wang J, Zhao Z, Chen J, Lu H, Liu G (2017) Fish meal application induces antibiotic resistance gene propagation in mariculture sediment. Environ Sci Technol 51:10850–10860. https://doi.org/10.1021/acs.est.7b02875
Hansson K, Sundström L, Pelletier A, Roy PH (2002) Int I 2 integron integrase in Tn7. J Bacteriol 184:1712–1721. https://doi.org/10.1128/JB.184.6.1712-1721.2002
Harikrishnan R, Kim JS, Balasundaram C, Heo MS (2012) Protection of Vibrio harveyi infection through dietary administration of Pueraria thunbergiana in kelp grouper, Epinephelus bruneus. Aquaculture 325:27–32. https://doi.org/10.1016/j.aquaculture.2011.10.019
Hatha M, Vivekanandhan AA, Joice GJ, Christol (2005) Antibiotic resistance pattern of motile aeromonads from farm raised freshwater fish. Int J Food Microbiol 98:131–134. https://doi.org/10.1016/j.ijfoodmicro.2004.05.017
Hesami S, Parkman J, MacInnes JI, Gray JT, Gyles CL, Lumsden JS (2010) Antimicrobial susceptibility of Flavobacterium psychrophilum isolates from Ontario. J Aquat Anim Health 22:39–49. https://doi.org/10.1577/H09-008.1
Heuer OE, Kruse H, Grave K, Collignon P, Karunasagar I, Angulo FJ (2009) Human health consequences of use of antimicrobial agents in aquaculture. Clin Infect Dis 49:1248–1253. https://doi.org/10.1086/605667
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices - a review. J Environ Manag 92:2304–2347. https://doi.org/10.1016/j.jenvman.2011.05.023
Hooper DC, Jacoby GA (2015) Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci 1354:12–31. https://doi.org/10.1111/nyas.12830
Huang Y, Michael GB, Becker R, Kaspar H, Mankertz J, Schwarz S, Runge M, Steinhagen D (2014) Pheno- and genotypic analysis of antimicrobial resistance properties of Yersinia ruckeri from fish. Vet Microbiol 171:406–412. https://doi.org/10.1016/j.vetmic.2013.10.026
Huang Y, Zhang L, Tiu L, Wang HH (2015) Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front Microbiol 6:914. https://doi.org/10.3389/fmicb.2015.00914
Huss HH, Reilly A, Embarek PKB (2000) Prevention and control of hazards in seafoods. Food Control 11:149–156. https://doi.org/10.1016/S0956-7135(99)00087-0
Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI (2012) Emerging Aeromonas species infections and their significance in public health. Sci World J 12:625023. https://doi.org/10.1100/2012/625023
Izumi S, Ouchi S, Kuge T, Arai H, Mito T, Fujii H, Aranishi F, Shimizu A (2007) PCR–RFLP genotypes associated with quinolone resistance in isolates of Flavobacterium psychrophilum. J Fish Dis 30:141–147. https://doi.org/10.1111/j.1365-2761.2007.00797.x
Jacobs L, Chenia HY (2007) Characterization of integrons and tetracycline resistance determinants in Aeromonas spp. isolated from south African aquaculture systems. Int J Food Microbiol 114:295–306. https://doi.org/10.1016/j.ijfoodmicro.2006.09.030
Jacoby GA, Hooper DC (2013) Phylogenetic analysis of chromosomally determined Qnr and related proteins. Antimicrob Agents Chemother 57:1930–1934. https://doi.org/10.1128/AAC.02080-12
Jagoda SS, Wijewardana TG, Arulkanthan A, Igarashi Y, Tan E, Kinoshita S, Watabe S, Asakawa S (2014) Characterization and antimicrobial susceptibility of motile aeromonads isolated from freshwater ornamental fish showing signs of septicaemia. Dis Aquat Org 109:127–137. https://doi.org/10.3354/dao02733
John N, Hatha AAM (2012) Prevalence, distribution and drug resistance of motile aeromonads in freshwater ornamental fishes. Indian J Fish 59:161–164
Kadowaki T, Yasui Y, Nishimiya O, Takahishi Y, Kohchi C, Soma GI, Inagawa H (2013) Orally administered LPS enhances head kidney macrophage activation with downregulation of IL-6 in common carp (Cyprinus carpio). Fish Shellfish Immunol 34:1569–1575. https://doi.org/10.1016/j.fsi.2013.03.372
Kang C-H, Shin Y, Jang S, Jung Y, So J-S (2016) Antimicrobial susceptibility of Vibrio alginolyticus isolate from oyster in Korea. Environ Sci Pollut Res 23:21106–21112. https://doi.org/10.1007/s11356-016-7426-2
Kim E, Aoki T (1993) Drug resistance and broad geographical distribution of identical R plasmids of Pasteurella piscicida isolated from cultured yellowtail in Japan. Microbiol Immunol 37:103–109
Kim EH, Yoshida T, Aoki TBB (1993) Detection of R plasmid encoded with resistance to florfenicol in Pasteurella piscicida. Fish Pathol 28:165–170. https://doi.org/10.3147/jsfp.28.165
Kim S-R, Nonaka L, Suzuki S (2004) Occurrence oftetracycline resistance genes tet(M) and tet(S) in bacteriafrom marine aquaculture sites. FEMS Microbiol Lett 237:147–156.
Kim MJ, Hirono I, Kurokawa K, Maki T, Hawke J, Kondo H, Santos MD, Aoki T (2008) Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R plasmids) from Photo- bacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob. Agents Chemother. 52: 606–611.
Ko WC, Lee HC, Chuang YC, Liu CC, Wu JJ (2000) Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Inf Secur 40:267–274. https://doi.org/10.1053/jinf.2000.0654
Kruse H, Sørum H (1994) Transfer of multiresistance plasmids between bacteria of diverse origin in natural micro-environments. Appl Environ Microbiol 60:4015–4021
Kumar SR, Ahmed VPI, Parameswaran V, Sudhakaran R, Babu VS, Hameed AS (2008) Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates clacrifer) to protect from Vibrio (Listonella) anguillarum. Fish Shellfish Immunol 25:47e56. https://doi.org/10.1016/j.fsi.2007.12.004
Kumar R, Swaminathan TR, Kumar RG, Dharmaratnam A, Basheer VS, Jena JK (2015) Mass mortality in ornamental fish, Cyprinus carpio koi caused by a bacterial pathogen, Proteus hauseri. Acta Trop 149:128–134. https://doi.org/10.1016/j.actatropica.2015.05.022
Kumar S, Lekshmi M, Parvathi A, Nayak BB, Varela MF (2017) Antibiotic resistance in seafood borne pathogens. In: Singh OV (ed) Food borne pathogens and antibiotic resistance. Wiley, Hoboken
Labbate M, Case RJ, Stokes HW (2009) The Integron/gene cassette system: an active player in bacterial adaptation. In: Gogarten MB, Gogarten JP, Olendzenski LC (eds) Horizontal gene transfer. Methods in molecular biology, vol 532, pp 103–125
Labee-Lund TM, Sorum H (2001) Class I integrons mediated antibiotic resistance in the fish pathogen Aeromonas salmonicida world-wide. Microb Drug Resist 7:263–272. https://doi.org/10.1089/10766290152652819
Labella A, Gennari M, Ghidini V, Trento I, Manfrin A, Borrego JJ, Lleo MM (2013) High incidence of antibiotic multi-resistant bacteria in coastal areas dedicated to fish farming. Mar Pollut Bull 70:197–203. https://doi.org/10.1016/j.marpolbul.2013.02.037
Lamari F, Chakroun I, Rtimi S (2017) Assessment of the correlation among antibiotic resistance, adherence to abiotic and biotic surfaces, invasion and cytotoxicity of Pseudomonas aeruginosa isolated from diseased gilthead sea bream. Colloids Surf B: Biointerfaces 158:229–236. https://doi.org/10.1016/j.colsurfb.2017.06.044
Larsson DGJ, Andremont A, Bengtsson-Palme J, Brandt KK, de Roda Husman AM, Fagerstedt P, Fick J, Flach CF, Gaze WH, Kuroda M, Kvint K, Laxminarayan R, Manaia CM, Nielsen KM, Plant L, Ploy M-C, Segovia C, Simonet P, Smalla K, Snape J, Topp E, van Hengel AJ, Verner-Jeffreys DW, Virta MPJ, Wellington EM, Wernersson AS (2018) Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ Int 117:132–138. https://doi.org/10.1016/j.envint.2018.04.041
Lewbart GA (2001) Bacteria and ornamental fish. Seminars in Avian and Exotic Pet Medicine 10:48–56. https://doi.org/10.1053/saep.2001.19543
Li XZ (2005) Quinolone resistance in bacteria: emphasis on plasmidmediated mechanisms. Int J Antimicrob Agents 25:453–463. https://doi.org/10.1016/j.ijantimicag.2005.04.002
Li D, Yu T, Zhang Y, Yang M, Li Z, Liu M, Qi R (2010) Antibiotic resistance characteristics of environmental bacteria from an oxytetracycline production wastewater treatment plant and the receiving river. Appl Environ Microbiol 76:3444–3451. https://doi.org/10.1128/AEM.02964-09
Li L, Lin S-L, Deng L, Liu Z-G (2013) Potential use of chitosan microparticles for oral delivery of DNA vaccine in black seabream Acanthopagrus schlegelii Bleeker to protect from Vibrio parahaemolyticus. J Fish Dis 36:987–995. https://doi.org/10.1111/jfd.12032
Liu X, Steele JC, Meng X-Z (2017) Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut 223:161–169. https://doi.org/10.1016/j.envpol.2017.01.003
Lozano I, Díaz NF, Muñoz S, Riquelme C (2018) Antibiotics in Chilean aquaculture: a review. Antibiotic Use in Animals p, In, p 21. https://doi.org/10.5772/intechopen.71780
Lulijwa R, Rupia EJ, Alfaro AC (2019) Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquacult (in press). https://doi.org/10.1111/raq.12344
Lunn AD, Fabrega A, Sanchez-Cespedes J, Vila J (2010) Prevalence of mechanisms decreasing quinolone-susceptibility among Salmonella spp. clinical isolates. Int Microbiol 13:15e20. https://doi.org/10.2436/20.1501.01.107
Luo Y, Mao D, Rysz M, Zhou Q, Zhang H, Xu L, Alvarez PJJ (2010) Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ Sci Technol 44:7220–7225. https://doi.org/10.1021/es100233w
Marshall SH, Arenas G (2003) Antimicrobial peptides: a natural alternative to chemical antibiotics and a potential for applied biotechnology. Electron J Biotechnol 6:271–284. https://doi.org/10.2225/vol6-issue3-fulltext-1
Marti E, Huerta B, Rodríguez-Mozaz S, Barceló D, Marcé R, Balcázar JL (2018) Abundance of antibiotic resistance genes and bacterial community composition in wild freshwater fish species. Chemosphere 196:115–119. https://doi.org/10.1016/j.chemosphere.2017.12.108
McPhearson RM, DePaola A, Zywno SR, Motes ML, Guarino AM (1991) Antibiotic-resistance in gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture 99:203–211. https://doi.org/10.1016/0044-8486(91)90241-X
Michel C, Kerouault B, Martin C (2003) Chloramphenicol and florfenicol susceptibility of fish—pathogenic bacteria isolated in France: comparison of minimum inhibitory concentration, using recommended provisory standards for fish bacteria. J Appl Microbiol 95:1008–1015. https://doi.org/10.1046/j.1365-2672.2003.02093.x
Michel C, Matte-Tailliez O, Kerouault B, Bernardet J-F (2005) Resistance pattern and assessment of phenicol agents’ minimum inhibitory concentration in multiple drug resistant Chryseobacterium isolates from fish and aquatic habitats. J Appl Microiol 99:323–332. https://doi.org/10.1111/j.1365-2672.2005.02592.x
Miranda CD, Kehrenberg C, Ulep C, Schwarz S, Roberts MC (2003) Diversity of tetracycline resistance genes in bacteria from Chilean salmon farms. Antimicrob Agents Chemother 47:883–888. https://doi.org/10.1128/aac.47.3.883-888.2003
Miranda CD, Tello A, Keen PL (2013) Mechanisms of antimicrobial resistance in finfish aquaculture environments. Front Microbiol 4:233. https://doi.org/10.3389/fmicb.2013.00233
Miranda CD, Godoy FA, Lee MR (2018) Current status of the use of antibiotics and their antimicrobial resistance in the chilean salmon farms. Front Microbiol 9:1284. https://doi.org/10.3389/fmicb.2018.01284
Mo WY, Chen Z, Leung HM, Leung AO (2017) Application of veterinary antibiotics in China's aquaculture industry and their potential human health risks. Environ Sci Pollut Res Int 24:8978–8989. https://doi.org/10.1007/s11356-015-5607-z
Musto J, Kirk M, Lightfoot D, Combs BG, Mwari L (2006) Multi drug resistant Salmonella Java infections acquired from tropical ornamental fish aquarium, Australia, 2003-04. Commun Diseas Intell 30:222–227
Naviner M, Gordon L, Giraud E, Denis M, Mangion C, Le Bris H, Ganiére J-P (2011) Antimicrobial resistance of Aeromonas spp. isolated from the growth pond to the commercial product in a rainbow trout farm following a flumequine treatment. Aquaculture 315:236–241. https://doi.org/10.1016/j.aquaculture.2011.03.006
Ndi OL, Barton MD (2011) Incidence of class 1 integron and other antibiotic resistance determinants in Aeromonas spp. from rainbow trout farms in Australia. J Fish Dis 34:589–599. https://doi.org/10.1111/j.1365-2761.2011.01272.x
Neela FA, Banu MNA, Rahman MA, Rahman MH, Alam MF (2014) Occurrence of antibiotic resistant bacteria in pond water associated with integrated poultry-fish farming in Bangladesh. Sains Malays 44:371–377. https://doi.org/10.17576/jsm-2015-4403-08
Newaj-Fyzul A, Austin B (2015) Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. J Fish Dis 14:937–955. https://doi.org/10.1111/jfd.12313
Ngamkala S, Futami K, Endo M, Maita M, Katagiri T (2010) Immunological effects of glucan and Lactobacillus rhamnosus GG, on probiotic bacterium, on Nile tilapia Oreochromis niloticus intestine with oral Aeromonas challenges. Fisheries Sci 76:833–840. https://doi.org/10.1007/s12562-010-0280-0
Nguyen TTT, Nguyen HT, Tsaia MA, Byadgi O, Wanga PC, Yoshida T, Chena SC (2017) Genetic diversity virulence genes and antimicrobial resistance of Streptococcus dysgalactiae isolates from different aquatic animal sources. Aquaculture 479:256–264. https://doi.org/10.1016/j.aquaculture.2017.06.002
Nonaka L, Suzuki S (2002) New Mg2+-dependent oxytetracycline resistance determinant Tet 34 in Vibrio isolates from marine fish intestinal contents. Antimicrob Agents Chemother 476:1550–1552. https://doi.org/10.1128/AAC.46.5.1550-1552.2002
Nonaka L, Ikeno K, Suzuki S (2007) Distribution of tetracycline resistance gene, tet(M), in Grampositive and gram-negative bacteria isolated from sediment and seawater at a coastal aquaculture site in Japan. Microbes Environ 22:355–364. https://doi.org/10.1264/jsme2.22.355
Novotny L, Dvorska L, Lorencova A, Beran V, Pavlik I (2004) Fish: a potential source of bacterial patho- gens for human beings. Vet Med – Czech 49:343–358. https://doi.org/10.1016/j.ijfoodmicro.2011.10.003
Nya EJ, Austin B (2010) Use of bacterial lipopolysaccharide (LPS) as an immunostimulant for the control of Aeromonas hydrophila infections in rainbow trout Oncorhynchus mykiss (Walbaum). J Appl Microbiol 108:686–694. https://doi.org/10.1111/j.1365-2672.2009.04464.x
Odeyemi OA, Ahmad A (2017) Antibiotic resistance profiling and phenotyping of Aeromonas species isolated from aquatic sources. Saudi J Biol Sci 24:65–70. https://doi.org/10.1016/j.sjbs.2015.09.016
Pan JC, Ye R, Wang HQ, Xiang HQ, Zhang W, Yu XF, Meng DM, He ZS (2008) Vibrio cholerae O139 multiple drug resistance mediated by Yersinia pestis pIP1202-like conjugative plasmids. Antimicrob Agents Chemother 52:3829–3836. https://doi.org/10.1128/AAC.00375-08
Partridge SR, Tsafnat G, Coiera E, Iredell JR (2009) Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev 33:757–784. https://doi.org/10.1111/j.1574-6976.2009.00175.x
Pathak SP, Bhattacherjee JW, Ray PK (1993) Seasonal variation in survival and antibiotic resistance among various bacterial populations in a tropical river. J Gen Appl Microbiol 39:4756
Petersen A, Dalsgaard A (2003) Antimicrobial resistance of intestinal Aeromonas spp. and Enterococcusspp. In fish cultured in integrated broiler-fish farms in Thailand. Aquaculture 219:71–82
Petersen A, Andersen JS, Kaewmak T, Somsiri T, Dalsgaard A (2002) Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl Environ Microbiol 68:6036–6042. https://doi.org/10.1128/AEM.68.12.6036-6042.2002
Piotrowska M, Popowska M (2014) The prevalence of antibiotic resistance genes among Aeromonas species in aquatic environments. Ann Microbiol 64:921–934. https://doi.org/10.1007/s13213-014-0911-2
Poirel L, Cattoir V, Nordmann P (2012) Plasmid-mediated quinolone resistance; interactions between human, animal, and environmental ecologies. Front Microbiol 3:24. https://doi.org/10.3389/fmicb.2012.00024 2
Preena PG, Dharmarathnam A, Raj NS, Kumar TVA, Raja SA, Swaminathan TR (2019a) Antibiotic susceptibility pattern of bacteria isolated from freshwater ornamental fish, guppy showing bacterial disease. Biologia (in press) doi: https://doi.org/10.2478/s11756-019-00261-8
Preena PG, Dharmarathnam A, Raj NS, Kumar TVA, Raja SA, Nair RR, Swaminathan TR (2019b) Diversity of antimicrobial resistant pathogens from a freshwater rnamental fish farm. Lett Appl Microbiol (in press) doi:https://doi.org/10.1111/lam.13231
Rahube TO, Yost CK (2010) Antibiotic resistance plasmids in wastewater treatment plants and their possible dissemination into the environment. Afr J Biotechnol 9:9183–9190. https://doi.org/10.1111/j.1749-7345.2008.00163.x
Rajendiran A, Natarajan E, Subramanian P (2008) Control of Aeromonas hydrophila infection in spotted snakehead Channa punctatus,by Solanum nigrum L., a medicinal plant. J World Aquacult Soc 39:375–383. https://doi.org/10.1111/j.1749-7345.2008.00163.x
Reboucas RH, de Sousa OV, Lima AS, Vasconcelos FR, de Carvalho PB, Silva dos Fernandes Vieira RH (2011) Antimicrobial resistance profile of Vibrio species isolated from marine shrimp farming environments (Litopenaeus vannamei) at Ceara, Brazil. Environ Res 111:21e24. https://doi.org/10.1128/aem.66.9.3883-3890.2000
Rhodes G, Huys G, Swings J, Mcgann P, Hiney M, Smith P, Pickup RW (2000) Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: implication of Tn1721 in dissemination of the tetracycline resistance determinant Tet a. Appl Environ Microbiol 66:3883–3890. https://doi.org/10.1128/aem.66.9.3883-3890.2000
Rico A, Jacobs R, Van den Brink PJ, Tello A (2017) A probabilistic approach to assess antibiotic resistance development risks in environ- mental compartments and its application to an intensive aquaculture production scenario. Environ Pollut 231:918–928. https://doi.org/10.1016/j.envpol.2017.08.079
Ripabelli G, Sammarco ML, McLauchlin J, Fanelli I (2003) Molecular characterization and antimicrobial resistance of Vibrio vulnificus and Vibrio alginolyticus isolated from mussels (Mytilus galloprovincialis). Syst Appl Microbiol 26:119–126. https://doi.org/10.1078/072320203322337407
Romero J, Feijoó CG, Navarrete P (2012) Antibiotics in aquaculture — use, abuse and alternatives. In: Carvalho ED David JS Silva RJ (eds) health and environment in aquaculture, pp 159
Rosewarne CP, Pettigrove V, Stokes HW, Parsons YM (2010) Class 1 integrons in benthic bacterial communities: abundance, association with Tn402-like transposition modules and evidence for coselection with heavy-metal resistance. FEMS Microbiol Ecol 72:35–46. https://doi.org/10.1111/j.1574-6941.2009.00823.x
Ruzauskas M, Klimiene I, Armalyte J, Bartkiene E, Siugzdiniene R, Skerniskyte J, Krasauskas R, Suziedeliene E (2018) Composition and antimicrobial resistance profile of gram-negative microbiota prevalent in aquacultured fish. J food safety 38:e12447. https://doi.org/10.1111/jfs.12447
Saavedra MJ, Guedes-Novais S, Alves A, Rema P, Tacão M, Correia A, Martínez-Murcia A (2010) Resistance to β-lactam antibiotics in Aeromonas hydrophila isolated from rainbow trout (Onchorhynchus mykiss). Int Microbiol 7:207–211
Sahoo PK, Swaminathan T, Abraham TJ, Kumar R, Pattanayaka S, Mohapatra A, Ratha SS, Patra A, Adikesavalu H, Sood N, Pradhan PK, Das BK, Jayasankar P, Jena JK (2016) Detection of goldfish haematopoietic necrosis herpes virus (cyprinid herpesvirus-2) with multi-drug resistant Aeromonas hydrophila infection in goldfish: first evidence of any viral disease outbreak in ornamental freshwater aquaculture farms in India. Acta Trop 161:8–17. https://doi.org/10.1016/j.actatropica.2016.05.004
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents 52:135. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Sarter S, Nguyen HNK, Hung LT, Lazard J, Montet D (2007) Antibiotic resistance in gram-negative bacteria isolated from farmed catfish. Food Control 18:1391–1396. https://doi.org/10.1016/j.foodcont.2006.10.003
Scarano C, Spanu C, Ziino G, Pedonese F, Dalmasso A, Spanu V, Virdis S, De Santis EP (2014) Antibiotic resistance of Vibrio species isolated from Sparus aurata reared in Italian mariculture. New Microbiol 37:329–337. https://doi.org/10.1093/jac/47.6.735
Schmidt AS, Bruun MS, Dalsgaard I, Larsen JL (2001a) Characterization of class 1 integrons associated with R-plasmids in clinical Aeromonas salmonicida isolates from various geographical areas. J Antimicrob Chemother 47:735–743. https://doi.org/10.1093/jac/47.6.735
Schmidt AS, Bruun MS, Dalsgaard I, Larsen JL (2001b) Incidence, distribution and spread of tetracycline resistance determinants and integron associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl Environ Microbiol 67:5675–5682. https://doi.org/10.1128/AEM.67.12.5675-5682.2001
Schmidt AS, Bruun MS, Dalsgaard I, Pedersen K, Larsen JL (2000) Occurrence of antimicrobial resistancein fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl Environ Microbiol 66: 4908–4915.
Shah SQ, Nilsen H, Bottolfsen K, Colquhoun DJ, Sørum H (2012) DNA gyrase and topoisomerase IV mutations in quinolone-resistant Flavobacterium psychrophilum isolated from diseased salmonids in Norway. Microb Drug Resist 18:207–214. https://doi.org/10.1089/mdr.2011.0142
Shah SQ, Cabello FC, L’abée-Lund TM, Tomova A, Godfrey HP, Buschmann AH, Sørum H (2014) Antimicrobial resistance and antimicrobial resistance genes in marine bacteria from salmon aquaculture and non-aquaculture sites. Environ Microbiol 16:1310–1320. https://doi.org/10.1111/1462-2920.12421
Sing CK, Khan MZI, Daud HHJM, Aziz AR (2016) Prevalence of Salmonella sp. in African catfish (Clarias gariepinus) obtained from farms and wet markets in Kelantan, Malaysia and their antibiotic resistance. Sains Malays 45:1597–1602
Singh AF, Rathore G, Singh V, Mani I, Singh RK, Mishra SK, Mishra BN, Verma OP (2009) Bacterial resistance to oxytetracycline in different life stages of Indian freshwater carp aquaculture system. Int J Microbiol Res 1:25–34. https://doi.org/10.9735/0975-5276.1.1.25-34
Slama KB, Jouini A, Sallem RB, Somalo S, Saenz Y, Estepa V, Boudabous A, Torres C (2010) Prevalence of broadspectrum cephalosporin-resistant Escherichia coli isolates in food samples in Tunisia, and characterization of integrons and antimicrobial resistance mechanisms implicated. Int J Food Microbiol 137:281–286. https://doi.org/10.1016/j.ijfoodmicro.2009.12.003
Smith P, Hiney MP, Samuelsen OB (1994) Bacterial resistance to antimicrobial agents used in fish farming: a critical evaluation of method and meaning. Annu Rev Fish Dis 4:273–313. https://doi.org/10.1016/0959-8030(94)90032-9
Snieszko SF, Bullock GL (1957) Treatment of sulfonamide-resistant furunculosis in trout and determination of drug sensitivity. Fish Bull 125:555–564
Sørum H (2006) Antimicrobial drug resistance in fish pathogens. In: Aarestrup FM (ed) Antimicrobial resistance in bacteria of animal origin. ASM Press, Washington, DC, pp 213–238
Sousa M, Torres C, Barros J, Somalo S, Igrejas G, Poeta P (2011) Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended –spectrum beta-lactamases-containing Escherichia coli isolates. Foodborne Pathog Dis 8:1139–1141. https://doi.org/10.1089/fpd.2011.0866
Sreedharan K, Philip R, Singh ISB (2011) Isolation and characterization of virulent Aeromonas veronii from ascetic fluid of oscar Astronotus ocellatus showing signs of infectious dropsy. Dis Aquat Org 1:29–39. https://doi.org/10.3354/dao02304
Sreedharan K, Philip R, Singh ISB (2012) Virulence potential and antibiotic susceptibility pattern of motile aeromonads associated with freshwater ornamental fish culture systems: a possible threat to public health. Braz J Microbiol 43:754–765. https://doi.org/10.1590/S1517-83822012000200040
Stalder T, Barraud O, Casellas M, Dagot C, Ploy MC (2012) Integron involvement in environmental spread of antibiotic resistance. Front Microbiol 3:119. https://doi.org/10.3389/fmicb.2012.00119
Stokes HW, Gillings MR (2011) Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into gram-negative pathogens. FEMS Microbiol Rev 35:790–819. https://doi.org/10.1111/j.1574-6976.2011.00273.x
Su HC, Ying GG, Tao R, Zhang RQ, Fogarty LR, Kolpin DW (2011) Occurrence of antibiotic resistance and characterization of resistance genes and integrons in Enterobacteriaceae isolated from integrated fish farms in South China. J Environ Monit 13:3229–3236. https://doi.org/10.1039/c1em10634a
Swain P, Nayak SK, Sasmal A, Behera T, Swain SK, Mishra SS, Sen AK, Das JK, Jayasankar P (2014) Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J Microbiol Biotechnol 30:2491–2502. https://doi.org/10.1007/s11274-014-1674-4
Tamminen M, Karkman A, Lohmus A, Muziasari WI, Takasu H, Wada S et al (2011) Tetracycline resistance genes persist at aquaculture farms in the absence of selec- tion pressure. Environ Sci Technol 45:386–391. https://doi.org/10.1021/es102725n
Tao W, Lee MH, Wu J, Kim NH, Kim J-C, Chung E, Hwang EC, Lee SW (2012) Inactivation of chloramphenicol and florfenicol by a novel chloramphenicol hydrolase. Appl Environ Microbiol 78:6295–6301. https://doi.org/10.1128/AEM.01154-12
Tendencia EA, Pena LD (2001) Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 195:193–204. https://doi.org/10.1016/S0044-8486(00)00570-6
Thornber K, Verner-Jeffreys D, Hinchliffe S, Rahman MM, Bass D, Tyler CR (2019) Evaluating antimicrobial resistance in the global shrimp industry. Rev Aquacult (in press). https://doi.org/10.1111/raq.12367
Tomova A, Ivanova L, Buschmann AH, Rioseco ML, Kalsi RK, Godfrey HP, Cabello FC (2015) Antimicrobial resistance genes in marine bacteria and human uropathogenic Escherichia coli from a region of intensive aquaculture. Environ Microbiol Rep 7:803–809. https://doi.org/10.1111/1758-2229.12327
Topp E, Larsson DGJ, Miller DN, Van den Eede C, Virta MPJ (2018) Antimicrobial resistance and the environment: assessment of advances, gaps and recommendations for agriculture, aquaculture and pharmaceutical manufacturing. FEMS Microbiol Ecol 94:fix185. https://doi.org/10.1093/femsec/fix185
Turkson P (2008) Use of drugs and antibiotics in poultry production in Ghana. Ghana J Agric Sci 41:23–33. https://doi.org/10.4314/gjas.v41i1.46142
Vaughan S, Coyne R, Smith P (1996) The critical importance of sample site in the determination of the frequency of oxytetracycline resistance in the effluent microflora of a freshwater fish farm. Aquaculture 139:47–54. https://doi.org/10.1016/0044-8486(95)01152-8
Vega-Sanchez V, Lat’ıf-Eugen’ın F, Soriano-Vargas E, Beaz Hidalgo R, Figueras MJ, Aguilera-Arreola MG, Castro-Escarpulli G (2014) Re-identification of Aeromonas isolates from rainbow troutand incidence of class 1 integron and β-lactamase genes. Vet Microbiol 172:528–533. https://doi.org/10.1016/j.vetmic.2014.06.012
Verner-Jeffreys DW, Welch TJ, Schwarz T, Pond MJ, Woodward MJ, Haig SJ, Rimmer GS, Roberts E, Morrison V, Baker-Austin C (2009) High prevalence of multidrug-tolerant bacteria and associated antimicrobial resistance genes isolated from ornamental fish and their carriage water. PLoS One 4:e8388. https://doi.org/10.1371/journal.pone.0008388
Wang Y, Leung PC, Qian PYGJ-D (2006) Antibiotic resistance and plasmid profile of environmental isolates of Vibrio species from Mai Po nature reserve, Hong Kong. Ecotoxicology 15:371–378. https://doi.org/10.1007/s10646-006-0078-0
Watts JEM, Schreier HJ, Lanska L, Hale MS (2017) The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mari Drugs 15:e158. https://doi.org/10.3390/md15060158
Weir M, Rajic A, Dutil L, Cernicchiaro N, Uhland FC, Mercier B, Tusevljak N (2012) Zoonotic bacteria, antimicrobial use and antimicrobial resistance in ornamental fish: a systematic review of the existing researchand survey of aquaculture-allied professionals. Epidemiol Infect 140:192–206. https://doi.org/10.1017/S0950268811001798
Witte W (1998) Medical consequences of antibiotic use in agriculture. Science 279:996–997. https://doi.org/10.1126/science.279.5353.996
World Health Organization (WHO) 2006. Report of a Joint FAO/OIE/WHO Expert Consultation on antimicrobial use in aquaculture and antimicrobial resistance, Seoul, Republic of Korea. Available at: http://www.who.int/topics/foodbornediseases/aquaculturerep1316june2006.pdf
Wu YR, Gong QF, Fang H, Liang WW, Chen M, He RJ (2013) Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol 34:220–227. https://doi.org/10.1016/j.fsi.2012.10.020
Xiao JF, Wang QY, Liu Q, Wang X, Liu H, Zhang YX (2009) Isolation and identification of fish pathogen Edwardsiella tarda from mariculture in China. Aquac Res 40:13e7. https://doi.org/10.1111/j.1365-2109.2008.02101.x
Yano Y, Hamano K, Tsutsui I, Aue-Umneoy D, Ban M, Satomi M (2015) Occurrence, molecular characterization, and antimicrobial susceptibility of Aeromonas spp. in marine species of shrimps cultured at inland low salinity ponds. Food Microbiol 47:21–27. https://doi.org/10.1016/j.fm.2014.11.003
Yoo MH, Huh MD, Kim EH, Lee HH, Jeong HD (2003) Characterization of chloramphenicol acetyltransferasegene by multiplex polymerase chain reaction in multi-drug-resistant strains isolated from aquatic environ-ments.Aquaculture 217:11–21.
Yu JE, Cho MY, Kim JW, Kang HY (2012) Large antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb Pathog 52:259–266. https://doi.org/10.1016/j.micpath.2012.01.006
Zhu YG, Zhao Y, Li B, Huang CL, Zhang SY, Yu S, Chen YS, Zhang T, Gillings MR, Su JQ (2017) Continental-scale pollution of estuaries with antibiotic resistance genes. Nat Microbiol 2:162–170. https://doi.org/10.1038/nmicrobiol.2016.270
Acknowledgments
The first author acknowledges Department of Science and Technology (DST, SERB) for the Fellowship under National Postdoctoral Fellowship Scheme with Grant number PDF/2017/000378 and Director, National Bureau of Fish Genetic Resources and National Centre for Aquatic Animal Health, Cochin University of Science and Technology for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Preena, P.G., Swaminathan, T.R., Kumar, V.J.R. et al. Antimicrobial resistance in aquaculture: a crisis for concern. Biologia 75, 1497–1517 (2020). https://doi.org/10.2478/s11756-020-00456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00456-4