Abstract
Purpose
Boreal peat soils comprise about 3% of the terrestrial environments, and when drained, they become sources of the greenhouse gas nitrous oxide (N2O). Ammonia oxidation can result in N2O emissions, either directly or by fuelling denitrification, but we know little about the ecology of ammonia-oxidizing bacteria (AOB) and archaea (AOA) in peat soils. Our aim was to determine temporal alterations in abundance and composition of these communities in a drained and forested peat soil in relation to N2O emissions and ammonia oxidation activity.
Materials and methods
The peat was sampled at three different depths in the upper 0.5 m over a period of 9 months covering two summer and two winter samplings. Community composition and abundance were determined by T-RFLP and quantitative real-time PCR of the bacterial and archaeal amoA genes. Potential ammonia oxidation rates were measured using the chlorate inhibition technique, and in situ N2O emission was determined using chambers.
Results and discussion
The soil parameters displayed little spatial and temporal heterogeneity, which probably explained why there were no depth-related effects on the abundance, composition, or activity of the ammonia oxidizers. In contrast to most terrestrial environments, the AOB dominated numerically over the AOA. Both groups changed in community composition between sampling occasions, although the AOB showed more significant seasonal signatures than the AOA. Temporal changes in abundance were only observed for the AOB, with a decrease in numbers from May to March. Such differences were not reflected by the activity or N2O emissions.
Conclusions
The high ammonium concentrations in the peat soil likely favored the AOB over the AOA, and we hypothesize that they were more active than the AOA and therefore responded to climatic and environmental changes. However, other processes rather than ammonia oxidation were likely responsible for N2O emissions at the site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrification is a central process in nitrogen cycling that involves the rate-limiting step of oxidation of ammonia to nitrite, which is then further oxidized to nitrate. This process has ramifications for the environment since it results in nitrate leaching from terrestrial ecosystems and contributes both directly to emissions of the greenhouse gas nitrous oxide (N2O) from soil (Bremner et al. 1981; Martikainen and de Boer 1993; Martikainen 1985; Schuster and Conrad 1992) and indirectly by providing the essential electron acceptor for denitrification. Unfortunately, N2O is not only a potent greenhouse gas but also predicted to be the dominant ozone-depleting substance of our century (Ravishankara et al. 2009). Both ammonia-oxidizing bacteria (AOB), belonging to the β-Proteobacteria, and the ammonia-oxidizing archaea (AOA), which are members of the mesophilic Thaumarchaeota, are involved in ammonia oxidation in soil. It has frequently been shown that the AOA are more abundant than the AOB in most soils (He et al. 2007; Jia and Conrad 2009; Leininger et al. 2006; Shen et al. 2008). A few studies have provided direct proof for the actual contribution of AOA or AOB to ammonia oxidation, and the conflicting results from these studies imply that the relative importance of AOB and AOA in soil may vary depending on the environmental conditions or soil type (Di et al. 2009; Jia and Conrad 2009; Leininger et al. 2006; Mertens et al. 2009; Offre et al. 2009; Tourna et al. 2008; Zhang et al. 2010).
Little is known about ammonia-oxidizing communities in boreal and subarctic peat soils. These soils cover nearly 3% of the Earth’s land surface (346 million hectares; Gorham 1991) and play a significant role in global climate change due to the emissions and uptake of greenhouse gasses. Natural peat soils are usually a source of methane and a sink for carbon dioxide (Regina et al. 1996); however, when drained for peat extraction, forestry, or agriculture, these soils become major sources of N2O. Understanding the ecology of bacterial and archaeal ammonia oxidizers in drained peat soils is therefore essential. If the AOB and AOA occupy different niches, as suggested from previous work (Wessén et al. 2011), changes in environmental conditions could result in temporal differences in the relative abundance of AOB and AOA. External factors like seasonality and other temporal alterations in soil moisture, temperature, and other climatic conditions were recently shown to affect the abundances of the AOB and AOA communities in temperate forests soils (Rasche et al. 2010). Our aim was to determine if we also have temporal differences in the abundance of the AOB and AOA and the ammonia oxidation capacity in a drained peat soil and if that would influence the in situ emissions of N2O. For that purpose, a forested soil was sampled at four occasions over a 9-month period at three different depths in the upper 0.5 m peat layer. Two of the sampling occasions were during the warm spring and summer seasons and the other two during the cold fall and winter seasons. Since the relatively wide ecophysiological diversity known within the AOB of the β-Proteobacteria (Koops et al. 2006; Kowalchuk and Stephen 2001) alone allow them to occupy different niches, the distribution of ecotypes within the AOB may also vary over time. Thus, we also determined temporal changes in the AOB and AOA community composition. The physiological breadth of AOA is less known, but the isolated representatives Nitrosopumilus maritimus and Nitrososphaera viennensis are adapted to lower ammonia concentrations than the bacterial ammonia oxidizers (Martens-Habbena et al. 2009; Tourna et al. 2011). The composition and size of the genetic pool of ammonia oxidizers were analyzed in terms of terminal-restriction fragment length polymorphism (T-RFLP) and quantitative PCR of bacterial and archaeal amoA genes, and substrate-induced ammonia oxidation rates were determined to estimate the pool of readily activated enzymes.
2 Materials and methods
2.1 Field site, sampling, and determination of soil properties
The sampling site is a forested peat soil situated in the southwest of Sweden (58°20′N; 13°30′ E). Soils were drained in the 1920s for peat extraction, and in the 1960s, the area was colonized by birch (Betula pendula). The average peat depth is 2.2 m with 80% organic matter. To represent the site, four replicated locations (A–D) with 3 m in between were sampled at four occasions over a 9-month period, with two sampling occasions each during cold and warm periods with and without freezing events. Samples taken in May and August 2008 corresponded to the warmer spring and summer seasons, and the daily mean temperatures during the week preceding the time of sampling were 14.2°C (4.2min–22.5max) and 21.6°C (13.0min–28.9max), respectively. For the cold fall and winter seasons, samples were taken in November 2008 and March 2009, and the mean temperatures were 4.7°C (−0.7min–9.2max) and −0.6°C (−4.5min–5.7max) with night temperatures below 0°C. Soil was sampled with an auger at three different depths (0–15, 15–30, and 30–45 cm). Directly after sampling, water content (overnight drying at 105°C), organic matter (OM) content (weight loss after ignition at 550°C for 4 h), and pH(H2O) were determined. Total organic nitrogen (TON), total organic carbon (TOC), NH4-N and NO3-N were determined by AgriLab (Uppsala, Sweden) using standard procedures. The soil samples were stored at 2°C for 2 weeks before measuring potential ammonia oxidation activities, and soil for DNA extraction was frozen until use.
2.2 Determination of N2O emissions and ammonia oxidation activity
Emissions were monitored as N2O accumulation over time in 7.4-l tin chambers with 0.037 m2 base area placed on each sampling location. Six 12-ml samples were withdrawn during 1 h according to Weslien et al. (2009). The gas samples were analyzed using a gas chromatograph (Perkin Elmer Clarus500, USA) equipped with an EC detector. Only regression lines with R 2 > 0.5 (linear fit) were regarded as emission. The sum of emitted N2O from the four replicate locations was used to estimate the emissions at each sampling occasion.
The potential for ammonia oxidation was measured as substrate-induced ammonia oxidation (SIAO) rates determined from accumulated nitrite induced by the addition of ammonium using the chlorate inhibition technique (Belser and Mays 1980) modified by Torstensson (1993). In brief, 100 ml of 25 mM potassium phosphate buffer (pH 7.2), containing 7.5 mM (di-) ammonium sulfate and 15 mM sodium chlorate, was added to 10 g of wet soil sample, and the soil slurries were incubated at 15°C for 4.5 h on a shaker at 180 rpm. Every hour, the supernatant was sampled and added to 4 mM KCl (1:1). Nitrite was analyzed spectrophotometrically by flow injection analysis with 2 M potassium chloride as the carrier (FIA, Tecator, Höganäs, Sweden; application note ASN 51-01/84), and the ammonia oxidation rate was calculated by linear regression of the accumulated nitrite over time.
2.3 DNA extraction
Total DNA was extracted from 150 mg dry peat according to Stralis-Pavese et al. (2004) except that no potassium acetate was added. The DNA concentration was determined using a Nanovue spectrophotometer (GE Healthcare,Uppsala, Sweden).
2.4 Real-time PCR quantification of amoA genes
Quantitative real-time PCR of amoA genes was performed to estimate the abundance of the ammonia-oxidizing bacterial and archaeal communities, respectively. The primers amoA-1F (5′-GGGGTTTCTACTGGTGGT-3′) and amoA-2R (5′-CCCCTCKGSAAAGCCTTCTTC-3′) were used for the AOB (Rotthauwe et al. 1997); and CrenamoA23f (5′-ATGGTCTGGCTWAGACG-3′) and CrenamoA616r (5′-GCCATCCATCTGTATGTCCA-3′) were used for the AOA (Tourna et al. 2008) which generated a 491-bp and 624-bp long fragment, respectively. The quantification was based on the fluorescence intensity of the SYBR Green dye and performed in a total volume of 20 μl using DyNAmo Flash SYBR Green qPCR Kit (Finnzymes), 0.5 μM of each primer, and 10 ng of soil DNA. Bovine serum albumin (BSA) was added to reach final concentrations of 0.8 μg μl–1. The amoA fragments were amplified using an initial denaturation step at 95°C for 15 min, followed by 35 cycles of 15 s at 95°C, 30 s at 55°C, and 30 s at 72°C and 30 s at 80°C in a Bio-Rad IQ5 thermal cycler. Data were retrieved at 80°C, and all reactions were finished with a melting curve starting at 60°C with an increase of 0.5°C up to 95°C. The PCR reaction runs had an efficiency of 103% and 97% for the AOB and AOA, respectively. Standard curves were obtained using serial dilutions of linearized plasmids (pGEM-T, Promega) containing cloned bacterial and archaeal amoA genes amplified from soil. Controls without templates resulted in undetectable values in all samples. Inhibitory effects on PCR performance were tested for all samples by adding a known amount of a circular plasmid mixed with a known amount of DNA, as well as samples with a known amount of circular plasmid mixed with water. There were no differences in the measured cycle threshold values for the different samples compared with those measured for the controls with water.
2.5 PCR amplification and T-RFLP analysis of amoA genes
The amoA genes from AOB and AOA were amplified in triplicate from each DNA extract using 10–20 ng DNA with 200 μM of the same primers as used for the quantification, except that the forward primers were 5-hexachlorofluorescein labeled (Rotthauwe et al. 1997; Tourna et al. 2008). Further, 1× PCR buffer, 2.5 U Taq polymerase, 1.5 mM MgCl2, and 1.5 μg μl–1 BSA were used. The PCR was run for 35 cycles following the procedure described in Rotthauwe et al. (1997) for the AOB and Sahan and Muyzer (2008) for the AOA, respectively. The amplicons from the triplicate reactions for each sample were pooled and purified with MinElute PCR purification kit (Qiagen, Hilden, Germany). For each sample, 50 ng DNA was digested using 10 units of HaeIII for the AOB and MboI for the AOA, following the instructions of the manufacturer (New England Biolabs, Ipswich, USA). The enzymes were selected based on in silico restriction analysis of 50 amoA sequences from AOB and AOA, respectively. Fluorescently labeled terminal restriction fragments (T-RFs) were separated and detected using an ABI 3730 capillary sequencer (Applied Biosystems, Foster City, USA), and the T-RF fingerprints were visualized using the Peak Scanner Software (Applied Biosystems). The T-RFs were normalized by column total, excluding those shorter than 50 bp or contributing with less than 0.5% of the total peak area. The average peak number in the community fingerprints was 7 for the AOB and 6 for the AOA.
2.6 Statistical analysis
Soil parameters, SIAO rates, and abundance of AOB and AOA at different depths and times were compared by ANOVA at P < 0.05. Pairwise relationships between soil parameters, N2O emissions, SIAO rates, and abundance of AOB and AOA were explored by Pearson correlations. Data that were not normally distributed were first Box–Cox transformed. The OM was excluded due to missing values for one sampling occasion.
For graphical representation of community relationships among samples, the T-RFLP profiles of the AOB and AOA communities were analyzed by nonmetric multidimensional scaling (NMS) using PC-ORD version 5.10 (MjM Software, Oregon, USA). The community matrices were based on presence–absence of T-RFs, and the Bray–Curtis distance measure was used. The NMS was run using a random starting configuration, a maximum of 500 iterations, and an instability criterion of 0.00001 and performed on 250 runs with the real data followed by 250 runs with random data to test for the null hypothesis. Soil parameters were combined into a secondary matrix and normalized by column totals before being incorporated into the analysis through the use of biplot ordinations. The correlations between soil parameters and NMS axes were shown as vectors indicating the direction and strength of the correlation. Seasonal differences in the ordinations were demonstrated by PerMANOVA. Thus, pairwise comparisons among seasons were performed using permutational multivariate analysis of variance as described by Anderson (2001) using the function adonis in the package vegan in R (Oksanen et al. 2009), and probabilities were corrected for multiple comparisons using the Hochberg correction (Hochberg 1988).
3 Results
3.1 Environmental parameters, N2O emissions, and SIAO rates
Water content, pH, TOC, organic matter content, and C/N ratio increased with depth at all sampling occasions (Table 1; Tables S1a and b of the Electronic supplementary material). The NH +4 concentration decreased with depth, with the 0–15-cm layer being significantly different from the 30–45-cm layer (P < 0.05). By contrast, the TON content remained the same at all occasions and depths, and the NO −3 did not show any consistent pattern with depth. The only temporal changes in soil properties were the NH +4 content that was significantly higher at the sampling occasion in March at all depths and the NO −3 content that was significantly lower at this occasion throughout the soil profile than at the other sampling times (P < 0.05).
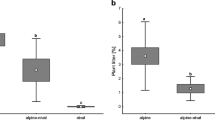
The N2O emissions displayed an increasing trend over the sampling period, with greater emissions in the winter than the summer period (Fig. 1). The emissions were negatively correlated to soil NO −3 content, which decreased over the period, but otherwise did not correlate significantly to any of the measured soil parameters (Table 2). In contrast to the emissions, the SIAO rates were similar at all sampling occasions with tendencies for lower rates in the deepest soil layer, but this difference was not significant. Further, the SIAO correlated negatively to pH, TON, and NH +4 (see Table 2).
3.2 Abundance of ammonia-oxidizing bacteria and archaea
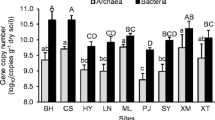
The amoA gene copy numbers, reflecting the AOB and AOA community sizes, were in the range of 9.1 × 106 to 8.0 × 108 and 1.0 × 106 to 6.5 × 108 copies per gram of dry weight soil, respectively (Fig. 2; Table S2 a and b of the Electronic supplementary material). The extreme values were only detected in single samples and in all, but 2 of the 48 samples, the AOB were more abundant than the AOA. The mean values for each soil layer show that the AOB numbers exceeded those of the AOA at all occasions (see Fig. 2). Neither the AOB nor the AOA abundance changed significantly according to depth, and no temporal differences were observed between depths. However, the total AOB abundance comprising all sampling depths decreased over the time period, resulting in significant differences between the samples from May and those from November and March. This was not observed for the total AOA abundance, which did not differ throughout the 9-month period. The AOB abundance was positively correlated to the C/N ratio and negatively correlated to the TON (see Table 2). Neither the AOB nor AOA abundance correlated to SIAO rates or N2O emission.
Abundance of amoA genes per gram dry weight soil in ammonia-oxidizing bacteria (AOB) and archaea (AOA) at different soil depths and sampling times. The ratio between the total AOB and AOA amoA gene abundance throughout the entire soil profile at each season is stated in boxes above the bars. The same letters indicate sampling occasions without significant differences within the bacterial and archaeal community, respectively (P < 0.05)
3.3 Community composition of ammonia-oxidizing bacteria and archaea
The AOB and AOA communities did not differ with depth (data not shown), but we observed significant temporal differences in both the AOB and AOA communities (Figs. 3 and 4). The NMS ordination of the AOB communities among samples indicated that both the May and August samples were clustered, whereas the November and March samples were scattered (see Fig. 3). Permutated multivariate analysis of variance (MANOVA) and pairwise comparisons of the distance matrices of the seasons verified that the May and August samples were significantly different from each other and November and March samples, but the November and March samples were not significantly different from each other. For the AOA communities, only the August samples formed a separate cluster in the NMS ordination, whereas the other samples overlapped (see Fig. 4). These findings were supported by a permutated MANOVA followed by pairwise comparison of sampling months, showing that only August was significantly different from the other sampling times. None of the soil parameters correlated with the AOB ordination, but pH correlated significantly to the AOA ordination (see Figs. 3 and 4). Correlations between the AOB or AOA communities to N2O emission and SIAO could not be observed.
Nonmetric multidimensional scaling of T-RFLP fingerprints of the archaeal amoA genes from all sampling locations and depths from May (white circle), August (black circle), November (white triangle), and March (black triangle) with a three-dimensional solution. The arrow refers to pH that correlated to the sample distribution in the ordination. Stress value 14.6
4 Discussion
In contrast to most other studies of ammonia oxidizers in soil, the AOB were more abundant than the AOA at all soil depths and sampling occasions. Most often, the AOA have been shown to dominate considerably over AOB when amoA gene copy numbers have been determined in various soil types (He et al. 2007; Jia and Conrad 2009; Leininger et al. 2006; Nicol et al. 2008; Shen et al. 2008). Results in agreement with ours have been reported from estuaries (Caffrey et al. 2007; Santoro et al. 2008) and a forest soil (Boyle-Yarwood et al. 2008). Interestingly, in an agricultural mineral soil subject to long-term peat amendment, the AOB exceeded the AOA in numbers, whereas the AOA were dominating in the other treatments amended with other organic or inorganic fertilizers at the same experimental site (Hallin et al. 2009; Wessén et al. 2010). The only other study so far quantifying ammonia oxidizers in organic soils showed that AOA were more abundant than AOB in a peat wetland, except in areas with high ammonium concentrations where the AOB dominated (Höfferle et al. 2010). The dominance of AOB in the present study could be explained by the stable and relatively high ammonium levels at the site throughout the sampling period and peat profile. In agreement, AOB have been shown to be the predominating ammonia oxidizers in nutrient-rich environments such as activated sludge (Wells et al. 2009) and proposed to be functionally more important in nitrogen-rich environments compared to the AOA (Di et al. 2009; Di et al. 2010b; Jia and Conrad 2009; Zhang et al. 2010). Moreover, Erguder et al. (2009) summarized the environmental conditions related to the dominance of AOA and suggested low-nutrient conditions as a potential niche for the AOA, which fits with the fact that the well-studied ammonia-oxidizing archaea N. maritimus, isolated from the marine environment, is adapted to low ammonia concentrations (Martens-Habbena et al. 2009). However, the recent soil isolate N. viennensis can use higher ammonia concentrations than the marine isolate, although this is still lower than what the bacterial ammonia oxidizers Nitrosospira spp. and Nitrosomonas spp. tolerate (Tourna et al. 2011).
We observed significant temporal differences in the abundance of bacterial ammonia oxidizers, with the AOB being more abundant during the warmer sampling occasions in comparison to the colder period, whereas the community size of archaeal ammonia oxidizers did not change over time. He et al. (2007) reported a decrease of both AOB and AOA abundance in winter in an agricultural soil. Seasonal dynamics were also observed for both AOB and AOA in a temperate beech forest soil, and they were tightly coupled with seasonal alterations in labile N pools as well as with variation in soil temperature and soil moisture (Rasche et al. 2010). In our study, the soil parameters exhibited minimal variation over time, but physicochemical soil parameters that were not determined may have influenced the bacterial ammonia oxidizers in addition to temperature and other climatic conditions. We hypothesize that the AOB are more active due to the relatively high ammonium concentrations in the peat soil, and therefore, the community is more dynamic in response to environmental change. With respect to community composition, the AOB also showed more significant seasonal signatures than the AOA. Soil bacterial communities in general have frequently been shown to change in composition with seasons (Bardgett et al. 1999; Lipson and Schmidt 2004; Smit et al. 2001). Nevertheless, Laverman et al. (2001) found temporal stability of the bacterial ammonia-oxidizing community structure in a nitrogen-saturated forest soil. In contrast to temporal changes, there were no depth-related differences in community abundance or composition. Others have shown that the AOA community structure differed with depth (Leininger et al. 2006) and that the community size of AOB decreased with increasing soil depth, while the AOA abundance varied only a little resulting in an increased relative abundance of archaeal ammonia oxidizers (Jia and Conrad 2009; Leininger et al. 2006). In addition, the ammonia oxidation activity has been shown to decrease with increasing soil depth in agricultural and mineral soils (Berg and Rosswall 1987; Bohrerova et al. 2004; Swensen and Bakken 1998), which contrast the similar ammonia oxidation capacity throughout the peat profile in our study. That the soil parameters had little spatial heterogeneity probably explains why we did not find any depth-related effects on the ammonia oxidizers.
The temporal differences in abundance and community structure in our study were not reflected by differences in activity, neither as N2O emissions or ammonia oxidation rates. However, Di et al. (2010a) reported that the total N2O emissions from grazed grasslands with high ammonium concentrations were significantly related to the amoA gene copy numbers from the AOB community. This suggests that the AOB rather than the AOA are responsible for N2O emissions from nitrogen-rich soils, although it still remains unclear if the AOA have the genetic makeup for N2O production (Schleper and Nicol 2010). In agreement with our study, Hermansson et al. (2004) detected an increased abundance of AOB between April and September that was uncoupled with ammonia oxidation capacity in a coniferous forest soil. Both the DNA-based approach to estimate community size and the substrate-induced potential for ammonia oxidation rely on past events determining growth of ammonia oxidizers, but the gene copy numbers may reflect even more historical rates of substrate supply. The stable SIAO rates in the present study were comparable to what has been measured in arable or forest soil (Berg and Rosswall 1985; He et al. 2007; Killham 1987), and gross nitrification rates determined in a peat soil were in the same order of magnitude as the substrate-induced rates presented in our study (Mørkved et al. 2007). In forest soils, temporal differences in nitrification activity have been observed (Laverman et al. 2000), and Reay et al. (2005) showed that seasonality of ammonia oxidation could be strongly dependent on which tree species that were present. In arable soil, ammonia oxidation capacity has been shown to be particularly high in spring and autumn (Berg and Rosswall 1987). The potential ammonia oxidation rates in the present study were not related to the in situ N2O emissions. The emissions were in the same range as reported for other organic soils (Kasimir-Klemedtsson et al. 1997; Maljanen et al. 2001; Nykanen et al. 1995; Regina et al. 2004; Weslien et al. 2009), and likely reflect not only N2O emissions from ammonia oxidation but also denitrification and other nitrogen-transforming processes producing N2O at the time of sampling. By contrast, the SIAO assay measures only the activity of ammonia oxidizers that are either active at the time of sampling or can be activated during the incubation and therefore rather reflects previous conditions determining growth of ammonia oxidizers than in situ conditions affecting N2O emissions.
In conclusion, the AOB dominated over the AOA in abundance and displayed greater temporal variation in both abundance and community composition. The high ammonium concentrations in the peat soil likely favored the AOB, and we hypothesize that they were more active than the AOA and therefore responded to climatic changes. However, we could not relate the AOB and AOA communities in the peat soil to N2O emissions from the site.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC (1999) Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biol Biochem 31:1021–1030
Belser LW, Mays EL (1980) Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol 39:505–510
Berg P, Rosswall T (1985) Ammonium oxidizer numbers, potential and actual oxidation rates in two Swedish arable soils. Biol Fertil Soils 1:131–140
Berg P, Rosswall T (1987) Seasonal variations in abundance and activity of nitrifiers in four arable cropping systems. Microb Ecol 13:75–87
Bohrerova Z, Stralkova R, Podesvova J, Bohrer G, Pokorny E (2004) The relationship between redox potential and nitrification under different sequences of crop rotations. Soil Till Res 77:25–33
Boyle-Yarwood SA, Bottomley PJ, Myrold DD (2008) Community composition of ammonia-oxidizing bacteria and archaea in soils under stands of red alder and Douglas fir in Oregon. Environ Microbiol 10:2956–2965
Bremner JM, Breitenbeck GA, Blackmer AM (1981) Effect of anhydrous ammonia fertilization on emission of nitrous oxide from soils. J Environ Qual 10:77–80
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geosci 2:621–624
Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS (2010a) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soils Sediments 10:943–954
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010b) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Hallin S, Jones CM, Schloter M, Philippot L (2009) Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J 3:597–605
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hermansson A, Bäckman JSK, Svensson BH, Lindgren P-E (2004) Quantification of ammonia-oxidizing bacteria in limed and non-limed acidic coniferous forest soil using real-time PCR. Soil Biol Biochem 36:1935–1941
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biomet 75:800–802
Höfferle S, Nicil G, Pal L, Hacin J, Prosser JI, Mandic-Mulec I (2010) Ammonium supply rate influences archaeal and bacterial ammonia oxidisers in a wetland soil vertical profile. FEMS Microbiol Ecol 74:302–315
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Kasimir-Klemedtsson Å, Klemedtsson L, Berglund K, Martikainen P, Silvola J, Oenema O (1997) Greenhouse gas emissions from farmed organic soils: a review. Soil Use Manage 13:245–250
Killham K (1987) A new perfusion system for the measurement and characterization of potential rates of soil nitrification. Plant Soil 97:267–272
Koops H-P, Purkhold U, Pommerening-Röser A, Timmermann G, Wagner M (2006) The lithoautotrophic ammonia-oxidizing bacteria. The Prokaryotes 5:778–811
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Laverman AM, Zoomer HR, van Verseveld HW, Verhoef HA (2000) Temporal and spatial variation of nitrogen transformations in a coniferous forest soil. Soil Biol Biochem 32:1661–1670
Laverman A, Speksnijder A, Braster M, Kowalchuk G, Verhoef H, van Verseveld H (2001) Spatiotemporal stability of an ammonia-oxidizing community in a nitrogen-saturated forest soil. Microb Ecol 42:35–45
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl Environ Microbiol 70:2867–2879
Maljanen M, Hytönen J, Martikainen PJ (2001) Fluxes of N2O, CH4 and CO2 on afforested boreal agricultural soils. Plant Soil 231:113–121
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–U234
Martikainen PJ (1985) Nitrous oxide emission associated with autotrophic ammonium oxidation in acid coniferous forest soil. Appl Environ Microbiol 50:1519–1525
Martikainen PJ, de Boer W (1993) Nitrous oxide production and nitrification in acidic soil from a Dutch coniferous forest. Soil Biol Biochem 25:343–347
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E (2009) Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J 3:916–923
Mørkved PT, Dörsch P, Bakken LR (2007) The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol Biochem 39:2048–2057
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Nykanen H, Alm J, Lang K, Silvola J, Martikainen PJ (1995) Emissions of CH4, N2O and CO2 from a virgin fen and a fen drained for grassland in Finland. J Biogeography 22:351–357
Offre PR, Prosser JI, Nicol GW (2009) Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70:99–108
Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H (2009) Vegan: community ecology package. R package version 1.16-12. http://vegan.r-forge.r-project.org/. Accessed Feb 2009
Rasche F, Knapp D, Kaiser C, Koranda M, Kitzler B, Zechmeister-Boltenstern S, Richer A, Sessitsch A (2010) Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J 5:389–402
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Reay DS, Nedwell DB, McNamara N, Ineson P (2005) Effect of tree species on methane and ammonium oxidation capacity in forest soils. Soil Biol Biochem 37:719–730
Regina K, Nykänen H, Silvola J, Martikainen P (1996) Fluxes of nitrous oxide from boreal peatlands as affected by peatland type, water table level and nitrification capacity. Biogeochem 35:401–418
Regina K, Syväsalo E, Hannukkala A, Esala M (2004) Fluxes of N2O from farmed peat soils in Finland. Europ J Soil Sci 55:591–599
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Sahan E, Muyzer G (2008) Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerschelde estuary. FEMS Microbiol Ecol 64:175–186
Santoro AE, Francis CA, de Sieyes NR, Boehm AB (2008) Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ Microbiol 10:1068–1079
Schleper C, Nicol GW (2010) Ammonia-oxidising archaea—physiology, ecology and evolution. In: Pool RK (ed) Advances in microbial physiology, vol 57. Academic, Oxford, pp 1–41
Schuster M, Conrad R (1992) Metabolism of nitric oxide and nitrous oxide during nitrification and denitrification in soil at different incubation conditions. FEMS Microbiol Lett 101:133–143
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Smit E, Leeflang P, Gommans S, van den Broek J, van Mil S, Wernars K (2001) Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl Environ Microbiol 67:2284–2291
Stralis-Pavese N, Sessitsch A, Weilharter A, Reichenauer T, Riesing J, Csontos J, Murrell CJ, Bodrossy L (2004) Optimization of diagnostic microarray for application in analysing landfill methanotroph communities under different plant covers. Environ Microbiol 6:347–363
Swensen B, Bakken LR (1998) Nitrification potential and urease activity in a mineral subsoil. Soil Biol Biochem 30:1333–1341
Torstensson L (ed) (1993) Guidelines—soil biological variables in environmental hazard assessment. Swedish Environmental Protection Agency report no. 4262. Swedish EPA, Stockholm
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108:8420–8425
Wells GF, Park HD, Yeung CH, Eggleston B, Francis CA, Criddle CS (2009) Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ Microbiol 11:2310–2328
Weslien P, Klemedtsson ÅK, Börjesson G, Klemedtson L (2009) Strong pH influence on N2O and CH4 fluxes from forested organic soils. EurJ Soil Sci 60:311–320
Wessén E, Nyberg K, Jansson JK, Hallin S (2010) Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl Soil Ecol 45:193–200
Wessén E, Söderström M, Stenberg B, Hellman M, Thomsen F, Klemedtson L, Philippot L, Hallin S (2011) Spatial distribution of ammonia oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J 5(7):1213–1225
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci USA 107:17240–17245
Acknowledgments
We thank C. Jones for statistical help. The Swedish University of Agricultural Sciences, The Swedish Research Council Formas financing the Uppsala Microbiomics Center, and the METHECO project (European Science Foundation) provided funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ji-Zheng He
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 163 kb)
Rights and permissions
About this article
Cite this article
Andert, J., Wessén, E., Börjesson, G. et al. Temporal changes in abundance and composition of ammonia-oxidizing bacterial and archaeal communities in a drained peat soil in relation to N2O emissions. J Soils Sediments 11, 1399–1407 (2011). https://doi.org/10.1007/s11368-011-0413-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0413-9