Abstract
It is important to understand the effects of temporal changes in microbial communities in the acidic soils of tea orchards with different fertilizers. A field experiment involving organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments was arranged to analyze the temporal changes in the bacterial and archaeal communities at bimonthly intervals based on the 16S ribosomal RNA (rRNA) gene using terminal restriction fragment length polymorphism (T-RFLP) profiling. The abundances of total bacteria, total archaea, and selected functional genes (bacterial and archaeal amoA, bacterial narG, nirK, nirS, and nosZ) were determined by quantitative polymerase chain reaction (qPCR). The results indicate that the structures of bacterial and archaeal communities varied significantly with time and fertilization based on changes in the relative abundance of dominant T-RFs. The abundancy of the detected genes changed with time. The total bacteria, total archaea, and archaeal amoA were less abundant in July. The bacterial amoA and denitrifying genes were less abundant in September, except the nirK gene. The OF treatment increased the abundance of the observed genes, while the CF treatment had little influence on them. The soil temperature significantly affected the bacterial and archaeal community structures. The soil moisture was significantly correlated with the abundance of denitrifying genes. Of the soil chemical properties, soil organic carbon was the most important factor and was significantly correlated with the abundance of the detected genes, except the nirK gene. Overall, this study demonstrated the effects of both temporal alteration and organic fertilizer on the structures of microbial communities and the abundance of genes involved in the nitrogen cycle.
概要
研究目的
研究化学肥料和有机肥处理条件下, 茶园酸性土壤细菌和古菌群落结构, 以及氮素转化相关功能酶基因丰度的时间变化规律。
创新要点
研究肥料、 土壤温度及土壤含水量对茶园酸性土壤细菌和古菌群落结构, 以及氮素转化相关功能酶基因丰度的影响。
研究方法
应用末端限制性片段长度多态性(T-RFLP)技术分析茶园酸性土壤中细菌和古菌群落结构随时间的变化规律, 应用荧光定量聚合酶链式反应(PCR)技术, 研究茶园酸性土壤细菌、 古菌、 硝化作用功能酶基因(细菌和古菌amoA 基因)和细菌反硝化作用功能酶基因(narG、 nirK、 nirS 和nosZ 基因)丰度的时间变化规律。
重要结论
茶园土壤细菌和古菌群落结构受到肥料的影响, 并随着取样时间有显著的变化。 细菌、 古菌和古菌的amoA 基因的丰度在7 月份最小, 而细菌的amoA 基因和反硝化作用功能酶基因(除nirK 基因)的丰度在9 月份最小。 有机肥处理增加了细菌、 古菌和氮素转化相关功能酶基因的丰度, 但化学肥料的施用对菌群及功能酶基因丰度的影响较小。 土壤温度显著影响了土壤细菌和古菌的群落结构。 土壤含水量与细菌反硝化作用功能酶基因有显著的相关性。 土壤有机碳含量与细菌、 古菌及功能酶基因丰度之间有显著的相关性。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil microbial communities can affect the crop quality, the sustainability of agro-ecosystems (Stark et al., 2008), and the biogeochemical cycle of soil nutrients (Buckley and Schmidt, 2003). The soil nitrogen (N) cycle contains two important processes: nitrification and denitrification. The oxidation of ammonia, the first and rate-limiting step of nitrification, is catalyzed by ammonia monooxygenase. This enzyme is encoded by the amoA gene from ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA), which may occupy different ecological niches (Prosser and Nicol, 2012). Denitrification, a four-step reducing process that is catalyzed by key enzymes encoded by the narG, nirK, nirS, and nosZ genes (Zumft, 1997), plays an important role in the reduction of nitrate to gaseous nitrogen. Studies of the nitrifiers and denitrifiers are commonly focused on functional genes, such as amoA, narG, nirK, and nirS (Szukics et al., 2010; Di et al., 2014).
The application of chemical fertilizer (CF) can increase the crop yield but changes the soil properties and results in N loss from agricultural systems (Galloway et al., 2003). CF increases the microbial biomass and changes the bacterial community structure (Peacock et al., 2001); however, it does not significantly increase the bacterial abundance (Chen et al., 2012) or decrease the bacterial and archaeal abundances in long-term experiments (Hallin et al., 2009; Chan et al., 2013). Organic fertilizer (OF), an alternative to CF, mitigates N pollution from agriculture ecosystems. The organic matter amendment not only increases the microbial biomass (Peacock et al., 2001) and total bacterial and archaeal abundance (Hallin et al., 2009; Chan et al., 2013), but also changes the structure of bacterial community through the increased soil organic carbon (SOC) content.
Shen et al. (2008) and Ai et al. (2013) found that chemical N fertilizer changed the AOB community structure and increased the soil potential nitrification activity (PNA) and AOB abundance, but He et al. (2007) reported that soil PNA and AOB abundances decreased with the application of a chemical N fertilizer. The effect of chemical N fertilizer on the abundance of AOA varies due to the soil pH (He et al., 2007; Shen et al., 2008; Ai et al., 2013). Manure amendment significantly increased the AOB abundance in a paddy field with long-term fertilization (Wang et al., 2014) but not in other systems (Wessén et al., 2010; Ai et al., 2013). For AOA, manure increased the AOA abundance and changed the community structure (Wessén et al., 2010; Ai et al., 2013). For denitrifiers, the influence of chemical N fertilizer and OF has been well studied but appears to have incongruent effects on denitrifier community composition (Wolsing and Priemé, 2004; Enwall et al., 2005; Clark et al., 2012). In a long-term experiment, ammonium fertilizer significantly decreased the abundance of denitrifiers (Hallin et al., 2009). Manure amendment increased the abundance of denitrifying genes both at the DNA and RNA levels (Hallin et al., 2009; Clark et al., 2012; Saunders et al., 2012).
It is important to study the temporal changes in soil microbial communities because temporal analyses may help to comprehensively explain the soil function and provide insight into the environmental niches that are inhabited by diverse soil microbes (Lauber et al., 2013). The abundances and community structures of bacteria and archaea (He et al., 2007; Rasche et al., 2011), including ammonia-oxidizing ones (He et al., 2007; Szukics et al., 2010), vary temporally due to changes in the soil properties. For denitrifiers, previous studies have demonstrated that the temporal effect on the abundance of denitrifying genes varies among different genes (Wolsing and Priemé, 2004; Dandie et al., 2008). A temporal shift in the denitrifier community composition has also been reported (Wolsing and Priemé, 2004; Dandie et al., 2008; Szukics et al., 2010).
Tea (Camellia sinensis) is an important cash crop in southern China. The soil pH in tea orchards is relatively low because of the tea plantations and high-level applications of chemical N fertilizers, especially urea (Yao et al., 2011). However, the study of the temporal changes in the soil microbial communities that are related to OF treatment is relatively rare in tea orchards. Therefore, this study focused on the temporal changes of the bacterial and archaeal communities and functional groups as related to nitrification and denitrification under different types of fertilizer treatments. The major hypotheses were (1) that fertilizer amendments would influence the structures of bacterial and archaeal communities and the abundances of the functional groups that are involved in nitrification and denitrification in tea orchards, and (2) that the temporal variability in microbial community structures and the abundance of detected genes may be related to soil climate changes, such as soil temperature and moisture variation. In this study, the abundances and community structures of total bacteria and total archaea, as well as the abundances of denitrifying (narG, nirS, nirK, and nosZ) and nitrifying (AOA and AOB) functional groups were investigated.
2 Materials and methods
2.1 Field site, experimental design, and soil sampling
The present study was conducted in the Jingshan tea production field (119°53′ E, 30°23′ N), Hangzhou, China. This area has a subtropical monsoon climate with an annual average air temperature of 17 °C and annual average rainfall of 1400 mm. The soil of the tea field is red clay, and the topsoil (0–10 cm) has a pH (H2O) value of 3.72, SOC of 35.80 g/kg, and total nitrogen (TN) level of 3.16 g/kg. The tea plants (C. sinensis L.) in the field were approximately 40 years old. The farmer applied CF, including urea and calcium superphosphate, three times (in March, June, and October) per year before the study was conducted to improve the tea yield.
The field experiment began in March 2012 with nine experimental plots that were 5 m wide and 20 m long. The plots were separated by a concrete wall that was 35 cm high aboveground and 30 cm deep underground to prevent water flow between the plots. The applied fertilizer treatments in this study included unfertilized control (CK), OF, and CF. The properties and application details of the fertilizers are shown in Table 1. To be consistent with the tea cultivation by the native farmers, the fertilizer was applied in three split applications in 2012. The experimental design was a completely randomized design, and each fertilizer treatment had three replicates. The fertilizer for each plot was applied equally to the rows between the tea plants.
Soil sampling was conducted every two months from May 17, 2012 to March 17, 2013. The soil sample of each plot was composed of 12 soil cores that were collected along zigzag paths (zigzag sampling) from the surface layer (0–10 cm). All of the soil cores were sampled from fertilized rows. A total of 12 soil cores from each plot were delivered on ice to the laboratory and were then pooled and sieved through 2- or 5-mm mesh (wet soils). Visible pieces of plant material and soil-dwelling animals were removed. To measure the soil ammonium and nitrate levels and perform a molecular analysis, the soil subsamples were stored at −20 °C. The soils were air-dried before the measurements of SOC, TN, and pH.
2.2 Soil property analysis
The soil moisture content was determined by oven-drying 10 g of soil at 105 °C for 24 h, and the results are presented based on the soil weight loss. The soil temperature was measured using a digital thermometer at a depth of 10 cm. The soil pH was determined by a digital pH meter after extracting with water (1 g soil extracted with 2.5 ml water). The SOC and TN were analyzed by oxidation with dichromate (Nelson and Sommers, 1982) and Kjeldahl determination (VELP Scientifica, Italy), respectively. The ammonium and nitrate contents were determined using the indo-phenol-blue colorimetric method (Sun, 2007) and dual-wavelength ultraviolet spectrophotometry (Norman et al., 1985), respectively, after extracting with 2 mol/L KCl solution (1 g soil extracted with 5 ml KCl solution).
2.3 DNA extraction and quantitative polymerase chain reaction (qPCR) analysis
DNA was extracted from 0.5 g of soil using the FastDNA Spin Kit for Soil (MP Biomedicals, USA).
The abundances of the total bacteria, total archaea, bacterial and archaeal amoA genes, and the bacterial narG, nirS, nirK, and nosZ genes were determined by qPCR with an ABI 7500 instrument (Applied Biosystems, USA). The PCR procedures and primers are shown in Table S1. Each reaction was performed in a volume of 25 µl containing 0.4 µl of each primer (10 µmol/L), 0.5 µl of ROXII (TaKaRa, Japan), 12.5 µl of 2× SYGB buffer (TaKaRa), 0.625 µl of DMSO, 0.875 µl of 3% bovine serum albumin (BSA), and 10 ng of template. For the standard curves, the amplicons of the detected genes were purified using the Cycle-Pure Kit (Omega Bio-Tek, USA), ligated into the vector pMD18-T (TaKaRa) and then transformed into Escherichia coli TOP10 competent cells. The plasmids were extracted using the Plasmid Mini Kit (Omega Bio-Tek) and checked using the basic local alignment search tool (BLAST). A NanoDrop 2000 (Thermo, USA) was used to measure the concentrations of the plasmids. The plasmids were serially diluted to generate the standard curves.
2.4 Microbial community analysis by terminal restriction fragment length polymorphism (T-RFLP)
For T-RFLP, the bacterial and archaeal 16S ribosomal RNA (rRNA) genes were amplified using FAM-labeled forward primers. The PCR was performed in a C1000 Thermal Cycler (Bio-Rad, USA). The primer sets and amplification details of the PCR for T-RFLP are shown in Table S1. The 50 µl PCR cocktail contained 10 ng of DNA, 0.15 µmol/L of each primer, 0.2 mmol/L dNTPs, 0.5 mg/ml BSA, 4 mmol/L Mg2+, 2 U of HS Taq (TaKaRa), and a 1× reaction buffer that was provided with the enzyme. Duplicate amplifications from each sample were mixed and purified using a gel extraction kit (Omega Bio-Tek). The bacterial and archaeal amplicons were digested with HhaI and AluI (NEB, USA), respectively, at 37 °C for 3 h. A 2-µl aliquot of each digest was mixed with 12 µl of formamide and 0.3 µl of the internal size standard (ROX-500, Applied Biosystems) and detected on an ABI 3730xl genetic analyzer (Applied Biosystems). The fragments were analyzed using the GeneMapper software (Version 4.0, Applied Biosystems). The height of each peak was expressed as the percentage of all of the peak heights in the profile. The peak was included for further analysis if its length was between 50 and 500 bp and its percentage was >1%.
2.5 Statistical analysis
For the soil properties and qPCR data that were not normally distributed, the data were log-transformed. A repeated-measure analysis of variance (ANOVA) was performed to analyze the effect of fertilizer and sampling time on the soil properties and qPCR data. We compared the significant differences among the fertilizers at each sampling time using Tukey’s test. A correlation analysis was used to examine the relationship between the soil properties and the abundance of phylogenetic and functional markers. Statistical analyses were performed with SPSS V16.0.
The T-RFLP data were square-root-transformed prior to analysis. A two-way interactive permutational multivariate ANOVA (PERMANOVA) (Anderson, 2001) was conducted to assess the effect of fertilizer and sampling time on the bacterial and archaeal communities. The pairwise comparisons in the PERMANOVA were used to determine whether the bacterial and archaeal communities were altered by fertilizer at each sampling time. The PERMANOVA generated a Monte Carlo asymptotic P-value (P_MC) when the pairwise comparisons were conducted. The microbial communities significantly differed among the fertilizer treatments if P_MC<0.05. Nonmetric multidimensional scaling (nmMDS) based on the Bray-Curtis similarity coefficients was used to analyze the relatedness of individual community profiles with all treatments conducted with Primer V5.0 software (PRIMER-E, UK). A redundancy analysis (RDA) with the Monte Carlo permutation test with 999 permutations was performed with Canoco (Version 4.5 for Windows, Biometrics Plant Research International, the Netherlands) to test the influence of the soil properties on the microbial community.
3 Results
3.1 Soil properties
The soil properties varied significantly with the fertilizers and sampling time (P<0.01). However, the interaction between the fertilizers and sampling time only significantly influenced the soil ammonium and nitrate levels (P<0.01). The soil TN, ammonium, and nitrate levels were higher under the CF treatment, but the soil pH was significantly lower under the CF treatment than under the OF or CK treatment, except on November 18th (Table S2). The OF treatment had a higher SOC content.
3.2 Abundances of bacteria and archaea
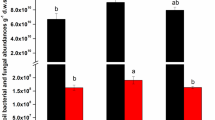
The abundances of total bacteria and archaea under the OF, CF, and CK treatments changed similarly with respect to the sampling time and was lowest on July 19th (Fig. 1). The OF treatment had the highest copy numbers of total bacterial and archaeal 16S rRNA genes, ranging from 1.7×109 to 4.9×109 and 1.3×107 to 1.1×108 copies per gram of dry soil, respectively (Fig. 1). A greater bacterial abundance was detected under the CF treatment than under the CK treatment with an exception on July 19th, but a significant difference was only shown on January 15th. No significant difference was detected for the archaeal abundance between the CF and CK treatments.
Quantitative PCR data of the total bacteria (a) and total archaea (b) in organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments over one year
Data are expressed as mean±SD, n=3. The letters above the bars indicate significant differences (P<0.05) among the fertilizers for each sampling time. The Y axis is a logarithmic coordinate axis
3.3 Abundances of the bacterial and archaeal amoA genes
Both of the bacterial and archaeal amoA gene copy numbers changed significantly with the sampling time (P<0.01). The archaeal amoA gene copy number changed from 3.7×105 to 2.3×107 copies per gram of dry soil and was lowest on July 19th (Fig. 2a). The population size of AOB was lower than that of AOA (Fig. 2b), the lowest AOB abundance was on September 16th, when no significant differences were detected among the three treatments. The OF treatment resulted in the greatest abundance of both the bacterial and archaeal amoA genes.
Quantitative PCR data of the archaeal (a) and bacterial (b) amoA genes in organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments over one year
Data are expressed as mean±SD, n=3. The letters above the bars indicate significant differences (P<0.05) among the fertilizers for each sampling time. The Y axis is a logarithmic coordinate axis
3.4 Abundance of denitrifiers
All of the detected denitrifying genes were more abundant under the OF treatment, but the effect was not always significant (Fig. 3). No significant difference was found when comparing the CF treatment with the CK treatment, with the exception of January 15th when the narG gene was more abundant under the CF treatment. The lowest denitrifying gene abundance was determined on September 16th except for that of nirK under the CF and CK treatments. The analysis of variance detected a significant variation of the abundance of denitrifying genes with the sampling time (P<0.01).
Quantitative PCR data of the bacterial narG (a), nosZ (b), nirS (c), and nirK (d) genes in organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments over one year
Data are expressed as mean±SD, n=3. The letters above the bars indicate significant differences (P<0.05) among the fertilizers for each sampling time. The Y axis is a logarithmic coordinate axis
3.5 Bacterial and archaeal communities
Bacterial community composition profiles showed 93 T-RFs across all of the profiles, of which three dominant fragments (58, 88, and 372 bp) and a further six fragments (63, 74, 211, 279, 294, and 298 bp) comprised >10% and 5% of the total abundance, respectively. The PERMANOVA analysis indicated a significant effect of the fertilizer and sampling time (P<0.01), which was further supported by nmMDS analysis (Fig. S1a). However, no significant differences were detected between the CF and OF treatments in July, January, or March (Table 2). The relative abundance of T-RF-58 varied significantly with the fertilizer except on July 19th and September 16th (Fig. 4a). The relative abundance of T-RF-88 changed significantly among the three treatments from July 19th to March 17th. While the relative abundance of T-RF-372 was significantly higher under the CK treatment on July 19th, this fragment was not detected from September to March.
Contributions of the major terminal restriction fragments (T-RFs) to the total bacterial (a) and archaeal (b) gene fragment diversities in organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments as determined over one year
The T-RFs that never contributed more than 5% were summarized as “others”
The archaeal community analysis revealed 88 peaks across all of the profiles, with four major peaks (141, 404, 405, and 435 bp) and six peaks (70, 116, 347, 350, 407, and 500 bp) forming >20% and >5% of the total abundance, respectively. The nmMDS plot indicated differences among the treatments throughout the entire sampling time with a stress value of 0.18 (Fig. S1b), which are supported by the PERMANOVA analysis (P<0.01). These dominant fragments varied significantly with the treatments. T-RF-404 was significantly more abundant under the CF treatment, while no significant difference was detected between the OF and CK treatments except on November 18th (Fig. 4b). The OF treatment but not the CF treatment significantly increased the relative abundance of T-RF-141. The relative abundance of T-RF-435 significantly decreased under the CF treatment except on January 15th, while the T-RF-405 fragment was more abundant under the CF treatment except on May 17th.
3.6 Relationships between soil properties and microbial abundance and microbial community structure
The SOC was significantly positively correlated with the abundance of all of the detected genes except for the archaeal amoA and nirK genes (Table 3). The abundance of AOA correlated negatively with nitrate but positively with the pH and the ratio of SOC to TN (C:N). The abundance of the nirK gene was only positively correlated with nitrate. The abundance of the nirS gene was negatively correlated with TN but positively correlated with the SOC and C:N. The pH was positively correlated with the abundances of total bacteria and the nosZ gene. No significant correlation was found between the soil ammonium content and the abundance of any of the detected genes. The soil temperature was negatively correlated with the abundance of total bacteria and positively correlated with the abundance of the nirK gene. The soil moisture was positively correlated with the abundances of narG, nirK, and nirS.
The relationship between the soil properties and the microbial communities under all of the treatments was analyzed using RDA (Fig. 5). The length of the arrow in the figure represents the effect of the soil properties on the microbial community distribution, which increased along the arrow’s direction. The bacterial communities were mainly influenced (P<0.05) by soil temperature, TN, C:N, ammonium, and moisture. Soil ammonium, temperature, SOC, pH, and C:N significantly (P<0.05) affected the archaeal communities. The cumulative variances of the species-environment relationship as explained by the first two axes were 69.60% and 76.70% for the bacterial and archaeal communities, respectively. The first axis explains the largest variation of the bacterial communities (P<0.01) and archaeal communities (P<0.01). For the bacterial communities, the first axis accounted for 13.40% of the variation in the species data and was strongly correlated with the soil temperature. The archaeal species data were changed mainly by the first axis, which attributed to 22.60% of the variation and was strongly correlated with the soil ammonium content, pH, and C:N.
Plots of a redundancy analysis of microbial communities based on the T-RFLP datasets that were obtained from the total bacteria (a) and total archaea (b) in the organic fertilizer (OF), chemical fertilizer (CF), and unfertilized control (CK) treatments
The numbers in the plots represent the sampling time: 1. May 17; 2. July 19; 3. Sept. 16; 4. Nov. 18; 5. Jan. 15; 6. Mar. 17. C:N is the ratio of soil organic carbon to total nitrogen. Only the statistically significant soil properties are shown
4 Discussion
4.1 Temporal changes in the soil bacterial and archaeal communities under different fertilizer treatments
We detected a significant effect of soil temperature on the community structures of the total bacteria and archaea, similar to other researchers (Rasche et al., 2011; Lauber et al., 2013). Temperature is an important factor in microbial community composition (Hayden et al., 2012). The Gram-positive (G+) bacterial abundance responds negatively to the winter months with lower soil temperatures (Bell et al., 2009). Elevated temperatures increase the abundance of G+ bacteria, including Firmicutes and Actinobacteria, but decrease the abundance of Gram-nagative (G−) bacteria (Gray et al., 2011; Hayden et al., 2012). For archaeal community composition, Bomberg et al. (2011) found that the crenarchaeotal denaturing gradient gel electrophoresis (DGGE) band numbers decreased with increasing temperatures, while the number of euryarchaeotal DGGE bands increased. Interestingly, no significant differences were detected in the bacterial community structure between the CF and OF treatments three months after the last fertilization, indicating that the dynamics of the soil bacterial community structure was unrelated to the applied materials after a certain duration of fertilization. Such a time-related variation in the bacterial community was also found by Calbrix et al. (2007) and may be attributed to a seasonal effect. The bacterial and archaeal abundances changed significantly with the sampling time and were lower in the summer than in the winter (Rasche et al., 2011; Jung et al., 2012). In agreement with He et al. (2007), the abundance of total bacteria did not change significantly among the three treatments in the summer. Very low soil microbial counts were detected when the soil was sampled following a heavy rainfall (Pandey and Palni, 1996). There was a heavy rainfall (as great as 57 mm) at the experimental site on July 17, which might be the reason for the lowest abundance of total bacteria and archaea being detected on July 19.
Fertilizer amendments increase the soil nutrient levels, including the soil SOC, TN, and ammonium contents, and thus influence the microbial community structure (Nicol et al., 2003; Böhme et al., 2005). However, long-term fertilization did not significantly change the bacterial community composition (He et al., 2008; Ogilvie et al., 2008). Of the tested soil chemical properties, the C:N and ammonium content significantly influenced the bacterial and archaeal community structures. OF directly increased the SOC and pH, changed the soil C:N and supported the growth of specific microorganisms (Marschner et al., 2003; Gattinger et al., 2007). For example, Marschner et al. (2003) found that OF increased the ratio of G+ to G− bacteria compared to the ratio under inorganic fertilizer. The mechanisms by which inorganic N fertilizer changes the microbial community structure are complicated (Clegg, 2006) and possibly include several actions. First, mineral nitrogen, as a substrate for growth or respiration, may affect the microbial community structure through the competition of microbes (Clegg, 2006). Second, soil pH, which decreased after ammonium nitrogen fertilization, is an important driver of the microbial community structure (Hansel et al., 2008). Third, CFs significantly increase the soil TN, which can influence the soil C:N ratio, thereby affecting the soil microbial community structure. In addition, CF influences the soil C:N ratio by stimulating root exudates and increasing the litter-fall quantity through improving the growth of tea plants.
In this study, the bacterial and archaeal abundances were in the same range as those of other studies in acidic soils of other agro-ecosystem (He et al., 2007; Kemnitz et al., 2007). OF amendment increased the bacterial and archaeal abundances (Gattinger et al., 2007; Zhang et al., 2012; Chan et al., 2013), perhaps due to the enhancement of the SOC content. In addition, organic manure may be beneficial to the proliferation of microbes (Sun et al., 2004). However, the CF treatment did not significantly affect the total abundance of bacteria and archaea. Application of CF not only increases the soil C content, which increases the total abundance of bacteria and archaea, but also decreases the soil pH, which decreases the total abundances of bacteria and archaea (He et al., 2007).
4.2 Temporal changes in the abundance of AOA and AOB under different fertilizer treatments
Previously, many studies have reported temporal changes in the abundance of AOA and AOB and attributed these changes to variations in the soil moisture and temperature (Rasche et al., 2011). However, Andert et al. (2011) found that the abundance of AOA did not change over a 9-month experiment. In this study, we found variations in the abundances of AOA and AOB with the sampling time that was not correlated with the soil moisture or temperature. Temporal effect is a comprehensive pattern that includes abiotic and biotic factors, such as soil temperature and moisture, rainfall, and tea plant growth. In addition, the soil temperature and moisture affect the plant growth and the transformation of soil nutrients (Gutknecht et al., 2012), and therefore influence the abundances of AOA and AOB indirectly. However, this indirect effect cannot always be detected using a correlation analysis. Interestingly, Rasche et al. (2011) reported that the AOA and AOB abundances were lower in the summer than in the winter, but He et al. (2007) found a contradicted result. The reason for this inconsistency may be differences in the soil types, soil pH, plant types, and other environmental factors.
The abundances of AOA and AOB could be affected by fertilizer amendments. OF, as a source of bioavailable SOC (Ai et al., 2013; Chan et al., 2013), stimulated the abundances of AOA and AOB (He et al., 2007; Shen et al., 2008; Ai et al., 2013). Compared to the CK treatment, CF decreased the soil pH and AOA abundance, as reported by He et al. (2007). However, Ai et al. (2013) found a contradicted result in a calcareous fluvo-aquic soil, possibly because chemical fertilization did not reduce the soil pH in the calcareous soil as the soil pH was significantly correlated with the abundance of AOA. CF treatment significantly enhances AOB abundance in alkaline soils (Shen et al., 2008; Ai et al., 2013) but not in acidic soils (He et al., 2007). In this study, CF increased the AOB abundance due to the increase of SOC, most likely because of the increased litter quantity and the tea root residue. In addition, AOA were more abundant than AOB, indicating that AOA have a greater role in acidic soils (Yao et al., 2011; Zhang et al., 2011).
4.3 Temporal changes in the abundance of denitrifiers under different fertilizer treatments
Previous studies have demonstrated that the abundance of denitrifying genes fluctuates with the seasons (Dandie et al., 2008; Wakelin et al., 2009; Rasche et al., 2011), but no consistent trend was found (Dandie et al., 2008; Rasche et al., 2011). We found temporal changes in the abundance of denitrifying genes, such as narG, nirK, and nirS, which may be due to changes in the soil moisture because the soil moisture was significantly correlated with the abundance of denitrifying genes, with the exception of nosZ. The copy numbers of denitrifying genes, such as nirK and nirS, increase with increasing the water content (Szukics et al., 2010; Di et al., 2014), but nosZ communities exhibit a great stability in response to changes in the soil moisture (Stres et al., 2008). Pastorelli et al. (2011) reported that the season did not affect the nosZ-gene-bearing denitrifiers, but we detected a variation of nosZ with the sampling time. Of the measured soil chemical properties, the soil SOC, pH and C:N were significantly correlated with the copy numbers of the nosZ gene. The temporal effect on the copy numbers of the nosZ gene might be due to the combined function of the soil properties and should be studied in future research.
The quantification of the denitrifying genes revealed a lower abundance in tea orchard soil (Hallin et al., 2009; Chen et al., 2012; Clark et al., 2012). The abundances of the denitrifying genes were in the following order: narG>nirK>nirS>nosZ. This phenomenon may be caused by the different gene copies within denitrifier genomes (Chen et al., 2012) and the decreased concentration of the denitrifying intermediates in the soil following the reduction order (Yu et al., 2008). The greater abundance of nirK than of nirS observed in tea orchard soil was consistent with Clark et al. (2012), but a contrasting phenomenon was reported in grassland (Čuhel et al., 2010). The bacteria carrying nirK genes become more abundant in response to increased N, while the bacteria carrying nirS may have an advantage in low-N soils (Clark et al., 2012). In tea orchards, the soil N increased due to the input of a large amount of CF used to enhance the tea yield. The qPCR analysis revealed that the OF increased the abundance of denitrifiers due to the added biomass C. Because most denitrifiers are heterotrophs (Saunders et al., 2012), we also found a significant correlation between the SOC as well as the C:N and the number of denitrifying genes. However, Hallin et al. (2009) reported that an OF treatment alone did not significantly affect the abundance of denitrifiers. In general, no significant effect of CFs was detected. The CF amendments significantly decreased the soil pH, which was a negative factor regarding the copy numbers of denitrifying genes and could counteract the positive effects of higher SOC induced by CFs (Hallin et al., 2009). In addition, the effect of CFs on denitrifier abundance is likely to be related to the soil C:N ratio, which was reduced by the N fertilizer amendments, changing the population of denitrifiers (Rösch and Bothe, 2009).
5 Conclusions
In conclusion, the results of our field study in the acidic soils of tea orchards indicated that temporal alteration and fertilizer applications significantly influence not only the bacterial and archaeal communities but also the abundances of phylogenetic and functional markers, which can be increased by the use of OF. Future studies should focus on the temporal response of the microbial-driven ecological processes to fertilizer, especially OF, and the relationship between the microbial community and microbial-driven ecological processes in tea crop soil.
Compliance with ethics guidelines
Hua WANG, Shao-hui YANG, Jing-ping YANG, Ya-min LV, Xing ZHAO, and Ji-liang PANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Ai, C., Liang, G., Sun, J., et al., 2013. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem., 57: 30–42. [doi:10.1016/j.soilbio.2012.08.003]
Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol., 26(1): 32–46. [doi:10.1111/j.1442-9993.2001.01070.pp.x]
Andert, J., Wessén, E., Börjesson, G., et al., 2011. Temporal changes in abundance and composition of ammonia-oxidizing bacterial and archaeal communities in a drained peat soil in relation to N2O emissions. J. Soils Sediments, 11(8):1399–1407. [doi:10.1007/s11368-011-0413-9]
Bell, C.W., Acosta-Martinez, V., Mcintyre, N.E., et al., 2009. Linking microbial community structure and function to seasonal differences in soil moisture and temperature in a Chihuahuan desert grassland. Microb. Ecol., 58(4): 827–842. [doi:10.1007/s00248-009-9529-5]
Böhme, L., Langer, U., Böhme, F., 2005. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosyst. Environ., 109(1–2):141–152. [doi:10.1016/j.agee.2005.01.017]
Bomberg, M., Münster, U., Pumpanen, J., et al., 2011. Archaeal communities in boreal forest tree rhizospheres respond to changing soil temperatures. Microb. Ecol., 62(1):205–217. [doi:10.1007/s00248-011-9837-4]
Buckley, D.H., Schmidt, T.M., 2003. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol., 5(6):441–452. [doi:10.1046/j.1462-2920.2003.00404.x]
Calbrix, R., Barray, S., Chabrerie, O., et al., 2007. Impact of organic amendments on the dynamics of soil microbial biomass and bacterial communities in cultivated land. Appl. Soil Ecol., 35(3):511–522. [doi:10.1016/j.apsoil.2006.10.007]
Chan, Y.K., Mccormick, W.A., Ma, B., 2013. Effects of inorganic fertilizer and manure on soil archaeal abundance at two experimental farms during three consecutive rotation-cropping seasons. Appl. Soil Ecol., 68:26–35. [doi:10.1016/j.apsoil.2013.03.004]
Chen, Z., Liu, J., Wu, M., et al., 2012. Differentiated response of denitrifying communities to fertilization regime in paddy soil. Microb. Ecol., 63(2):446–459. [doi:10.1007/s00248-011-9909-5]
Clark, I.M., Buchkina, N., Jhurreea, D., et al., 2012. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the broadbalk wheat experiment. Philos. Trans. R. Soc. B Biol. Sci., 367(1593):1235–1244. [doi:10.1098/rstb.2011.0314]
Clegg, C.D., 2006. Impact of cattle grazing and inorganic fertiliser additions to managed grasslands on the microbial community composition of soils. Appl. Soil Ecol., 31(1–2):73–82. [doi:10.1016/j.apsoil.2005.04.003]
Čuhel, J., Šimek, M., Laughlin, R.J., et al., 2010. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol., 76(6):1870–1878. [doi:10.1128/AEM.02484-09]
Dandie, C.E., Burton, D.L., Zebarth, B.J., et al., 2008. Changes in bacterial denitrifier community abundance over time in an agricultural field and their relationship with denitrification activity. Appl. Environ. Microbiol., 74(19): 5997–6005. [doi:10.1128/AEM.00441-08]
Di, H.J., Cameron, K.C., Podolyan, A., et al., 2014. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol. Biochem., 73:59–68. [doi:10.1016/j.soilbio.2014.02.011]
Enwall, K., Philippot, L., Hallin, S., 2005. Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl. Environ. Microbiol., 71(12):8335–8343. [doi:10.1128/AEM.71.12.8335-8343.2005]
Galloway, J.N., Aber, J.D., Erisman, J.W., et al., 2003. The nitrogen cascade. Bioscience., 53(4):341–356. [doi:10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2]
Gattinger, A., Höfle, M.G., Schloter, M., et al., 2007. Traditional cattle manure application determines abundance, diversity and activity of methanogenic archaea in arable European soil. Environ. Microbiol., 9(3):612–624. [doi:10.1111/j.1462-2920.2006.01181.x]
Gray, S.B., Classen, A.T., Kardol, P., et al., 2011. Multiple climate change factors interact to alter soil microbial community structure in an old-field ecosystem. Soil Sci. Soc. Am. J., 75(6):2217–2226. [doi:10.2136/sssaj2011.0135]
Gutknecht, J.L., Field, C.B., Balser, T.C., 2012. Microbial communities and their responses to simulated global change fluctuate greatly over multiple years. Glob. Change Biol., 18(7):2256–2269. [doi:10.1111/j.1365-2486.2012.02686.x]
Hallin, S., Jones, C.M., Schloter, M., et al., 2009. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J., 3(5):597–605. [doi:10.1038/ismej.2008.128]
Hansel, C.M., Fendorf, S., Jardine, P.M., et al., 2008. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol., 74(5):1620–1633. [doi:10.1128/AEM.01787-07]
Hayden, H.L., Mele, P.M., Bougoure, D.S., et al., 2012. Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil. Environ. Microbiol., 14(12):3081–3096. [doi:10.1111/j.1462-2920.2012.02855.x]
He, J.Z., Shen, J.P., Zhang, L.M., et al., 2007. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol., 9(9):2364–2374. [doi:10.1111/j.1462-2920.2007.01358.x]
He, J.Z., Zheng, Y., Chen, C.R., et al., 2008. Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. J. Soils Sediments, 8(5):349–358. [doi:10.1007/s11368-008-0025-1]
Jung, J., Yeom, J., Han, J., et al., 2012. Seasonal changes in nitrogen-cycle gene abundances and in bacterial communities in acidic forest soils. J. Microbiol., 50(3): 365–373. [doi:10.1007/s12275-012-1465-2]
Kemnitz, D., Kolb, S., Conrad, R., 2007. High abundance of crenarchaeota in a temperate acidic forest soil. FEMS Microbiol. Ecol., 60(3):442–448. [doi:10.1111/j.1574-6941.2007.00310.x]
Lauber, C.L., Ramirez, K.S., Aanderud, Z., et al., 2013. Temporal variability in soil microbial communities across land-use types. ISME J., 7(8):1641–1650. [doi:10.1038/ismej.2013.50]
Marschner, P., Kandeler, E., Marschner, B., 2003. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem., 35(3): 453–461. [doi:10.1016/S0038-0717(02)00297-3]
Nelson, D., Sommers, L., 1982. Total carbon, organic carbon and organic matter. In: Page, A., Miller, R., Keeney, D. (Eds.), Methods of Soil Analysis. American Society of Agronomy, Madison, p.539–580.
Nicol, G.W., Glover, L.A., Prosser, J.I., 2003. The impact of grassland management on archaeal community structure in upland pasture rhizosphere soil. Environ. Microbiol., 5(3):152–162. [doi:10.1046/j.1462-2920.2003.00399.x]
Norman, R.J., Edberg, J.C., Stucki, J.W., 1985. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J., 49(5):1182–1185. [doi:10.2136/sssaj1985.03615995004900050022x]
Ogilvie, L.A., Hirsch, P.R., Johnston, A.W., 2008. Bacterial diversity of the broadbalk ‘classical’ winter wheat experiment in relation to long-term fertilizer inputs. Microb. Ecol., 56(3):525–537. [doi:10.1007/s00248-008-9372-0]
Pandey, A., Palni, L.M.S., 1996. The rhizosphere effect of tea on soil microbes in a himalayan monsoonal location. Biol. Fertil. Soils., 21(3):131–137. [doi:10.1007/BF00335924]
Pastorelli, R., Landi, S., Trabelsi, D., et al., 2011. Effects of soil management on structure and activity of denitrifying bacterial communities. Appl. Soil Ecol., 49:46–58. [doi:10.1016/j.apsoil.2011.07.002]
Peacock, A.G., Mullen, M., Ringelberg, D., et al., 2001. Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol. Biochem., 33(7–8): 1011–1019. [doi:10.1016/S0038-0717(01)00004-9]
Prosser, J.I., Nicol, G.W., 2012. Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol., 20(11): 523–531. [doi:10.1016/j.tim.2012.08.001]
Rasche, F., Knapp, D., Kaiser, C., et al., 2011. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J., 5(3):389–402. [doi:10.1038/ismej.2010.138]
Rösch, C., Bothe, H., 2009. Diversity of total, nitrogen-fixing and denitrifying bacteria in an acid forest soil. Eur. J. Soil Sci., 60(6):883–894. [doi:10.1111/j.1365-2389.2009.01167.x]
Saunders, O.E., Fortuna, A.M., Harrison, J.H., et al., 2012. Gaseous nitrogen and bacterial responses to raw and digested dairy manure applications in incubated soil. Environ. Sci. Technol., 46(21):11684–11692. [doi:10.1021/es301754s]
Shen, J.P., Zhang, L.M., Zhu, Y.G., et al., 2008. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol., 10(6):1601–1611. [doi: 10.1111/j.1462-2920.2008.01578.x]
Stark, C.H., Condron, L.M., O’Callaghan, M., et al., 2008. Differences in soil enzyme activities, microbial community structure and short-term nitrogen mineralisation resulting from farm management history and organic matter amendments. Soil Biol. Biochem., 40(6):1352–1363. [doi:10.1016/j.soilbio.2007.09.025]
Stres, B., Danevčič, T., Pal, L., et al., 2008. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol., 66(1): 110–122. [doi:10.1111/j.1574-6941.2008.00555.x]
Sun, B., 2007. Protocols for Standard Soil Observation and Measurement in Terrestrial Ecosystems. China Environmental Science Press, Beijing, China, p.165–166 (in Chinese).
Sun, H., Deng, S., Raun, W., 2004. Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl. Environ. Microbiol., 70(10):5868–5874. [doi:10.1128/AEM.70.10.5868-5874.2004]
Szukics, U., Abell, G.C., Hödl, V., et al., 2010. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol., 72(3):395–406. [doi:10.1111/j.1574-6941.2010.00853.x]
Wakelin, S.A., Gregg, A.L., Simpson, R.J., et al., 2009. Pasture management clearly affects soil microbial community structure and N-cycling bacteria. Pedobiologia, 52(4):237–251. [doi:10.1016/j.pedobi.2008.10.001]
Wang, Y., Zhu, G., Song, L., et al., 2014. Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. J. Basic Microbiol., 54(3):190–197. [doi:10.1002/jobm.201200671]
Wessén, E., Nyberg, K., Jansson, J.K., et al., 2010. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol., 45(3):193–200. [doi:10.1016/j.apsoil.2010.04.003]
Wolsing, M., Priemé, A., 2004. Observation of high seasonal variation in community structure of denitrifying bacteria in arable soil receiving artificial fertilizer and cattle manure by determining T-RFLP of nir gene fragments. FEMS Microbiol. Ecol., 48(2):261–271. [doi:10.1016/j.femsec.2004.02.002]
Yao, H., Gao, Y., Nicol, G.W., et al., 2011. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microbiol., 77(13):4618–4625. [doi:10.1128/AEM.00136-11]
Yu, K., Struwe, S., Kjøller, A., et al., 2008. Denitrification rate determined by nitrate disappearance is higher than determined by nitrous oxide production with acetylene blockage. Ecol. Eng., 32(1):90–96. [doi:10.1016/j.ecoleng. 2007.09.006]
Zhang, L.M., Hu, H.W., Shen, J.P., et al., 2011. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J., 6(5):1032–1045. [doi:10.1038/ismej.2011.168]
Zhang, Q.C., Shamsi, I.H., Xu, D.T., et al., 2012. Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl. Soil Ecol., 57:1–8. [doi:10.1016/j.apsoil.2012.02.012]
Zumft, W.G., 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev., 61(4): 533–616.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Research Fund for the Doctoral Program of Higher Education of China (No. 20130101110127) and the Project of Zhejiang Key Scientific and Technological Innovation Team (No. 2010R50039), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1400114) contains supplementary materials, which are available to authorized users
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, H., Yang, Sh., Yang, Jp. et al. Temporal changes in soil bacterial and archaeal communities with different fertilizers in tea orchards. J. Zhejiang Univ. Sci. B 15, 953–965 (2014). https://doi.org/10.1631/jzus.B1400114
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1400114