Abstract
Genetic stability is highly important in terms of endangered species gene banks. Culture and cold stresses under in vitro conditions may lead to genetic variability. Many methods and possibilities to design appropriate starters for DNA-fingerprinting purposes increase cost and time consumption. Furthermore, multiplicity of possible primers makes it difficult to standardize plant research. The aim of this study was to verify effectiveness of various methods in assessing the clonal homogeneity of Taraxacum pieninicum plantlets regenerated after long-term in vitro cold-storage and to simplify the selection of the genetic stability analysis for Asteraceae family. Inter Simple Sequence Repeats (ISSR) and Start Codon Targeted (SCoT) polymorphism assays were performed to detect DNA sequence variation. No differences were observed using 16 ISSR markers and 12 SCoT markers between regrown plantlets after storage and plants cultivated from seeds in a soil. Furthermore, SCoT markers were most effective for screening T. pieninicum genome and appeared to be highly useful for different micropropagated and endangered species of Asteraceae family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DNA based molecular markers become a versatile tools applied in various fields like taxonomy, genetic engineering and plant breeding (Chittora 2018). They are also increasingly used in research focused on the protection of endangered species by storage in in vitro culture. Quality control of micropropagated plant material has to be verify before introduction of any species into the natural environment (Kaçar et al. 2006). In vitro culture may be associated with changes in physiological, epigenetic and genetic quality, what is contrary to the main assumption of retaining the genetic integrity of the propagated plants. Genetic variations might occur in undifferentiated cells, protoplast or calli. They are generated in uncontrollable and unpredictable ways and most variations are not suitable for further usage (Butiuc-Keul et al. 2016). The most frequent changes occurring in in vitro culture refers to polyploidization, aneuploidization, chromosomal breakage, deletion, translocation, gene amplifications and mutations (Teixeira da Silva et al. 2007). Different markers and techniques are available for assess genetic stability or diversity of plants propagated both in vitro and in vivo. Techniques such as Amplified Fragment Length Polymorphism (AFLP), Inter Simple Sequence Repeats (ISSR) and Random Amplified Polymorphic DNA (RAPD) have been termed as Arbitrarily Amplified Dominant (AAD) markers. AAD markers are used for genomic DNA- fingerprinting, genetic and qualitative trait loci (QTL) mapping, population studies, phylogenetic inference and systematic studies (Gorji et al. 2011). Collard and Mackill (2009) validated in rice method based on the short conserved region flanking the ATG start codon. This method was called Start Codon Target (SCoT) polymorphism and its most significant advantage is that the primers are considered to be universal in plants. SCoT marker PCR amplification profiles indicated also dominant marker. Knowledge of the plant sequence is not required for mentioned RAPD, ISSR and SCoT techniques, that is why these methods are chosen predominatingly. For epigenetic variation conventional markers are not suitable. In this case markers such as Methylation Sensitive Amplified Polymorphism (MSAP) or methylated-sensitive Restriction Fragment Length Polymorphism (RFLP) need to be used. Moreover, in vitro culture may induce transpositions of transposable elements, what could be detected using retrotransposon-based molecular markers (Sequence-Specific Amplified Polymorphism S-SAP; Inter-Retrotransposon Amplified Polymorphism IRAP; Retrotransposon-Microsatellite Amplified Polymorphism REMAP) (Teixeira da Silva et al. 2007). Despite the large number of available techniques, the knowledge of the sequence and amount of the isolated DNA are decisive.
Taraxacum pieninicum Pawł. is critically endangered species of the Asteraceae family, occurring only in one locality in The Pieniny Mts. (Poland) (Zarzycki and Szeląg 2006). Classical methods of T. pieninicum protection appear to be insufficient due to the number of individuals, limited availability and vitality of the seeds. Micropropagation procedure for T. pieninicum has been developed (Trejgell et al. 2013), although this method is labor-consuming, costly and fraught with the risk of contamination every 4 weeks. Therefore, system for shoot tips and synthetic seeds storage in slow growth conditions was presented in previous studies (Kamińska et al. 2016, 2018), in which plant material could be stored even for 12 months without passages.
The aim of this study was (1) to compare ISSR and SCoT profiles obtained with chosen primers and (2) to assess the suitability of the in vitro cultivation and storage systems of T. pieninicum to plantlets introduction into in vivo conditions. Additionally we want to verify universality of the SCoT markers using sequences from the research on chrysanthemums from Asteraceae family.
Materials and methods
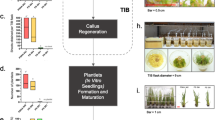
Synthetic seeds of T. pieninicum were obtained by encapsulation of the shoot tips using 3% sodium alginate and 100 mM CaCl2. Synseeds were stored on MS medium (Murashige and Skoog 1962) for 9 and 12 months at 4 °C under reduced light or in the darkness as it was described in our previous paper (Kamińska et al. 2018). For genetic analysis plantlets after storage were regrown in optimal in vitro conditions and acclimatized to ex vitro conditions according to the procedure shown in Fig. 1. DNA was isolated from young leaves of randomly chosen plants (100 mg) using modified Doyle and Doyle (1987) method. Plants germinated from natural seeds and grown in pots were used as a control. The reaction mixture for PCR contained: 1× Ammonium-Reaction buffer, 3 mM MgCl2, 10 nmol of each dNTP, 0.5 μM primer, 50 mg DNA and 1 unit of Maximo DFS-Plus Taq DNA Polymerase DNA-free (GeneOn). PCR reactions were carried out in a thermocycler (AG 22331, Eppendorf, Hamburg, Germany) with an initial denaturation of the DNA at 94 °C for 4 min and then 35 cycles of amplification consisting of denaturation at 94 °C for 20 s, primer annealing at Tm calculated for each primer for 20 s and primer extension at 72 °C for 3 min. The final extension was performed at 72 °C for 5 min. The PCR products were electrophoresed in 1.5% agarose gel (carried out at 100 V) using 1× TBE running buffer and SYBR® Green Master Mix (Applied Biosystems, CA, USA). The size of amplicons was visualized under UV light and estimated using DNA ladder (O’GeneRuler™ DNA ladder Mix, Fermentas). Images were captured using ChemiDoc™XRS Gel Documentation system (Bio-Rad, CA, USA). Bands were analyzed using Quantity One® version 4.6.2. software (Bio-Rad, CA, USA). All the generated patterns were repeated at least three times in order to obtain reproducible data. The primers for ISSR were chosen on the basis of analysis of the genus Taraxacum collected in Korea (Ryu and Bae 2012) and for SCoT analysis primers were chosen from paper focused on genetic diversity of Chrysanthemum morifolium belonging to the Asteraceae family (Feng et al. 2016). After primer verification on control plants, 16 from 19 ISSR markers and each of 12 SCoT markers were used in analyses.
Plant material for DNA isolation; schematic procedure of storage and regrowth of Taraxacum pieninicum synthetic seeds (Kamińska et al. 2018) (BA – Benzylaminopurine, NAA - Naphthalene acetic acid, PGR – Plant Growth Regulator)
Results and discussion
The oligonucleotide sequences of the used ISSR and SCoT primers and the results were summarized in Table 1. Examples of ISSR and SCoT amplifications were shown in Fig. 2, representing band patterns obtained with primers No. 14, 15 and No. 3, 5, respectively. Analysis with ISSR and SCoT markers gave a total of 51 and 83 monomorphic bands, regardless of the storage conditions. In none of the analyzed plantlets and markers, polymorphic bands were obtained. A single ISSR marker generated an average of 2.7 bands. The number of product varied from 1 (ISSR – 5, 7, 8 and 16) to 7 (ISSR - 14) (Fig. 2a). In this analysis length of obtained product ranged from 400 to 3500 bp (Table 1). In the SCoT analysis 6.9 bands were generated per primer, with length ranged between 300 and 3500 bp depending on the primer sequence. In this case a minimum number of bands was 4 (SCoT - 8), in turn maximum was 10 (SCoT – 5) (Table 1, Fig. 2d). In the previous paper 21 RAPD markers generated 67 products, what in average gave 3.2 bands per primer (Kamińska et al. 2018). Comparing all these 3 techniques, SCoT markers were the most effective for screening T. pieninicum genome. Similar results, where ScoT analysis was most effective than for ISSR and RAPD, were obtained for Alhagi maurorum (Agarwal et al. 2015) and Solanum sp. (Gorji et al. 2011).
PCR-amplified band pattern generated in ISSR analysis by primers No. 14 (a) and 15 (b) and in SCoT analysis by primers No. 3 (c) and 5 (d) on DNA of Taraxacum pieninicum plantlets regrown after cold-storage: molecular marker (100–10,000 bp) (M), control plants (non-stored, conventionally cultivated in soil) (lines 1), plants stored for 9 months under light conditions (lines 2–3), in the dark (lines 4–5), and for 12 months under light conditions (lines 6–7) and in the dark (8–9)
Conditions of in vitro culture, especially long-term exposure of plant material to cytokinins, and indirect regeneration may lead to somaclonal variations (Leva et al. 2012; Kaeppler and Phillips 1993). Somaclonal variation can be manifested at various levels: phenotypic, cytological, biochemical and genetic/epigenetic (Kaeppler et al. 2000). However, complex analysis of genetic stability is necessary due to the fact that visible morphological variation may occur less frequently than at the DNA level (Smýkal et al. 2007; Machczyńska et al. 2015). Tissue culture-induced variation was observed in regenerants (Cao et al. 2016), but it could be also observed in progeny of the in vitro regenerated plants (Machczyńska et al. 2015; Lorz and Scowcroft 1983). Shoot tips and nodal segments are considered as the most genetic stable explant (Upadhyay et al. 2014). However, despite the fact that this organized tissue was used for micropropagation and storage of T. pieninicum, in our studies callus was formed at the shoot clusters base, therefore cytokinin (benzylaminopurine) stimulated not only proliferation of pre-existing axillary meristems, but also might induced de novo differentiation of adventitious buds from callus. Disruption of normal cellular control during callus phase may increase frequency of variation (Guo et al. 2007) what was reported in Vanilla planifolia (Ramírez-Mosqueda and Iglesias-Andreu 2015). Additionally exposure to cold stress might result in the excessive generation of reactive oxygen species (ROS) what can increase probability of the somaclonal variation (Cassells and Curry 2001) and it seems to be a reasonable view that prolonged cold exposure may increase the likelihood of genetic changes. In Pistacia lentiscus genetic variation was reported after multiple microshoots storage for 6 months in the darkness (Koç et al. 2014). Here we demonstrated that even 12-months-long storage of encapsulated T. pieninicum shoot tips under different light conditions did not generate any genetic instability in terms of used primers in the different technique. Usage more than one DNA-fingerprinting techniques ensure a much wider coverage of the analyzed genome (Devi et al. 2014) especially that results generated with ISSR or RAPD are usually based on the non-coding regions of DNA, whereas SCoT technique is correlated to functional genes (Collard and Mackill 2009). Exclusion of possible deviance from true-to-type mother plant is particularly important in terms of endangered species gene banks. In our previous work RAPD analysis indicated no genetic differences in plants obtained from stored synseeds (Kamińska et al. 2018). However, RAPD is considered as a non-repetitive technique and standardization of procedure is needed every time because of its sensitivity to the reaction conditions. Band profiles obtained in RAPD analysis may vary between (and even within) laboratories after conditions, personnel or equipment changes (Arif et al. 2010). A comparable number and size of products to previous study was reported here for ISSR analysis, while with SCoT markers over 2 times more products per primer were obtained. Compared to RAPD, ISSR and SCoT techniques are more reproducible due to the use of the longer primers (Xiong et al. 2011). Furthermore, ISSR markers are related to microsatellite regions, which mostly showed higher rate of mutations in comparison to the whole genome (Bublyk et al. 2013). Our findings indicated that each SCoT primer designed according to Feng et al. (2016) was suitable for T. pieninicum. All the ISSR and SCoT profiles from acclimatized plantlets after storage and regrowth were monomorphic and comparable to the control plants. Considering number of bands obtained for each ISSR marker high genetic diversity was noted among Taraxacum family. Here, different band pattern was formed for T. pieninicum than for T. officinale and T. coreanum (Ryu and Bae 2012). In this work it was also noted that plants collected from different areas showed high polymorphism.
According to the results, it can be concluded that micropropagation and long-term synseeds storage systems used for T. pieninicum do not generate genetic changes on the basis of used primers. Therefore, plants after storage in in vitro culture might be used for the reintroduction of this endangered species. It is essential to broaden and use the genetic base for rare plants, that will improve the work focused on maintaining plants biodiversity without introducing unstable species. Furthermore, SCoT primers seems to be universal for members of Asteraceae family among which there are lots of critically endangered species conserved in in vitro culture. Our results might help in the initial phase of regular control of genetic homogeneity of micropropagated plantlets without increasing costs of the plants conservation systems.
Abbreviations
- AAD:
-
Arbitrarily Amplified Dominant
- AFLP:
-
Amplified Fragment Length Polymorphism
- IRAP:
-
Inter-Retrotransposon Amplified Polymorphism
- ISSR:
-
Inter Simple Sequence Repeats
- MSAP:
-
Methylation Sensitive Amplified Polymorphism
- QTL:
-
Qualitative Trait Loci
- RAPD:
-
Random Amplified Polymorphic DNA
- REMAP:
-
Retrotransposon-Microsatellite Amplified Polymorphism
- RFLP:
-
Restriction Fragment Length Polymorphism
- ScoT:
-
Start Codon Targeted
- S-SAP:
-
Sequence-Specific Amplified Polymorphism
References
Agarwal T, Gupta AK, Patel AK, Shekhawat NS (2015) Micropropagation and validation of genetic homogeneity of Alhagi maurorum using SCoT, ISSR and RAPD markers. Plant Cell Tissue Organ Cult 120:313–323. https://doi.org/10.1007/s11240-014-0608-z
Arif IA, Bakir MA, Khan HA, Al Farhan AH, Al Homaidan AA, Bahkali AH, Al Sadoon M, Shobrak M (2010) A brief review of molecular techniques to assess plant diversity. Int J Mol Sci 11:2079–2096. https://doi.org/10.3390/ijms11052079
Bublyk OM, Andreev IO, Kalendar RN, Spiridonova KV, Kunakh VA (2013) Efficiency of different PCR-based marker systems for assessment of Iris pumila genetic diversity. Biologia 68:613–620. https://doi.org/10.2478/s11756-013-0192-4
Butiuc-Keul A, Farkas A, Cristea V (2016) Genetic stability assessment of in vitro plants by molecular markers. Studia Universitatis Babeş-Bolyai Biologia 61:107–114
Cao Z, Sui S, Cai X, Yang Q, Deng Z (2016) Somaclonal variation in ‘Red Flash’ caladium: morphological, cytological and molecular characterization. Plant Cell Tissue Organ Cult 126:269–279. https://doi.org/10.1007/s11240-016-0996-3
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tiss Organ Cult 64:145–157. https://doi.org/10.1023/A:1010692104861
Chittora M (2018) Assessment of genetic fidelity of long term micropropagated shoot cultures of Achras sapota L. var. ‘Cricket Ball’ as assessed by RAPD and ISSR markers. Indian J Biotechnol 17:492–495
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86–93. https://doi.org/10.1007/s11105-008-0060-5
Devi SP, Kumania S, Rao SR, Tandon P (2014) Single primer amplification reaction (SPAR) methods reveal subsequent increase in genetic variations in micropropagated plants of Nepenthes khasiana Hook. f. maintained for three consecutive regenerations. Gene 538:23–29. https://doi.org/10.1016/j.gene.2014.01.028
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Feng SG, He RF, Jiang MY, Lu JJ, Shen XX, Liu JJ, Wang ZA, Wang HZ (2016) Genetic diversity and relationship of medicinal Chrysanthemum morifolium revealed by start codon targeted (SCoT) markers. Sci Hort 201:118–123. https://doi.org/10.1016/j.scienta.2016.01.042
Gorji AM, Poczai P, Polgar Z, Taller J (2011) Efficiency of arbitrarily amplified dominant markers (SCOT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato. Am J Potato Res 88:226–237. https://doi.org/10.1007/s12230-011-9187-2
Guo WL, Wu R, Zhang YF, Liu XM, Wang HY, Gong L, Zhang ZH, Liu B (2007) Tissue culture-induced locus-specific alteration in DNA methylation and its correlation with genetic variation in Codonopsis lanceolata Benth. et hook. f. Plant Cell Rep 26:1297–1307. https://doi.org/10.1007/s00299-007-0320-0
Kaçar YA, Byrne PF, Teixeira da Silva JA (2006) Molecular markers in plant tissue culture. In: Teixeira da Silva JA (ed) Floriculture, Ornamental and plant biotechnology: advances and topical issues, Vol II. Global Science Books, United Kingdom, Isleworth, pp 444–449
Kaeppler SM, Phillips RL (1993) DNA methylation and tissue culture-induced variation in plants. In vitro Cell Dev Biol Plant 29:125–130. https://doi.org/10.1007/BF02632283
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188. https://doi.org/10.1023/A:1006423110134
Kamińska M, Skrzypek E, Wilmowicz E, Tretyn A, Trejgell A (2016) Effect of light conditions and ABA on cold storage and post-storage propagation of Taraxacum pieninicum. Plant Cell Tissue Organ Cult 127:25–34. https://doi.org/10.1007/s11240-016-1026-1
Kamińska M, Gołębiewski M, Tretyn A, Trejgell A (2018) Efficient long-term conservation of Taraxacum pieninicum synthetic seeds in slow growth conditions. Plant Cell Tissue Organ Cult 132:469–478. https://doi.org/10.1007/s11240-017-1343-z
Koç İ, Akdemir H, Onay A, Çiftçi YÖ (2014) Cold-induced genetic instability in micropropagated Pistacia lentiscus L. plantlets. Acta Physiol Plant 36:2373–2384. https://doi.org/10.1007/s11738-014-1610-0
Leva AR, Petruccelli R, Rinaldi LMR (2012) Somaclonal variation in tissue culture: a case study with olive. In: Leva A, Rinaldi LMR (eds) Recent advances in plant in vitro culture. InTech Pub, pp 123–150. https://doi.org/10.5772/50367
Lorz H, Scowcroft WR (1983) Variability among plants and their progeny regenerated from protoplasts of Su/su heterozygotes of Nicotiana tabacum. Theor Appl Genet 66:67–75. https://doi.org/10.1007/BF00281851
Machczyńska J, Zimny J, Bednarek PT (2015) Tissue culture-induced genetic and epigenetic variation in triticale (x Triticosecale spp. Wittmack ex A. Camus 1927) regenerants. Plant Mol Biol 89:279–292. https://doi.org/10.1007/s11103-015-0368-0
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ramírez-Mosqueda MA, Iglesias-Andreu LG (2015) Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia jacks. Plant Cell Tissue Organ Cult 123:657–664. https://doi.org/10.1007/s11240-015-0868-2
Ryu J, Bae CH (2012) Genetic diversity and relationship analysis of genus Taraxacum accessions collected in Korea. Korean J Plant Res 25:329–338. https://doi.org/10.7732/kjpr.2012.25.3.329
Smýkal P, Valledor L, Rodríguez R, Griga M (2007) Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.). Plant Cell Rep 26:1985–1998. https://doi.org/10.1007/s00299-007-0413-9
Teixeira da Silva JA, Bolibok H, Rakoczy-Trojanowska M (2007) Molecular markers in micropropagation, tissue culture and in vitro plant research. Genes Genom Genom 1:66–72
Trejgell A, Chernetskyy M, Podlasiak J, Tretyn A (2013) An efficient system for regenerating Taraxacum pieninicum Pawł. from seedling explants. Acta Biol Cracov Ser Bot 55:73–79. https://doi.org/10.2478/abcsb-2013-00013
Upadhyay R, Kashyap P, Singh C, Tiwari KN, Singh K, Singh M (2014) Assessment of factors on shoot proliferation potential of nodal explants of Phyllanthus fraternus and assessment of genetic fidelity of micropropagated plants using RAPD marker. Biologia 69:1685–1692. https://doi.org/10.2478/s11756-014-0484-3
Xiong FQ, Zhong RC, Han ZQ, Jiang J, He LQ, Zhuang WJ, Tang RH (2011) Start codon targeted polymorphism for evaluation of functional genetic variation and relationship in cultivated peanut (Arachis hypogaea L.) genotypes. Mol Biol Rep 38:3487–3494. https://doi.org/10.1007/s11033-010-0459-6
Zarzycki K, Szeląg Z (2006) Red list of the vascular plants in Poland. In: Mirek Z (ed) Red list of plants and fungi in Poland. W. Szafer Institute of Botany, Polish Academy of Sciences, Cracow, pp 9–20
Acknowledgments
This project was supported by funds from Ministry of Science and Higher Education (PL) for young scientists from Nicolaus Copernicus University in Toruń (2827-B).
Author information
Authors and Affiliations
Contributions
Monika Kamińska designed and carried out all the experiments, analyzed the data and wrote the manuscript, Alina Trejgell helped in analysis of the data and in writing the manuscript, and Andrzej Tretyn helped in preparing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamińska, M., Tretyn, A. & Trejgell, A. Genetic stability assessment of Taraxacum pieninicum plantlets after long-term slow growth storage using ISSR and SCoT markers. Biologia 75, 599–604 (2020). https://doi.org/10.2478/s11756-019-00377-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00377-x