Abstract
Transposable elements constitute a large fraction of plant genomes and represent a powerful marker tool for genetic diversity studies. Here, the retrotransposon-based marker method inter primer binding sites (iPBS) was used to assess the genetic variation and intergeneric hybrid dynamics in the family Asteraceae by studying genera Helianthus, Echinaceae, Tagetes, Tithonia and Verbesina. Two selected iPBS primers (2222 and 2224) detected intergeneric polymorphism in the range 44.8% - 93.3% (mean 70%) and 85.7% - 100% (mean 89.5%) respectively. Moreover, iPBS markers allowed the genetic discrimination at within-species level between varieties of H. annuus (35.7% and 19.1%) but also between single cross’s segregating intergeneric hybrids (28.6% and 40%). The inheritance of iPBS markers and the parental genomes respectively in intergeneric hybrids of H. annuus has been manifested by the non-random elimination of markers mainly of origin of wild species and the preferential inheritance of markers unique to H. annuus. Such instability evidences genomic reconstruction involving LTR elements. In conclusion, the iPBS method stands as a reliable approach for the evaluation of genetic diversity of Asteraceae germplasms and perspective for use in the breeding practice of sunflower and related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wide (intergeneric and interspecific) hybridizations commonly have a great potential for crop improvement by widening the genetic base from which plant breeder can select desirable traits (Liu et al. 2005). In genus Helianthus, there has been an increasing interest in the use of wild sunflower relatives - a valuable source of desirable agronomic traits (Breton et al. 2012; Vassilevska-Ivanova et al. 2013, 2014, 2015, 2018; Liu et al. 2017; Seiler et al. 2017). However, most of the wild relative species remain untapped as usable germplasm. The reason for this is that the genus Helianthus has no close relatives; the pattern of distributions of phylogenetic markers suggested that wide hybridization is not uncommon within the larger group to which the common sunflower belongs (Seiler et al. 2017).
Transposable elements (TEs) are well suited as molecular markers to monitor natural and stress-induced genetic diversity (Schulman et al. 2004). The reason for this is their ubiquitous distribution in plant genomes (Schnable et al. 2009; Choulet et al. 2014) and susceptibility to activation and transposition in response to stress such as pathogen attacks, wounding, extreme temperature etc. (Wessler 1996; Grandbastien 1998). In this line, intergeneric hybridizions often appears as a „genome shock “capable of triggering changes in gene regulation and chromosome rearrangements (Adams et al. 2003; Paun et al. 2007; Morgan et al. 2011) and a substantial part of these genome alterations are attributed to the mobilization of TEs (Otto 2007; Kawakami et al. 2011).
The genome of sunflower H. annuus is composed of more than 81% of TEs, mainly represented by long terminal repeat (LTR)-retrotransposons (Giordani et al. 2014). Retrotransposition events have been and are probably still occurring since the origin of this species thus playing a major role in shaping its DNA landscape (Vukich et al. 2009; Kawakami et al. 2011; Staton et al. 2012). Furthermore, genome expansion and proliferation of TEs in the genus Helianthus have been largely influenced by interspecific hybridization events as shown for hybrids of H. annuus and annual wild sunflower species H. petiolaris (Staton et al. 2009; Ungerer et al. 2006, 2009). Therefore, retrotransposon-based marker methods appear attractive to be used as a fingerprinting tool in Asteraceae. However, beside two studies addressing the application of IRAP (Inter Retrotransposon Amplified Polymorphism) markers to infer patterns of evolution in the genus Helianthus (Vukich et al. 2009; Basirnia et al. 2014), comprehensive information on TE variability between different genera of the family Asteraceae is still missing particularly in relation to genome dynamics in intergeneric hybrids. Kalendar et al. (2010) developed the DNA marker system iPBS (inter primer binding sites) which allows the detection of polymoprphisms in the insertional pattern of multiple retrotransposon copies and the method has been recently used to explore the genetic diversity in various plant species (Smýkal et al. 2011; Baranek et al. 2012; Andeden et al. 2013; Mehmood et al. 2013; Guo et al. 2014; Baloch et al. 2015a, b; Nemli et al. 2015; Demirel et al. 2018; Yaldiz et al. 2018).
This study was aimed to explore: 1/ the potential of iPBS markers for fingerprinting the genetic variability at within-species and intergeneric level in the family Asteraceae and 2/ to screen patterns of intergeneric hybrid dynamics with the cultivated Helianthus annuus as a parent.
Materials and methods
Plant material and DNA extraction
Species of five Asteraceae (Compositae) genera – the sunflower Helianthus annuus (cultivars Favorit and 1114), Echinacea purpurea, Tagetes sp., Verbesina encelioides and Tithonia rotundifolia were assessed by iPBS markers. Also, five intergeneric hybrids developed after crossing H. annuus cv 1114 and wild species above were investigated: H. annuus cv 1114 x E. purpurea (HAxEp), H. annuus cv 1114 x V. encelioides (HAxVe1 and HAxVe2), H. annuus cv 1114 x T. rotundifolia (HAxTr), and H. annuus cv 1114 x Tagetes sp. (HAxTag). The hybrids between the common H. annuus and related species of Asteraceae family included here were previously described by Vassilevska-Ivanova et al. (2013, 2014, 2015, 2016). The total genomic DNA of samples was extracted from fresh 6-day-old etiolated leaves using Dneasy Plant Mini kit (Qiagen).
iPBS-retrotransposon analysis
The iPBS method identifies diverse LTR sequences and directly visualizes their polymorphism among cultivars (Kalendar et al. 2010). This method focuses on the PBS region, which is adjacent to the 5′ LTR and is conserved among different LTR retrotransposon families. Because the 3′ terminal sequence of tRNA is complementary and binds to the PBS region to initiate reverse transcription, the latter sequence is conserved across nearly all LTR retrotransposon families. Therefore, the primers for the PBS region allow the simultaneous detection of almost all types of LTR transposable elements compared with other transposon-based marker methods such as IRAP and REMAP where transposon-specific primers have to be used. Four iPBS primers previously described by Kalendar et al. (2010) were tested for amplification efficiency (Table 1). Polymerase chain reaction (PCR) was performed in 25 μL of the reaction mixture containing 50 ng of DNA template, 1X PCR buffer DreamTaq buffer, 0.25 mM dNTPs, 1 μM of primer for 12 nt primers or 0.6 μM for 18 nt primers, 1 units Taq DNA polymerase (DreamTaq, Fermentas) and 0.04 units Pfu DNA Polymerase (Fermentas). The PCR program consisted of: 1 cycle at 95 °C for 3 min; 35 cycles of 95 °C for 15 s, 53–55 °C (depending on the primer) for 60 s, and 68 °C for 60 s. The reaction was completed by a final extension at 72 °C for 5 min. Fifteen-micro-liter aliquots of PCR products were resolved by 1.7% (w/v) agarose gel electrophoresis at 80 V for 7 h in 1X STBE buffer (10X STBE: 0.25 M Tris–H3BO3, 40 mM Na2B4O7, 10 mM EDTA, pH 8.6) and detected by ethidium bromide staining. Each PCR reaction was performed in three replicates.
Data analysis
LTR-retrotransposon dynamics between Asteraceae species was evaluated through the following parameters describing the abundance and diversity of TEs: number of amplified loci, band frequencies among segregating loci and percentage of polymorphism. We also estimated the relative inheritance of iPBS markers from parents in their corresponding hybrids by monitoring the number of bands inherited from each parent, number of bands shared by both parents and number of new and lost bands (rearranged bands). The distance between samples was calculated based on the Jaccard coefficient of dissimilarity using the Excel application XLSTAT v. 2014.5.03 (Addinsoft, NY, USA). The iPBS marker binary data were resolved into networks trees with the NeighborNet algorithm (Bryant and Moulton 2004) obtained on Jaccard distances’ parameter implemented in the program SplitsTree 4 v. 4.14.4 (Huson and Bryant 2006).
Results
iPBS banding pattern and genetic diversity among investigated genera of Asteraceae
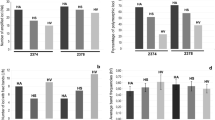
The discrimination power of four iPBS primers was assessed based on the reproducibility of banding patterns, parameters of band dynamics and the level of generated polymorphism. After the initial screening, two primers (2222 and 2224) that produced the largest number of easily scorable and reproducable bands were chosen for subsequent analysis. The amplification range was 600–4000 bp (primer 2222) and 550–5000 bp (primer 2224) (Fig. 1). A significant variability in the insertional pattern of LTR-retrotransposons both between parental species and among hybrid lines was also observed. The number of amplified loci varied between different genera with the highest number scored for both cultivars of the common sunflower H. annuus cv Favorit (24) and H. annuus cv 1114 (22). The lowest number of amplified loci was observed for wild species E. purpurea (17) and Tagetes sp. (14).
iPBS fingerprints of species of five Asteraceae genera and their intergeneric hybrids with the common sunflower H. annuus. The analyzed samples by primers 2222 (panel a) and 2224 (panel b) are as follow: 1 – HA 1114, 2 – HA Favorit 3 – E. purpurea, 4 – Tagetes sp., 5 – T. rotundifolia, 6 – V. encelioides, 7 – HAxTag, 8 – HAxEp, 9 – HAxTr, 10 – HAxVe1, 11 – HAxVe2. M – 1 kb GeneRuler (Fermentas)
The resolution power of iPBS markers allowed the genetic differentiation of analyzed species as it is illustrated by the Jaccard coefficients of dissimilarity (Table S1) and the percentage of polymorphism (number of polymorphic loci) between Asteraceae species (Table 2) presented in a pairwise manner. The iPBS markers allowed to reveal a variability at within-species level as evident for cultivars H. annuus cv 1114 and H. annuus cv Favorit. In this line, the degree of the observed polymorphisms between both H. annuus varieties was 35.7% (primer 2222) and 19.1% (primer 2224). Furthermore, segregating hybrids from one and the same cross - H. annuus x V. encelioides1 (HAxVe1) and H. annuus x V. encelioides2 (HAxVe2) are distinguished and the respective polymorphism values between HAxVe1 and HAxVe2 are 28.6% and 40%. The between-species polymorphism was in the range 44.8% - 93.3% for primer 2222 (mean 70%), and 85.7% - 100% for primer 2224 (mean 89.5%).
To further visualize the degree of genetic relationships among the representatives of the Asteraceae family and the intergeneric hybrids of H. annuus cv 1114, the binary data were used to construct a network tree with the NeighborNet algorithm (Fig. 2). iPBS analysis provided consistent and similar clustering trees where hybrids and parental species spitted into separate branches. The common sunflower H. annuus 1114 was significantly closer to its hybrids and their genetic similarity is also supported by the higher number of markers unique to this parent and transmitted to hybrids (Fig. 3).
Inheritance of iPBS markers in hybrids assessed by primers 2222 (a) and 2224 (b). The sunflower H. annuus cv 1114 was the male parent (P1) in all crosses. P1 – markers unique for P1, P2 – markers unique for the second parent, P1/P2 – markers present in both parents, New- markers appeared in hybrids but not presented in parents
Patterns of TE inheritance in intergeneric hybrids of sunflower Helianthus annuus
We compared the iPBS banding patterns of intergeneric hybrids with respective parents H. annuus cv 1114 and wild species Echinacea purpurea, Verbesina encelioides, Tagetes sp. and Tithonia rotundifolia. The data allowed us to estimate the pattern of inheritance of parental iPBS markers in hybrid plants. In all hybrids, the markers which are unique for H. annuus cv 1114 (P1) were predominantly inherited by the hybrids (Fig. 3). In addition, the second major fraction comprised markers inherited from both parents (biparental inheritance) and this trend was more pronounced when using primer 2222. A characteristic feature of hybrid genomes is the amplification of new bands that are not present in both parents. Similarly, there were bands lost (not transferred to the progeny of each cross) in the hybrids but present in one or both parents.
The overall pattern of band rearrangement (loss and gain of new bands) in hybrids in reference to their respective parents is shown in Table 3. The loss of parental bands in hybrids was found to be more frequent than the gain of new ones. For both primers 2222 and 2224, the fraction of rearranged bands in the hybrid H. annuus cv 1114 x Tagetes sp. (HAxTag) was solely manifested by lost bands. The genetic distance between parents has been generally assumed to be a factor affecting genetic disbalance in respective hybrids. In our study, the hybrid H. annuus x T. rotundifolia (HAxTr) displayed the highest number of lost bands with highest genetic distance detected between parents.
Discussion
The iPBS method is a high-throughput approach for identification of genetic variability related to the insertion/loss of LTR retrotransposons and/or to DNA sequence variations (nucleotide substitutions or indels) in adjacent regions. To our knowledge, this pilot study is the first attempt to apply iPBS markers for fingerprinting among-genera diversity within the family Asteraceaе and to assess genome dynamics in response to intergeneric hybridizations. The iPBS technique allowed the amplification of a large number of loci and a substantial polymorphism was observed at both intergeneric and within-species level. The hybrids were found to be genetically closer to the female parent H. annuus cv 1114 than to the respective male parent (pollen source) - E. purpurea, Tagetes sp., V. encelioides and T. rotundifolia. These finding are in good agreement with previous studies on these hybrids manifesting phenotype features that are intermediate between both parents or closer to the cultivated sunflower (Vassilevska-Ivanova et al. 2015, 2016). High efficiency of genetic discrimination was observed between single species cultivars but also between hybrid lines segregating from one and the same cross (common parental origin). This fact highlights the usefulness of the iPBS method for fingerprinting and related genetic analyses for the purpose of breeding programs involving representatives of the family Asteraceae.
According to the “genome shock” hypothesis of Barbara McClintock (1984), genetic incompatibilities unmasked by hybridization are assumed to induce a programmed response leading to chromosomal rearrangements, activation of silent transposable elements, elimination of DNA sequences and epigenetic silencing (Barton 2001; Chen 2007; Xiong et al. 2011; Delgado et al. 2017). Although alterations in gene expression and cytosine methylation have been previously reported in wide crosses between intergeneric species in Asteraceae (Hegarty et al. 2006, 2008; Tate et al. 2006; Wang et al. 2014), patterns of TE dynamics in interegeneric hybrids having H. annuus as a parent have not been investigated by TE marker methods so far. In our study, the hybrids are characterized by a significant rate of band rearrangements. Although several novel bands were detected, the loss of parental bands is predominant in intergeneric hybrids. The amplification and removal of LTR-retrotransposons are one of the earliest responses of the genome to wide hybridization or allopolyploidy (Shaked et al. 2001). Sequence elimination has been reported in rice (Ma et al. 2004), wheat (Shaked et al. 2001; Kashkush et al. 2002), Tragopogon spp. (Tate et al. 2006; Koh et al. 2010), Cucumis sp. (Chen et al. 2007), and Brassica sp. (Song et al. 1995). It is likely that the non-random elimination of sequences might constitute a way to homogenize the divergent genomes (Feldman et al. 1997; Comai 2000). This process is supposed to be highly dependent on the divergence level of parental genomes. The highest values of lost fragments were observed for the hybrid H. annuus x T. rotundifolia whose parents possess the highest genetic distance (Table 3). However, further studies on a larger collection of hybrid progenies should be performed in order to make conclusions about the actual presence of such a trend.
A crucial step in the application of the iPBS method is the preliminary screening and selection of iPBS primers that are specific and informative for a particular plant species in aspect of the amplification rate and the level of polymorphism (Kalendar et al. 2010). The fingerprinting analysis is based on the scoring of fingerprinting bands and it assumes that bands with the same size correspond to the same locus. However, similarity in band size does not necessarily indicate identity in sequence content, especially when interspecific or intergeneric data are compared. The reason for this is the chance of occurrence of homoplasy among different genera for dominant marker systems such as the iPBS method. The use of a larger dataset (larger number of scored markers) is the way that may reduce this limitation and minimize the bias in the interpretation of genetic relationships. Although such a bias may exist to some extent in our study, we are confident that the use of iPBS data from primers 2222 and 2224 that generated reproducible and clear patterns with high number of bands provides a reliable estimation of the real genetic patterns of analyzed species and hybrids. Furthermore, the selected primers are from the size group of 18-mers and it was previously reported that iPBS primers of this size were more efficient than 12–13-mer primers (Guo et al. 2014; Kalendar et al. 2010).
The interspecific and intergeneric hybridizations have diversified the genome of the cultivated sunflower H. annuus and represent a breeding approach to employ wild relatives as donors of new allele diversity for better biotic and abiotic stress resistance, plant architecture, oil content and yield. In this process, the introduction of novel genetic markers systems is of great importance for sunflower breeders to speed up the selection process. The present study showed that the iPBS method, upon optimization, can be efficiently used in molecular breeding of Helianthus annuus and its wild genera both for shedding light on genome composition in hybrids but also to study the interplay between level of parental distances and expression of agricultural traits. The gathered knowledge from the present investigation constitutes a platform for further studies on genetic impacts of intergeneric hybridizations that may be important for evolutionary studies and resolving phylogenetic relationships not only in the genus Helianthus but also in other genera.
Abbreviations
- TEs:
-
Transposable Elements
- IRAP:
-
Inter Retrotransposon Amplified Polymorphism
- iPBS:
-
inter Primer Binding Sites
- REMAP:
-
Retrotransposon Microsatellite Amplified Polymorphism
References
Adams KL, Cronn R, Percifield R, Wendel JF (2003) Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proc Natl Acad Sci U S A 100:4649–4654. https://doi.org/10.1073/pnas.0630618100
Andeden EE, Baloch FS, Derya M, Kilian B, Özkan H (2013) iPBS-Retrotransposons-based genetic diversity and relationship among wild annual Cicer species. J Plant Biochem Biotechnol 22:453–466. https://doi.org/10.1007/s13562-012-0175-5
Baloch FS, Alsaleh A, de Miera LES, Hatipoğlu R, Ciftci V, Karakoy T, Yıldız M, Ozkan H (2015a) DNA based iPBS-retrotransposon markers for investigating the population structure of pea (Pisum sativum) germplasm from Turkey. Biochem Syst Ecol 61:244–252. https://doi.org/10.1016/j.bse.2015.06.017
Baloch FS, Derya M, Andeden EE, Alsaleh A, Comertpay G, Kilian B, Ozkan H (2015b) Inter-primer binding site retrotransposon and inter-simple sequence repeat diversity among wild Lens species. Biochem Syst Ecol 58:162–168. https://doi.org/10.1016/j.bse.2014.12.002
Baranek M, Meszaros M, Sochorova J, Čechova J, Raddova J (2012) Utility of retrotransposon-derived marker systems for differentiation of presumed clones of the apricot cultivar Velkopavlovicka. Sci Hortic 143:1–6. https://doi.org/10.1016/j.scienta.2012.05.022
Barton NH (2001) The role of hybridization in evolution. Mol Ecol 10:551–568. https://doi.org/10.1046/j.1365-294x.2001.01216.x
Basirnia A, Darvishzadeh R, Abdollahi MB (2014) Retrotransposon insertional polymorphism in sunflower (Helianthus annuus L.) lines revealed by IRAP and REMAP markers, Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology: Official Journal of the Societa Botanica Italiana. https://doi.org/10.1080/11263504.2014.970595
Breton C, Gil A, Wargnier J, Seriyes H, Berville A (2012) Transfer of architectural traits from perennial Helianthus mollis Lam. to sunflower (H. annuus L.) and localisation of introgression. Euphytica 186(2):557–572. https://doi.org/10.1007/s10681-012-0656-6
Bryant D, Moulton V (2004) Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol 21:255–265. https://doi.org/10.1007/3-540-45784-4_28
Chen ZJ (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58:377–406. https://doi.org/10.1146/annurev.arplant.58.032806.103835
Chen L, Lou Q, Zhuang Y, Chen J, Zhang X, Wolukau JN (2007) Cytological diploidization and rapid genome changes of the newly synthesized allotetraploids Cucumis x hytivus. Planta 225:603–614. https://doi.org/10.1007/s00425-006-0381-2
Choulet F, Alberti A, Theil S et al (2014) Structural and functional partitioning of bread wheat chromosome 3B. Science 345:1249721. https://doi.org/10.1126/science.1249721
Comai L (2000) Genetic and epigenetic interactions in allopolyploid plants. Plant Mol Biol 43:387–399. https://doi.org/10.1023/A:1006480722854
Delgado A, Carvalho A, Martín AC, Martín A, Lima-Brito J (2017) Genomic restructuring in F1 Hordeum chilense × durum wheat hybrids and corresponding hexaploid tritordeum lines revealed by DNA fingerprinting analyses. J Genet 96:e13–e24. https://doi.org/10.1007/s12041-017-0772-0
Demirel U, Tındaş I, Yavuz C, Baloch FS (2018) Assessing genetic diversity of potato genotypes using inter-PBS retrotransposon marker system. Plant Genet Res 16:137–145. https://doi.org/10.1017/S1479262117000041
Feldman M, Liu B, Segal G, Abbo S, Levy AA, Vega JM (1997) Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics 147:1381–1387
Giordani T, Cavallini A, Natali L (2014) The repetitive component of the sunflower genome. Curr Plant Biol 1:45–54. https://doi.org/10.1016/j.cpb.2014.05.001
Grandbastien MA (1998) Activation of plant retrotransposons under stress conditions. Trends Plant Sci 3:181–187. https://doi.org/10.1016/S1360-1385(98)01232-1
Guo DL, Guo MX, Hou XG, Zhang GH (2014) Molecular diversity analysis of grape varieties based on iPBS markers. Bioc Syst Ecol 52:27–32. https://doi.org/10.1016/j.bse.2013.10.008
Hegarty MJ, Barker GL, Wilson ID, Abbott RJ, Edwards KJ, Hiscock SJ (2006) Transcriptome shock after interspecific hybridization in Senecio is ameliorated by genome duplication. Curr Biol 16:1652–1659. https://doi.org/10.1016/j.cub.2006.06.071
Hegarty MJ, Barker GL, Brennan AC, Edwards KJ, Abbott RJ, Hiscock SJ (2008) Changes to gene expression associated with hybrid speciation in plants: further insights from transcriptomic studies in Senecio. Philos Trans R Soc Lond Ser B Biol Sci 363:3055–3069. https://doi.org/10.1098/rstb.2008.0080
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. https://doi.org/10.1093/molbev/msj030
Kalendar R, Antonius K, Smýkal P, Schulman AH (2010) iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theor Appl Genet 121:1419–1430. https://doi.org/10.1007/s00122-010-1398-2
Kashkush K, Feldman M, Levy AA (2002) Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160:1651–1659
Kawakami T, Dhakal P, Katterhenry AN, Heatherington CA, Ungerer MC (2011) Transposable element proliferation and genome expansion are rare in contemporary sunflower hybrid populations despite widespread transcriptional activity of LTR retrotransposons. Genome Biol Evol 3:56–167. https://doi.org/10.1093/gbe/evr005
Koh J, Soltis PS, Soltis DE (2010) Homeolog loss and expression changes in natural populations of the recently and repeatedly formed allotetraploid Tragopogon mirus (Asteraceae). BMC Genomics 11:97. https://doi.org/10.1186/1471-2164-11-97
Liu J, Xu X, Xiuxin DX (2005) Intergeneric somatic hybridization and its application to crop genetic improvement. Plant Cell Tissue Organ Cult 82:19–44. https://doi.org/10.1007/s11240-004-6015-0
Liu Z, Seiler GJ, Gulya TJ, Feng J, Rashid KY, Cai X, Jan CC (2017) Triploid production from interspecific crosses of two diploid perennial Helianthus with diploid cultivated sunflower (Helianthus annuus L.). G3 (Bethesda) 37(4):1097–1108. https://doi.org/10.1534/g3.116.036327
Ma J, Devos KM, Bennetzen JL (2004) Analyses of LTR retrotransposon structures reveal recent and rapid genomic DNA loss in rice. Genome Res 14:860–869. https://doi.org/10.1101/gr.1466204
McClintock B (1984) The significance of responses of the genome to challenge. Science 226:792–801. https://doi.org/10.1126/science.15739260
Mehmood A, Jaskani MJ, Ahmad S, Ahmad R (2013) Evaluation of genetic diversity in open pollinated guava by iPBS primers. Pak J Agric Sci 50:591–597
Morgan ER, Timmerman-Vaughan GM, Conner AJ, Griffin WB, Pickering R (2011) Plant interspecific hybridization: outcomes and issues at the intersection of species. Plant Breed Rev 34:161–220. https://doi.org/10.1002/9780470880579.ch5
Nemli S, Kianoosh T, Tanyolac MB (2015) Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) accessions through retrotransposon based interprimer binding sites (iPBSs) markers. Turk J Agric For 39:940–948. https://doi.org/10.3906/tar-1505-59
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462. https://doi.org/10.1016/j.cell.2007.10.022
Paun O, Fay MF, Soltis DE, Chase MW (2007) Genetic and epigenetic alterations after hybridization and genome doubling. Taxon 56:649–656. https://doi.org/10.2307/25065850
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S et al (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326:1112–1115. https://doi.org/10.1126/science.1178534
Schulman AH, Flavell AJ, Ellis THN (2004) The application of LTR retrotransposons as molecular markers in plants. Methods Mol Biol 260:145–173. https://doi.org/10.1007/978-1-61779-603-6_7
Seiler G, Qi LL, Marek LF (2017) Utilization of sunflower crop wild relatives for cultivated sunflower improvement. Crop Sci 57:1083–1101. https://doi.org/10.2135/cropsci2016.10.0856
Shaked H, Kashkush K, Ozkan H, Feldman M, Levy AA (2001) Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13:1749–1759. https://doi.org/10.1105/TPC.010083
Smýkal P, Bačová-Kerteszová N, Kalendar R, Corander J, Schulman AH, Pavelek M (2011) Genetic diversity of cultivated flax (Linum usitatissimum L.) germplasm assessed by retrotransposon-based markers. Theor Appl Genet 122:1385–1397. https://doi.org/10.1007/s00122-011-1539-2
Song K, Lu P, Tang K, Osborn TC (1995) Rapid genome change in synthetic polyploids ofBrassica and its implications for polyploid evolution. Proc Natl Acad Sci U S A 92:7719–7723. https://doi.org/10.1073/pnas.92.17.7719
Staton SE, Ungerer MC, Moore RC (2009) The genomic organization of Ty3/gypsy-like retrotransposons in Helianthus (Asteraceae) homoploid hybrid species. Am J Bot 96:1646–1655. https://doi.org/10.3732/ajb.0800337
Staton SE, Bakken BH, Blackman BK, Chapman MA, Kane NC, Tang S, Ungerer MC, Knapp SJ, Rieseberg LH, Burke JM (2012) The sunflower (Helianthus annuus L.) genome reflects a recent history of biased accumulation of transposable elements. Plant J 72:142–153. https://doi.org/10.1111/j.1365-313X.2012.05072.x
Tate JA, Ni Z, Scheen AC, Koh J, Gilbert CA, Lefkowitz D, Chen ZJ, Soltis PS, Soltis DE (2006) Evolution and expression of homeologous loci in Tragopogon miscellus (Asteraceae), a recent and reciprocally formed allopolyploid. Genetics 173:1599–1611. https://doi.org/10.1534/genetics.106.057646
Ungerer MC, Strakosh SC, Zhen Y (2006) Genome expansion in three hybrid sunflower species is associated with retrotransposon proliferation. Curr Biol 16:R872–R873. https://doi.org/10.1016/j.cub.2006.09.020
Ungerer MC, Strakosh SC, Stimpson KM (2009) Proliferation of Ty3/gypsy-like retrotransposons in hybrid sunflower taxa inferred from phylogenetic data. BMC Biol 7:40. https://doi.org/10.1186/1741-7007-7-40
Vassilevska-Ivanova R, Kraptchev B, Stancheva I, Geneva M (2013) A compact sunflower line produced after cross Helinathus annuus x Verbesina encelioides. Centr Eur J Biol 8:492–498. https://doi.org/10.2478/s11535-013-0147-8
Vassilevska-Ivanova R, Kraptchev B, Stancheva I, Geneva M, Iliev I, Georgiev G (2014) Utilization of related wild species (Echinacea purpurea) for genetic enhancement of cultivated sunflower (Helianthus annuus L.). Turk J Agric For 38:15–22. https://doi.org/10.3906/tar-1210-91
Vassilevska-Ivanova R, Kraptchev B, Shtereva L (2015) An intergeneric hybrid line produced after cross Helianthus annuus x Echinacea purpurea. Genet Resour Crop Evol 62:829–836. https://doi.org/10.1007/s10722-015-0281-z
Vassilevska-Ivanova R, Shtereva L, Stancheva I, Geneva M (2016) Salt stress response of sunflower breeding lines developed after wide hybridization. Turk J Agric Nat Sci 3:197–204
Vassilevska-Ivanova R, Stancheva I, Geneva M, Tcekova Z (2018) Evaluating an interspecific Helianthus annuus x Helianthus nuttallii line for use in sunflower breeding. Turkish JAF Sci Tech 6:1684–1689. https://doi.org/10.24925/turjaf.v6i12.1684-1689.1361
Vukich M, Giordani T, Natali L, Cavallini A (2009) Copia and gypsy retrotransposons activity in sunflower (Helianthus annuus L.). BMC Plant Biol 9:150. https://doi.org/10.1186/1471-2229-9-150
Wang H, Jiang J, Chen S, Qi X, Fang W, Guan Z, Teng N, Liao Y, Chen F (2014) Rapid genetic and epigenetic alterations under intergeneric genomic shock in newly synthesized Chrysanthemum morifolium x Leucanthemum paludosum hybrids (Asteraceae). Genome Biol Evol 6:247–259. https://doi.org/10.1093/gbe/evu008
Wessler SR (1996) Turned on by stress. Plant retrotransposons. Curr Biol 6:959–961. https://doi.org/10.1016/S0960-9822(02)00638-3
Xiong Z, Gaeta RT, Pires JC (2011) Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc Natl Acad Sci U S A 108:7908–7913. https://doi.org/10.1073/pnas.1014138108
Yaldiz G, Camlica M, Nadeem MA, Nawaz MA, Baloch FS (2018) Genetic diversity assessment in Nicotiana tabacum L. with iPBS-retrotransposons. Turk J Agric For 42:154–164. https://doi.org/10.3906/tar-1708-32
Acknowledgments
This work was supported by IAEA National TC Project Bul 5/014 “Screening of Cereal Germplasm Stress Response and Adaptation Potential by Advanced Nuclear, Omics and Physiological Approaches”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 43 kb)
Rights and permissions
About this article
Cite this article
Bonchev, G.N., Vassilevska-Ivanova, R. Fingerprinting the genetic variation and intergeneric hybrid dynamics in the family Asteraceae (genera Helianthus, Echinaceae, Tagetes and Verbesina) using iPBS markers. Biologia 75, 457–464 (2020). https://doi.org/10.2478/s11756-019-00363-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00363-3