Abstract
The influence of a 3-years cultivation of legumes on macroarthropod community composition was investigated. Soil samples were taken from a field experiment which was conducted for three years to characterize the agronomic characteristics of eight cover crops: Crotalaria juncea L., Crotalaria spectabilis Roth, Crotalaria ochroleuca G. Don., Canavalia ensiformis (L.) DC., Mucuna pruriens (L.) DC., Dolichos lablab L., Neonotonia wightii (Wight & Arn.) J.A. Lackey, and Brachiaria decumbens Stapf. cv. Basilisk. The highest values of diversity in the macroarthropod community were found on the plot where M. pruriens was cultivated. Brachiaria decumbens cultivation showed lower values of species richness, Shannon’s diversity index and Simpson’s dominance index compared to the other studied plant species. Our findings suggest that the cultivation of legume cover crops can change positively the macroarthropod community composition. The results highlight the importance of considering the effects of legumes on the diversity of this biological component and their effect on plant growth and soil organic carbon contents. Thus, legumes may exploit positive feedback in semiarid conditions on a Regosol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the effect of the addition of legumes that may regulate the macroarthropod diversity and richness from a 3-years field experiment on a Regosol is essential to explain why the long-term cultivation of non-legume plant species becomes less beneficial to belowground soil organisms and their interaction with plant biomass production, soil organic carbon, and biodiversity than the cultivation of legume plant species in the same conditions (Coyle et al. 2017; Manwaring et al. 2018; Roy et al. 2018). Over time, the cultivation of non-legume plant species under conventional farming system may result in a decline of soil organic carbon, plant biomass production and macroarthropod richness (Amazonas et al. 2017; Souza et al. 2018).
In this context, the macroarthropod abundance (e.g., assessing ecosystem services provided by this functional group) is the key to determine positive plant-soil feedback. According to Souza et al. (2015) and Zhang et al. (2018), the abundance of soil macroarthropods depends on continuous input of soil organic matter combined with both adequate plant composition and vegetation structure. Sustainable soil practices/management, as the cultivation of legumes, increases macroarthropod richness (average 50.0% higher species richness and 70.3% higher abundance than in non-legumes) (Mauda et al. 2018). Ecosystem engineers, litter transformers and predators respond negatively to conventional farming system using non-legume plant species, while root pathogens respond positively (Ng et al. 2018). These soil fauna groups contribute to ecosystem services, such as biomass production, nutrient cycling, water supply and soil erosion control (Li et al. 2018). Thus, they play an important role in the soil quality and plant biomass production (Yang et al. 2018).
In a conventional farming system using legumes, the ecological stability and biodiversity could be improved in many ways. In this work, we hypothesized that the continuous cultivation of plant species from the Fabaceae family promotes positive effects on macroarthropod community diversity. Legume cover crops are recognized by their abilities to fix nitrogen (N) and support high biomass production (Souza et al. 2018). Based on the soil quality and nutrient content hypothesis, we expected to find high species richness and diversity of macroarthropod in plots where plant species from Fabaceae family that presents high biomass production were cultivated (Manwaring et al. 2018; Roy et al. 2018). There are evidences that plant composition, plant diversity and vegetation structure can affect both trophic structure and macroarthropod community composition based on both enemies and resource concentration hypotheses (Ng et al. 2017, 2018).
It may be argued that the cultivation of legumes over time could be a sustainable alternative to improve macroarthropod diversity and its services to the ecosystem in areas from smallholder farming system in the Brazilian Northeast, increasing soil fertility and improving annual plant yield. In fact, the macroarthropod community (e.g., Araneae, Coleoptera, Hymenoptera, Isoptera, Mantodea, and Scorpionidae) can contribute to fundamental services for terrestrial ecosystems (Coyle et al. 2017; Li et al. 2018; Ng et al. 2018; Yang et al. 2018). However, there is limited information on how legumes and non-legumes may affect macroarthropod diversity under semiarid conditions on a Regosol. Our aim was to determine whether the addition of legumes and non-legumes affects macroarthropod community diversity. To accomplish this, we performed field experiment and investigated whether the influence of legumes on above- and belowground community composition on a Regosol changed the macroarthropod diversity by its continuous cultivation.
Materials and methods
Study system and climatic conditions
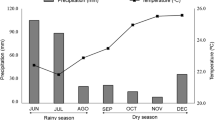
The field experiment was carried out at the “Chã-de-Jardim” Experimental Station, Agrarian Science Centre, Federal University of Paraiba (CCA-UFPB), located in Areia, Paraíba, Brazil (06°58′12” S, 35°42′15” W, altitude 619 m above sea level). The climate in the area is As′ (Köppen), with average annual precipitation and temperature of 1500 mm and + 21 °C, respectively. Climate data, monthly rainfall and mean temperatures from Areia, Paraíba, Brazil (2016–2017) (see Fig. 1) were obtained from the website: http://www.inmet.gov.br.
Monthly data of air temperature (dotted line) and rainfall (black line) from the site near to downtown Areia, Paraiba, Brazil (2016–2017); data were obtained from the website: http://www.inmet.gov.br
Soil sampling and characterization
The soil type of the experimental site was classified as Regosol (WRB 2006). Soil samples were collected at the beginning of August 2016 during the dry period and when the plants were in flowering stage. Samples (N = 40) were collected from a depth of 0–20 cm, air-dried and passed through a 2-mm sieve. We determined soil pH in a suspension of soil and distilled water (Black 1965). Total soil nitrogen and soil organic carbon were estimated according to the methodology described by Okalebo et al. (1993). Available phosphorus was determined using the Olsen’s P protocol (Olsen et al. 1954). The soil-chemical data at the site before starting the experiment are given in Table 1.
Experimental design and plant dry biomass data
We performed a 3-years field study with different plant species which were allocated in a randomized block design that consisted of eight plant species, namely Crotalaria juncea L., Crotalaria spectabilis Roth, Crotalaria ochroleuca G. Don., Canavalia ensiformis (L.) DC., Mucuna pruriens (L.) DC., Dolichos lablab L., Neonotonia wightii (Wight & Arn.) J.A. Lackey, and Brachiaria decumbens Stapf. Cv. Basilisk (for more details on plant species characteristics see Table 1; and fertilizers, doses, and application mode, see Souza et al. 2018). Each treatment plot (6 × 4 m) was replicated in five blocks, and to our analysis we used the central portion (5 × 3 m) of each plot. The studied plants were sown at a seedling rate of 300 seeds m−2 at a 3-cm depth and the plots were spaced 0.5 m between them. We selected 40 plants per plot during the flowering stage to quantify their dry biomass. Plants were harvested from each plot at 8–10 cm above the ground level. The biomass production was determined following Souza et al. (2018). We determined dry weight in ton per hectare.
Macroarthropod community data
To sample the soil macroarthropod community, we used the Tropical Soil Biology and Fertility (TSBF) protocol described by Anderson and Ingram (1989). At the end of the experiment, we installed two Provid-type traps per each plot to collect soil macroarthropod specimens. Into the traps, we added 200 ml of a detergent solution at a concentration of 10% and 10 ml of 70% alcohol. The traps remained in the field for a period of 48 h. Samplings were performed at each plot of the experiment area, totalling 80 sampling points (N = 80). Only the macroarthropod individuals longer than 10 mm were considered in our analyses. They were removed manually and stored in containers with 70% alcohol. These were later counted and identified under a stereoscopic microscope, at the level of major taxonomic group. The term group was used in the soil macroarthropod study, meaning either a family, a class, or an order, with the objective of comprising a set of individuals with a similar life form. The communities were characterized based on the following parameters: (a) Richness, number of groups per studied plot; (b) Shannon Diversity Index (H) (Shannon and Weaver 1949); and (c) Simpson dominance index (C) (Simpson 1949). In addition, we assessed the order occurrence frequency of every macroarthropod order by each studied treatment.
Statistical analyses
The Shapiro-Wilk test was applied to assess the normality of the data distribution. One-way ANOVA was used for analyzing differences between the studied cover crops in macroarthropod richness, Shannon diversity index, and Simpson dominance index. Data sets were arcsin square root transformed for percentage variables and log10(x) for the remaining (Zar 1984). Notwithstanding, the results are presented in their original scale of measurement. When necessary, Bonferroni’s test was conducted. A data matrix was used to compare the relationships between soil properties, the plant dry biomass, and macroarthropod community composition of the cover crop species by Jaccard similarity coefficient. Similarities derived from this coefficient were also transformed to distance measures (e.g. Euclidean coefficient). Dendrogram for Jaccard similarity coefficient was constructed using the unweighted paired-groups method of averaging (Jackson et al. 1989). In order to evaluate the relationships between soil properties, plant dry biomass, and macroarthropod community composition of the cover crop species, a principal component analysis (PCA) was performed. All statistical analyses were conducted using R (R Core Team 2018).
Results
Plant dry biomass production
The one-way ANOVA results showed significant differences between the studied plant species for plant dry biomass (F7,32 = 22.75, p < 0.001). The highest plant dry biomass was found on the plots where D. lablab and C. ochroleuca plants were cultivated (14.53 ± 2.05 T ha−1; and 10.53 ± 2.01 T ha−1, respectively), whereas the lowest value of this variable was found on the plots where B. decumbens was cultivated (2.48 ± 0.27 T ha−1). We did not find any significant differences between C. juncea and C. ensiformis for plant dry biomass production (Fig. 2).
Macroarthropod community composition
Thirteen different macroarthropod orders were identified belonging to four functional groups/types: Ecosystems engineers (2), litter transformers (1), predators (4) and herbivores (6). The most abundant taxa in all studied plant species were Hymenoptera. Macroarthropods from Araneae, Coleoptera and Orthoptera were also mostly found in all studied plant species. Hemiptera were observed only on plots where B. decumbens was cultivated (Table 2). Additional data about macroarthropod community composition are given in Online Resource 1.
Ecological indexes
The one-way ANOVA results showed significant differences among the studied plant species on macroarthropod richness (F7,72 = 19.21, p < 0.001), Shannon diversity index (F7,72 = 32.93, p < 0.001), and Simpson dominance index (F7,72 = 31.23, p < 0.001). The highest values of macroarthropod richness, Shannon diversity index and Simpson dominance index were found on plots where M. pruriens was cultivated (9.0 ± 0.2; 1.16 ± 0.01; and 0.58 ± 0.01, respectively), whereas B. decumbens presented the lowest values of these variables (Fig. 3a, b).
Multivariate analyses
Dendrogram was constructed based on the Jaccard’s similarity coefficient (Fig. 4). In this dendrogram three clusters were formed at the similarity coefficient of 0.30 which consisted of the macroarthropod community composition (MCC) of all studied plant species. Two lines, namely C. juncea and C. spectabilis, remained ungrouped. This shows that the MCC presented in C. juncea and C. spectabilis plots are quite distinct from the other plant species. Cluster-1 consisted of MCC from B. decumbens and C. ensiformis plots. Cluster-2 comprised of MCC from N. wightii and D. lablab. Cluster-3 consisted of MCC from M. pruriens and C. ochroleuca. Among all the studied plant species, Cluster-1 was found most diverse as it separated from all other plant species at a very low similarity coefficient of 0.30.
The PCA analyses showed that plant dry biomass, total organic carbon, frequency of occurrence of Hymenoptera, Scorpiones, Dermaptera, Coleoptera and Orthoptera were the main factors contributing to the variance of the samples (Fig. 5). The analysis also showed: (1) a strong negative relationship between the frequency of occurrence of Hymenoptera, Coleoptera and Orthoptera with plant dry biomass; (2) a negative relationship between soil pH and the frequency of occurrence of Scolopendromorpha; and (3) a positive relationship between total organic carbon and total nitrogen (Fig. 5).
PCA score plot of soil properties, plant biomass production, and macroarthropod community composition for the eight studied cover crops. PDB = Plant dry biomass; N = total nitrogen; Corg = soil organic carbon; SCOP = Scolopendromorpha; DIP = Diptera; LLEP = Lepidoptera larvae; ARA = Araneae; MAN = Mantodea; ODO = Odonata; HEM = Hemiptera; THY = Thysanoptera; COL = Coleoptera; ORT = Orthoptera; HYM = Hymenoptera; SCOR = Scorpiones; DER = Dermaptera. Points represent samples from each plot by studied cover crop
Discussion
Our results emphasize the influence of different plant species on the presence of soil macroarthropod community composition (i.e., ecosystems engineer, herbivore, litter transformer and predator functional groups). Essentially, we wanted to understand how the continuous use of legumes changes the macroarthropod communities. The results of this study revealed that there were differences between each studied plant species on macroarthropod richness, Shannon diversity index, and Simpson dominance index. According to Moura et al. (2015), plant species with the following characteristics: (i) fast-growing, (ii) nitrogen fixation, (iii) high soil reaction in their rhizosphere by the extrusion of H+, and (iv) high biomass production may enhance soil rootability and improve the fauna’s habitat. However, Roy et al. (2018) highlight the importance to consider the negative effects of monoculture (i.e., low plant diversity) that may lead to farming system that produce less residue with lower C: N and lignin: N ratios than natural ecosystems. Our hypothesis that plant species from Fabaceae family promotes positive effects on the macroarthropod community composition was supported for all studied plant species from Fabaceae family, except for C. ensiformis, which presented the same values of richness of the B. decumbens. The macroarthropod community and plant status in C. ensiformis plots were characterized by a lower abundance of predators, litter transformers, ecosystem engineers, and leaf transpiratory rate (data not shown) that created a negative feedback into the trophic structure. On the other hand, we identified a high presence of herbivores (i.e., Hymenoptera and Orthoptera) and low presence of predators (i.e., Araneae) on the plots where C. ensiformis was cultivated. It agrees with the work done by Delgado-Baquerizo et al. (2018) and Solen et al. (2018) that reported soil macroarthropod community response in relation to quality of the plant residue. Although our experiment was not designed to directly test whether precipitation and air temperature affect macroarthropod diversity, we must consider that the highest monthly precipitation was registered one month before macroarthropod sampling.
For macroarthropod richness, Shannon diversity index, and Simpson dominance index, C. spectabilis and M. pruriens, showed a higher value of these three variables than the other studied plant species. Thus, these results support our hypothesis that plant species from Fabaceae family that presents higher biomass production (C. spectabilis) or higher N content in plant tissue (M. pruriens) than plant species from Poaceae family may positively affect macroarthropod community composition. The higher richness and diversity presented by C. spectabilis and M. pruriens compared to C. ensiformis and B. decumbens may be related with their high growth rate and cover rate. We cannot exclude the hypotheses described by Moura et al. (2015) that in agricultural soils the macroarthropod abundance is driven by the quantity of plant biomass (i.e., habitat quality hypothesis), while macroarthropod diversity is driven by litter quality (i.e., nutrient content hypothesis), which can be supported by the results on the plots of C. spectabilis and M. pruriens on richness and Shannon’s diversity index. These results agree with previous studies done by Zhang et al. (2018), Souza et al. (2018), Forbes et al. (2018), and Siqueira et al. (2014), which reported that soil ecosystems with constant organic amendments increase soil organic carbon which directly influence soil organisms (i.e. Coleoptera, Scolopendromorpha and Araneae). According to Phophi et al. (2017), plant species with spreading (M. pruriens) or dense (C. spectabilis) cover-like form lead to both high richness and abundance of soil macroarthropods.
Plant biomass production and litter quality are important to soil macroarthropods and these variables act as food resource and refuge site, respectively (Silva et al. 2018; Souza et al. 2016; Vukicevich et al. 2016). Macroarthropods groups, especially orders with high abundance, were determinants in our study to separate cover crop groups. Cluster-1 (B. decumbens and C. ensiformis) characterized by low biomass production and low plant N content, respectively, presented four similar orders (i.e., Araneae, Coleoptera, Hymenoptera and Orthoptera) and we did not find any differences for the frequency of occurrence of these orders among the cover crops from Cluster-1. However, C. spectabilis remained ungrouped, because of their good production of plant residues (10.8 ± 1.4 T ha−1) associated with high values of plant N, P, and K content (data not shown), and presented eight different macroarthropod orders with different ecosystem services. Among the orders that we observed in our study, the most frequent order was Hymenoptera (Formicidae family) for all studied treatments. These results agree with Forbes et al. (2018) who reported Hymenoptera as the largest order of soil macroarthropods and suggest a model with data from real hot systems where Hymenoptera may have 2.5–3.2 times more species than Coleoptera. Wink et al. (2005) also found Formicidae as a dominant group studying different ecosystems and habitats, and Luz et al. (2013) reported higher abundance of ants in habitats with high litter deposition than in disturbed habitats. Although our experiment was not designed to directly test whether macroarthropod diversity affect plant biomass production through changes in ecosystem services that in turns affect soil chemical and physical properties, the results of the PCA analyses shown that the plant dry biomass could be affected by the abundance of Hymenoptera, Coleoptera and Orthoptera. Our results agree with the works done by Souza et al. (2015) and Luz et al. (2013) that reported Hymenoptera (Formicidae) and Coleoptera (Scarabaeidae) associated with both high production of biomass and soil organic carbon in preserved areas .
Conclusions
Over the long-term experiment, the use of plant species from Fabaceae family (e.g., C. spectabilis, and M. pruriens) as green manuring showed high macroarthropod richness, Shannon’s diversity index, and Simpson’s dominance index on a Regosol in field conditions. Our findings suggest that plant species with high biomass production, high quality of their residues, high root activity and fast growth had positive effects on macroarthropod community composition. The results of our study highlight the importance of considering the long-term effect of cover crops utilization as green manuring, based on a sustainable way to improve soil quality and soil biodiversity. Thus, the long-term utilization of plant species from Fabaceae family may exploit a positive feedback among plant, soil and soil biota.
References
Amazonas NT, Viani RAG, Rego MGA, Camargo FF, Fujihara RT, Valsechi OA (2017) Soil macrofauna density and diversity across a chronosequence of tropical forest restoration in southeastern Brazil. Braz J Biol 78:449–456. https://doi.org/10.1590/1519-6984.169014
Anderson JN, Ingram JSI (1989) Tropical soil biology and fertility: a handbook of methods. CAB International, Wallingford
Black CA (1965) Methods of soil analysis, part 2. In: Black CA (ed) Agronomy monograph, vol 9. WI, Madison, pp 771–1572
Coyle DR, Nagendra UJ, Taylor MK, Campbell JH, Cunard CE, Joslin AH, Mundepi A, Phillips CA, Callaham MA Jr (2017) Soil fauna responses to natural disturbances, invasive species, and global climate change: current state of the science and a call to action. Soil Biol Biochem 110:116–133. https://doi.org/10.1016/j.soilbio.2017.03.008
Delgado-Baquerizo M, Eldridge DJ, Maestre FT, Ochoa V, Gozalo B, Reich PB, Singh BK (2018) Aridity decouples C:N:P stoichiometry across multiple trophic levels in terrestrial ecosystems. Ecosystem 21:459–468. https://doi.org/10.1007/s10021-017-0161-9
Forbes AA, Bagley RK, Beer MA, Hippee AC, Widmayer HA (2018) Quantifying the unquantifiable: why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol 18:21. https://doi.org/10.1186/s12898-018-0176-x
Jackson DA, Somers KM, Harvey HH (1989) Similarity coefficients: measures of co-occurrence and association or simply measures of occurrence? Am Nat 133:436–453. https://doi.org/10.1086/284927
Li FR, Liu JL, Ren W, Liu LL (2018) Land-use change alters patterns of soil biodiversity in arid lands of northwestern China. Plant Soil 428:371–388. https://doi.org/10.1007/s11104-018-3673-y
Luz RA, Fontes LS, Cardoso SRS, Lima ÉFB (2013) Diversity of the arthropod edaphic fauna in preserved and managed with pasture áreas in Teresina, Piauí, Brazil. Braz J Biol 73(3):483–489
Manwaring M, Wallace HM, Weaver HJ (2018) Effects of a mulch layer on the assemblage and abundance of mesostigmatan mites and other arthropods in the soil of a sugarcane agro-ecosystem in Australia. Exp Appl Acarol 74:291–300. https://doi.org/10.1007/s10493-018-0227-1
Mauda EV, Joseph GS, Seymour CL, Munyai TC, Foord SH (2018) Changes in landuse alter ant diversity, assemblage composition and dominant functional groups in African savannas. Biodivers Conserv 27:947–965. https://doi.org/10.1007/s10531-017-1474-x
Moura EG, Aguiar ACF, Piedade AR, Rousseau GX (2015) Contribution of legume tree residues and macrofauna to the improvement of abiotic soil properties in the eastern Amazon. Appl Soil Ecol 86:91–99. https://doi.org/10.1016/j.apsoil.2014.10.008
Ng K, Dricoll DA, Macfadyen S, Barton PS, McIntyre S, Lindenmayer DB (2017) Contrasting beetle assemblage responses to cultivated farmlands and native woodlands in a dynamic agricultural landscape. Ecosphere 8:e02042. https://doi.org/10.1002/ecs2.2042
Ng K, McIntyre S, Macfadyen S, Barton PS, Driscoll DA, Lindenmayer DB (2018) Dynamic effects of ground-layer plant communities on beetles in a fragmented farming landscape. Biodivers Conserv 27:2131–2153. https://doi.org/10.1007/s10531-018-1526-x
Okalebo JR, Gathua KW, Woomer PL (1993) Laboratory methods of plant and soil analysis: A working manual. Technical Bulletin No 1, Soil Science Society East Africa, Nairobi
Olsen SR, Cole CV, Watanable FS, Dean LA (1954) Estimation of available phosphorous in soil by extraction with sodium bicarbonate. Department of Agriculture, Washigton
Phophi MM, Mafongoya PL, Odindo AO, Magwaza LS (2017) Screening cover crops for soil macrofauna abundance and diversity in conservation agriculture. Sustain Agric Res 6:142–149. https://doi.org/10.5539/sar.v6n4p142
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Roy S, Roy MM, Jaiswal AK, Baitha A (2018) Soil arthropods in maintaining soil health: thrust areas for sugarcane production systems. Sugar Tech (4):376–391. https://doi.org/10.1007/s12355-018-0591-5
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Silva RA, Siqueira GM, Costa MKL, Guedes Filho O, Silva EFF (2018) Spatial variability of soil fauna under different land use and managements. Rev Bras Cienc Solo 42:e0170121. https://doi.org/10.1590/18069657rbcs20170121
Simpson EH (1949) Measurement of diversity. Nature 163:688
Siqueira GM, Silva EFF, Paz-Ferreiro J (2014) Land use intensification effects in soil arthropod community of an entisol in Pernambuco state, Brazil. Sci World J 2014:625856. https://doi.org/10.1155/2014/625856
Solen LC, Nicolas J, Xavier AS, Thibaud D, Simon D, Michel G, Johan O (2018) Impacts of agricultural practices and individual life characteristics on ecosystem services: a case study on family farmers in the context of an Amazonian Pioneer front. Environ Manag 61:772–785
Souza TAF, Rodrígues AF, Marques LF (2015) Long-term effects of alternative and conventional fertilization on macroarthropod community composition: a field study with wheat (Triticum aestivum L.) cultivated on a Ferralsol. Org Agric 6:323–330. https://doi.org/10.1007/s13165-015-0138-y
Souza ST, Cassol PC, Baretta D, Bartz MLC, Klauberg Filho O, Mafra AL, Rosa MG (2016) Abundance and diversity of soil macrofauna in native forest, eucalyptus plantations, perennial pasture, integrated crop-livestock, and no-tillage cropping. Rev Bras de Cienc Solo 40:e0150248. https://doi.org/10.1590/18069657rbcs20150248
Souza AVSS, Souza TAF, Santos D, Rios ES, Souza GJL (2018) Agronomic evaluation of legume cover crops for sustainable agriculture. Russ Agric Sci 44:31–38. https://doi.org/10.3103/S1068367418010093
Vukicevich E, Lowery T, Bower P, Úrbez-Torres JR, Hart M (2016) Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture: a review. Agron Sustain Dev 36:48. https://doi.org/10.1007/s13593-016-0385-7
Wink C, Guedes JVC, Fagundes CK, Rovedder AP (2005) Insetos edáficos como indicadores da qualidade ambiental / soil borne insects as indicators of environmental quality. Rev Cienc Agrovet 4:60–71
WRB (2006) (IUSS Working Group) World Reference Base for Soil World Soil Resources Reports, Rome: FAO (103):128
Yang B, Zhang W, Xu H, Wang S, Xu X, Fan H, Chen HYH, Ruan H (2018) Effects of soil fauna on leaf litter decomposition under different land used in eastern coast of China. J For Res 29:973–982. https://doi.org/10.1007/s11676-017-0521-5
Zar JH (1984) Biostatistical analysis. Prentice Hall, USA
Zhang P, Neher DA, Li B, Wu J (2018) The impacts of above- and belowground plant input on soil microbiota: invasive Spartina alterniflora versus native Phragmites australis. Ecosystem 21:469–481
Acknowledgments
Special thanks to Dr. Helena Freitas for valuable discussions and checking of English grammar. The authors also thank the anonymous reviewers for helpful comments, which greatly improved a previous version of the manuscript. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – “Finance Code 001”.
Author information
Authors and Affiliations
Contributions
We declare that all the authors made substantial contributions to the conception, design, acquisition, analysis, and interpretation of the data. All the authors participate in drafting the article, revising it critically for important intellectual content; and finally, the authors gave final approval of the version to be submitted to Biologia.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 101 kb)
Rights and permissions
About this article
Cite this article
de Melo, L.N., de Souza, T.A.F. & Santos, D. Cover crop farming system affects macroarthropods community diversity in Regosol of Caatinga, Brazil. Biologia 74, 1653–1660 (2019). https://doi.org/10.2478/s11756-019-00272-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00272-5