Abstract
Understanding fundamental mechanisms for surface electronic excitation is of great importance in surface chemistry. Charge transport through metal-oxide interfaces plays a significant role in heterogeneous catalysis. Over the last several decades, a number of experimental and theoretical results suggest that this charge flow through metal-support interfaces leads to catalytic enhancement often observed in mixed catalysts. Direct measurement of charge flow on actual catalysts is a rather challenging task because it requires the use of an electronic circuit. This approach has been enabled by a catalytic nanodiode that is mainly composed of a catalytic metal and semiconducting oxides that form a Schottky contact. In this article, we describe the advances in this approach. We show that there is close connection between the phenomena of hot-electron creation and chemical reaction that occur at both gas-solid and liquid-solid interfaces. The intensity of hot-electron flow is well correlated with the turnover rates of corresponding reactions, which opens the possibility for developing new operando methodologies to monitor catalytic reactions as well as a novel scheme for the electronic control of chemical reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In heterogeneous catalysis, one of the key technologies for increasing the activity and selectivity of a chemical reaction is the use of catalytically active metal nanoparticles (NPs) in combination with a suitable support.1–6 This approach is based on earlier work that revealed significant changes in the activity of fine metal particles when placed on the support of a metal oxide, even though the support itself is inactive for this reaction. Specifically, in the 1960s, Schwab et al. discovered significant changes in the activity of fine metal particles when placed on the support of a metal oxide,7,8 and attributed this effect to the formation of a Schottky barrier at the metal-oxide interface with the subsequent transfer of charge carriers through the barrier, which affects the course of the surface reaction.3,7,9 This effect was also investigated by Boffa et al.10 using rhodium deposited on various reducible oxides. They observed a remarkable 14-fold increase in catalytic activity for CO2 hydrogénation on three different oxides—TiOx, NbOx, and TaOx. The activity was highest at a half monolayer of oxide coverage where the oxide–metal interface area was at a maximum. Later, these phenomena were referred to as the strong metal-support interaction (SMSI) effect,11 which indicates the enhancement of catalytic activity when Group VIII metal catalysts (including Pt, Pd, Rh, Fe, Ni, and Ir) are supported on reducible oxides such as CeO2, Nb2O5, and TiO2.
To understand the electronic origin of the SMSI effect, it is desirable to directly measure the flow of charge between the metal and the oxide. To achieve this goal, metal-oxide catalysts need to be combined with a Schottky diode, and thus, the catalytic nanodiode was developed.1,2
This article considers the main aspects of research aimed at developing methods for studying the transfer of charge carriers through metal-support interfaces under catalytic reaction conditions. First, we review the mechanisms for hot-electron excitation and transport through metal-oxide interfaces. We then show various schemes for detecting hot electrons that are generated during catalytic processes. We provide an overview of the latest results from detecting hot electrons in supported catalysts during chemical reactions at both gas-solid and liquid-solid interfaces. Hot electrons can be generated by photon absorption on the surface, therefore, the photocatalytic process amplified by surface plasmons is quite relevant to hot electron flow. We discuss this aspect as well.
Principle of catalytic nanodiode
One explanation to justify the generation of hot electrons is related to the difference in the heat capacity of electrons and phonons. The electronic heat capacity (Celectmn) of most metals in thermal equilibrium is about one hundred times smaller than the lattice heat capacity (Clattice) at 300 K. For example, for copper,
where R is the gas constant, T is the temperature, and γ is the Sommerfeld constant (0.70 mJ/mol-K2 for Cu). When heat is deposited from exothermic surface reactions or photon flux, the electrons heat up much faster (femtoseconds) than the lattice (picoseconds) because their heat capacity is much lower. Another mechanism to explain hot-electron generation is described in the framework of a nonadiabatic mechanism. The transition of the system to a new state occurs with one of the electronically excited states leading to the creation of an electron-hole (e–h) pair.
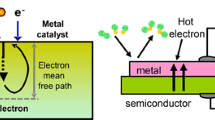
Relaxation of hot electrons happens on the femtosecond to picosecond time scale, and their mean free path is on the order of tens of nanometers.12 This implies two detection strategies. The first is to obtain sufficient time resolution for observing this excitation. The second is to use a nanometer-scale energy barrier for irreversible transfer of the hot-electron flux. For the first approach, two-photon time-resolved photoemission spectroscopy has been employed to directly study the dynamics of optically excited electrons at metal and semiconductor surfaces. This technique has been applied to the direct measurement of hot electron relaxation in noble and transition metals13 and surface-state dynamics on clean and adsorbate-covered metal surfaces,14 as well as charge-carrier dynamics in semiconductors, where much work has been performed. For the second approach, Nienhaus et al. used Schottky diodes in high-vacuum experiments aimed at detecting e–h pairs excited during the chemi-sorption of atomic hydrogen on the surface of Ag thin films supported on n-Si and p-Si substrates, as shown in Figure 1a.12,15,16 The hot-electron flow generated by hydrogen adsorption was detected as the current, as shown in Figure 1b.
It is important to consider the question of how to detect hot charge carriers that are excited in metal catalysts by an exothermic chemical reaction. As previously mentioned, in the case of highly exothermic reactions taking place on the surface of metals with a low work function, nonadiabatic reactions can be detected by observing chemiluminescence or the emission of excited electrons into a vacuum. However, for conventional catalytic reactions, this approach does not work because the work function of most catalytic metals (e.g., Pt, Pd, and Rh) is much larger than the excess energy released by the surface reaction. Therefore, for a long time, it was unclear if low-energy chemical reactions create hot charge carriers in metal catalysts.
A solution to this problem was found by Somorjai and Park, who proposed the use of thin-film Schottky diodes, called catalytic nanodiodes, for detecting hot electrons during catalytic reactions.17–19 An example of this scheme for the planar Pt/Si catalytic nanodiode under H2O2 decomposition reaction is shown in Figure 1c.20 A thin metal film acts as both the catalyst and electrode, thus allowing the flux of the chemically excited hot electrons to be measured. To ensure a reliable supply of hot carriers from the surface of the film to the Schottky contact, the film thickness should not exceed the ballistic mean free path of the electrons in the metal.20
An exothermic chemical reaction catalyzed on a metal surface creates distributions of hot charge carriers (electrons and holes) located between levels corresponding to the Fermi energy in the metal and the maximum chemical energy liberated by the reaction. To detect hot carriers via a catalytic nanodiode, excited electrons are detected because of internal emission through the Schottky barrier formed at the metal-semiconductor interface, as shown in the energy-band diagram (Figure 1d) of the Pt/Si catalytic nanodiode. Since the thickness of the metal film is on the order of the mean free path of the hot electrons (i.e., typically ≤15 nm), these excited charge carriers cross the film without significant attenuation and reach the metal-semiconductor interface while still energetic enough to overcome the Schottky barrier.
(a) Ag/Si Schottky diode used to measure hot-electron flow generated by chemisorption of atomic hydrogen, (b) Chemicurrent as a function of time measured with a Ag/Si diode. Reprinted with permission from Reference 12. © 2002 Elsevier, (c) Scheme of the Pt/Si catalytic nanodiodes during the H2O2 decomposition process, and (d) the corresponding energy-band diagram. From Reference 20.
Various schemes of catalytic nanodiodes
In earlier experiments on the detection of hot electrons during catalytic chemical reactions, planar Schottky nanodiodes were used that consist of an ultrathin Pt film deposited on an n-TiO2 or w-GaN substrate. 19,21–23 Since the detection of hot electrons occurs during their transfer through the Schottky contact, a necessary condition for the operation of a catalytic nanodiode is a properly sized Schottky barrier.12,24 The height of the barrier must be large enough to limit the transfer of thermal carriers through the metal-semiconductor interface, which allows the detection of only the hot charge carriers excited in nonadiabatic reactions. However, the Schottky barrier must not exceed the exothermicity of the surface reaction. Thus, considerations for the transparency of the barrier for charge carriers with a certain excess energy should be taken into account when choosing a semiconductor support for the nanodiodes.24,25
For an ideal Schottky diode, the height of the barrier, φb, can be found using φb = Φm − χs, where Φm is the metal work function and χs is the electron affinity of the semiconductor.26 However, for real nanodiodes, significant deviations from this equation can be observed. The height of the barrier is determined by fitting an experimentally measured current-voltage curve to the diode equation:
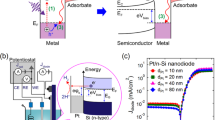
(a) Detection of chemically excited hot electrons based on a planar metal-semiconductor Schottky diode (catalytic nanodiode). (b) Schematic of hot-electron detection during a catalytic reaction on Pt nanoparticles (NPs) supported on a Au/TiO2 catalytic nanodiode.27 (c) Schematic of a metal-mesoporous Pt/TiO2 semiconductor Schottky diode.34,35 (d) Inverse catalyst of CoO NPs on a Pt/TiO2 catalytic nanodiode.37
where \(I_s = AA*T^{2}exp \left( - \frac{e_0\varphi_b}{k_B{T}} \right)\) is the saturation current, A is the area of a Schottky contact, A* is the Richardson constant, T is the temperature, e0 is the elementary charge, φb is the Schottky barrier height, kB is the Boltzmann constant, V is the voltage, Rser is the series resistance, and n is the ideality factor, which is a measure of how closely the diode follows the ideal diode equation.
The use of planar Schottky nanodiodes (Figure 2a) has made it possible to carry out proof-of-concept experiments demonstrating the feasibility of studying nonadiabatic processes in catalytic reactions using solid-state devices. However, the structure of such thin-film nanodiodes differs significantly from the supported metal-semiconductor catalysts used in industrial processes. To fill this gap, several research groups have experimented with more complex nanodiodes whose structures simulate real-world catalysts.
For instance, Park et al. demonstrated the possibility of detecting nonadiabatic electronic excitation in colloid NPs of platinum (Pt) using the catalytic H2 and CO oxidation reactions.27,28 The idea behind this experiment is seen in Figure 2b—Pt NPs of a defined size were deposited as two-dimensional arrays on an Au thin film supported on the TiO2 surface of the nanodiode. The Au film creates an electrical connection between the Pt NPs and the external circuit, thus allowing continuous hot-electron flow. Another idea for making nanodiodes that are close to real three-dimensional (3D) catalysts was proposed by Schierbaum et al., who studied the effects of charge carrier creation during catalytic reactions on Pt/TiO2 nanodiodes based on porous titanium oxide layers fabricated using plasma electrolytic oxidation of a Ti metal foil,29,30 as shown in Figure 2c. Karpov et al. and Jeon et al. also conducted chemicurrent studies using similar nanodiodes consisting of a Pt mesh supported on mesoporous TiO2 and ZrO2 layers.31–34 This approach is related to building a 3D catalytic nanodiode that allows a higher flux of hot electrons and catalytic activity. Recently, Goddeti et al. demonstrated a semi-3D catalytic diode using Pt/TiO2 nanotubes that resulted in much higher hot-electron generation.35,36 In addition, an inverse catalyst with cobalt oxide NPs on Pt/TiO2 catalytic nanodiodes was demonstrated by Lee et al. (Figure 2d).37 The interface between Pt and CoO revealed a higher chemicurrent as well as higher catalytic activity during hydrogen oxidation, emphasizing the importance of the metal-oxide interface in heterogeneous catalysis and hot-electron generation.38
Hot-electron detection during gas-phase and liquid-phase reactions
Using a catalytic nanodiode, hot-electron fluxes were detected during various gas-phase reactions (Figure 3a), including CO oxidation,19 NO/CO reaction,39 methanol oxidation,40 and hydrogen oxidation.23,28,41 A typical chemicurrent signal that corresponds to the transfer of hot electrons excited by the catalytic reaction in a H2 (15 Torr) + O2 (745 Torr) gas mixture on 1.7-nm Pt NPs on Au/TiO2 nanodiodes is shown in Figure 3b.28 The chemicurrent demonstrates a noticeable temperature dependence that is caused by an increase in the rate of H2 oxidation at the elevated temperature of the Pt catalyst. The chemicurrent value is also dependent on the metal thickness. As can be seen, an increase in both the Pt NPs size and Au film thickness leads to a sharp decrease in the chemicurrent, which is associated with a decrease in hot charge carrier flux because of electron-electron and electron-phonon scattering.28
The Au layer that is used for the ohmic contact can be replaced by other conductive thin layers such as graphene. To study the chemicurrent effect on Pt NPs supported on a graphene layer, a graphene-based catalytic nanodiode was developed.42 Graphene has several extraordinary properties that make it a promising candidate for use as a catalytic support. Graphene is a particularly excellent conductor of ballistic electrons and is thus an interesting material for detecting nonadiabatic electronic excitation in catalytic reactions using Schottky nanodiodes.
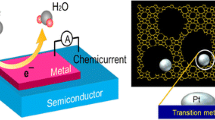
As mentioned earlier, the detection of nonadiabatic reactions using catalytic nanodiodes occurs because of internal emission of hot electrons through the Schottky barrier and therefore does not depend on the density of the reacting medium. This provides an opportunity to study catalytic reactions occurring at liquid-solid interfaces that are of great practical importance for heterogeneous catalysis.43–47 The scheme for nanodiode-based hot-electron detection during solid-liquid reactions is shown in Figure 3c. To date, most data from the experimental detection of chemically excited hot electrons during liquid-phase reactions were reported for the catalytic decomposition reaction of hydrogen peroxide \({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_2}\mathop \to \limits^{{\text{catalyst}}} {\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}\,{\text{ + }}\,{{\text{O}}_2}\), which is commonly used in industry as an oxidizing agent for pulp and paper bleaching, wastewater treatment, textile production, chemical synthesis, and many other applications.48,49 In addition, the decomposition of H2O2 is highly exothermic (ΔH = −25.3 kJ/mol)47 and can therefore serve as a model system for mechanistic studies of energy and charge transfer in catalysts at liquid-solid interfaces.
Typical chemicurrent signals measured on planar 10-nm Pt/n-Si, Au/n-Si, and Ag/n-Si nanodiodes in an aqueous solution containing H2O2 are shown in Figure 3d. The chemicurrent is measured when the nanodiode is immersed in the H2O2 solution.43,47 The shape of the chemicurrent signal reflects the surface reaction rate. Initially, when the surface reaction rate is the greatest, the chemicurrent reaches a peak value. Then, as the steady-state reaction is established, the chemicurrent decreases and acquires a value that varies only little with time. The value of this steady-state chemicurrent is a clear function of the H2O2 concentration in the solution.46 Thus, chemicurrent measurements provide an easy-to-implement method for studying catalytic processes at the liquid-solid interface in real time.
Surface plasmon driven hot-electron flux and its influence on photocatalytic reactions
The application of plasmonics to the field of heterogeneous catalysis has received a lot of attention in recent years because of the potential to efficiently harvest and utilize solar energy for chemical processes. Plasmonic metal nanostructures or hybrid materials (composed of catalytically active semiconductor and plasmonic metal NPs) under light irradiation are efficient sources for generating hot electrons. This concept has been demonstrated by several groups who observed the amplification of hot-electron flux on plasmonic nanodiodes.50–52
(a) Schematic of hot-electron detection during solid-gas reactions. (b) Typical chemicurrent signals attributed to the transfer of hot electrons excited by catalytic H2 oxidation on Au/TiO2 nanodiodes deposited with different sizes of Pt nanoparticles.28 (c) Schematic of hot-electron detection during solid-liquid reactions, (d) Chemicurrent obtained on Ag/Si, Pt/Si, and Au/Si catalytic nanodiodes during H2O2 decomposition.43
(a) Schematic of the MAPbl3/plasmonic-Au/TiO2 nanodiode, and cross-sectional elemental energy-dispersive spectroscopy mapping image of the MAPbl3-covered plasmonic nanodiode. (b) Incident photon to current efficiency (IPCE) as a function of incident photon energy measured on the MAPbl3/plasmonic-Au/TiO2 nanodiodes according to the number of MAPbl3 layers.53 (c) Schematic of photoanode composed of Au nanoparticles (NPs) on TiO2 nanotube arrays (TNAs) and the working principle of the photoelectrochemical cell, (d) Incident-photon-to-electron conversion plots of electrodes composed of Au NPs on TNA with different sized Au NPs and bare TNA collected at 1.23 V versus reversible hydrogen electrode (RHE).54 Note: CB, conduction band; VB, valence band.
The efficiency of hot-electron generation is also influenced by the lifetime of hot electrons. This aspect was investigated by Park et al. who modified a plasmonic nanodiode with perovskite materials. In this study, an MAPbI3-modified plasmonic nanodiode combining MAPbI3 (perovskite) with the local surface plasmon resonance effect of nanostructured Au was utilized, as shown in Figure 4a.53 A higher hot-electron flux was observed in this device (as shown in Figure 4b) due to the presence of the perovskite material; this was attributed to the extended lifetime of the hot electrons, which was measured using femtosecond transient absorption experiments.
Ahigher hot-electron flux can influence the rates of catalytic chemical reactions through excitation by the photoinduced surface plasmon resonances of the metal nanostructures. Moon et al.54 investigated this aspect by studying photocat-alytic water splitting mediated by the injection of hot carriers using TiO2 nanotube arrays decorated with various sized plasmonic Au NPs, as shown in the experimental scheme in Figure 4c. The internal quantum efficiency of the Au NPs on TiO2 nanotube arrays photoanodes for the oxygen evolution reaction in visible light increased from 1.96 to 7.68%, when the size of the Au NPs decreased from 30 to 5 nm (Figure 4d). Moreover, the smaller NP-decorated TiO2 nanotube arrays showed about a ninefold lower Au loading compared with the larger NPs with the same coverage.54
Enhancement of the local electric field that serves as “hot spots” in plasmonic photocatalysis boosts the generation of electrons and holes. An increased photoinduced charge is generated locally in the TiO2 via local field enhancement of the plasmonic NPs.55,56 Considering that strong electric enhancement occurs at the edges of the NPs, smaller NPs will increase the total perimeter length compared with larger sizes with the same coverage; thus, more hot carriers can travel over the interface. Interesting future studies involve the direct probing of hot-electron transfer using ultrafast studies or metal-semiconductor diodes during photocata-lytic processes.
Conclusion
In this article, we highlighted recent studies aimed at understanding the nature of charge transfer arising during catalytic reactions on supported metal catalysts. First, we discussed the basic mechanisms of catalytic nanodiodes where nonadiabatic effects could be potentially responsible for creating hot charge carriers in metal catalysts during exothermic reactions and photon irradiation. We also provided an overview of the various schemes and applications for catalytic nanodiodes, including hot-electron detection during solid-gas and solid-liquid reactions. The detection of hot electrons in the form of a chemicurrent allows for operando studies of nonadiabatic effects during gas-solid and liquid-solid reactions. Because of the intrinsic relation between surface plasmons and hot-electron flux, the direct measurement of hot electrons can be a useful tool to monitor the local electric field and “hot spot” during photocatalytic reactions.
A more fundamental understanding of hot-electron phenomena is required at both theoretical and experimental levels. Hot electrons on a metallic surface can be created by external energy deposition in the form of photons, ions, electrons, and chemical reactions. Therefore, we can consider hot electrons to be a major mediator for general energy conversion. The scheme of energy conversion from chemical (i.e., catalytic reactions) and photon-to-electrical (i.e., hot-electron current) energy may give insight into other fields, including solar cells and electrochemical and photocatalytic devices.
References
G.A. Somorjai, J.Y. Park, Angew. Chem. Int. Ed. Engl. 47, 9212 (2008).
J.Y. Park, Current Trends of Surface Science and Catalysis (Springer, New York, 2014).
G.T.K.K. Gunasooriya, E.G. Seebauer, M. Saeys, ACS Catal. 7, 1966 (2017).
Y. Lykhach, S.M. Kozlov, T. Skala, A. Tovt, V. Stetsovych, N. Tsud, F. Dvorak, V. Johanek, A. Neitzel, J. Myslivecek, S. Fabris, V. Matolin, K.M. Neyman, J. Libuda, Nat. Mater. 15, 284 (2016).
M.G. Willinger, W. Zhang, O. Bondarchuk, S. Shaikhutdinov, H.-J. Freund, R. Schlögl, Angew. Chem. Int. Ed. Engl. 53, 5998 (2014).
C.R. Henry, Surf. Sci. Rep. 31, 231 (1998).
G.-M. Schwab, K. Koller, J. Am. Chem. Soc. 90, 3078 (1968).
G.-M. Schwab, Surf. Sci. 13, 198 (1969).
G.A. Somorjai, H. Frei, J.Y. Park, J. Am. Chem. Soc. 131, 16589 (2009).
A. Boffa, C. Lin, A.T. Bell, G.A. Somorjai, J. Catal. 149, 149 (1994).
S.J. Tauster, Acc. Chem. Res. 20, 389 (1987).
H. Nienhaus, Surf. Sci. Rep. 45, 1 (2002).
S. Ogawa, H. Petek, Surf. Sci. 363, 313 (1996).
T. Hertel, E. Knoesel, M. Wolf, G. Ertl, Phys. Rev. Lett. 76, 535 (1996).
H. Nienhaus, H.S. Bergh, B. Gergen, A. Majumdar, W.H. Weinberg, E.W. McFarland, Surf. Sci. 445, 335 (2000).
B. Gergen, H. Nienhaus, W.H. Weinberg, E.W. McFarland, Science 294, 2521 (2001).
J.Y. Park, G.A. Somorjai, J. Vac. Sci. Technol. B 24, 1967 (2006).
J.Y. Park, G.A. Somorjai, ChemPhysChem 7, 1409 (2006).
J.Y. Park, J.R. Renzas, B.B. Hsu, G.A. Somorjai, J. Phys. Chem. C 111, 15331 (2007).
J.Y. Park, L.R. Baker, G.A. Somorjai, Chem. Rev. 115, 2781 (2015).
G.A. Somorjai, Catal. Lett. 101, 1 (2004).
X. Ji, A. Zuppero, J.M. Gidwani, G.A. Somorjai, Nano Lett. 5, 753 (2005).
A. Hervier, J.R. Renzas, J.Y. Park, G.A. Somorjai, Nano Lett. 9, 3930 (2009).
I.I. Nedrygailov, J.Y. Park, Chem. Phys. Lett. 645, 5 (2016).
H. Nienhaus, B. Gergen, W.H. Weinberg, E.W. McFarland, Surf. Sci. 514, 172 (2002).
S.M. Sze, K.K. Ng, Physics of Semiconductor Devices (Wiley, Hoboken, NJ, 2006).
J.Y. Park, H. Lee, J.R. Renzas, Y. Zhang, G.A. Somorjai, Nano Lett. 8, 2388 (2008).
H. Lee, I.I. Nedrygailov, C. Lee, G.A. Somorjai, J.Y. Park, Angew. Chem. Int. Ed. Engl. 54, 2340 (2015).
K. Schierbaum, M. El Achhab, Phys. Status Solidi A 208, 2796 (2011).
Ö. Cakabay, M. El Achhab, K. Schierbaum, Appl. Phys. A 118, 1127 (2014).
E.G. Karpov, M.A. Hashemian, S.K. Dasari, J. Phys. Chem. C 117, 15632 (2013).
M.A. Hashemian, E. Palacios, I.I. Nedrygailov, D. Diesing, E.G. Karpov, ACS Appl. Mater. Interfaces 5, 12375 (2013).
N.J. Ray, M.A. Hashemian, E.G. Karpov, ACS Appl. Mater. Interfaces 7, 27749 (2015).
B. Jeon, H. Lee, K.C. Goddeti, J.Y. Park, ACS Appl. Mater. Interfaces 11, 15152 (2019).
K.C. Goddeti, H. Lee, B. Jeon, J.Y. Park, Chem. Commun. 54, 8968 (2018).
K.C. Goddeti, C. Lee, Y.K. Lee, J.Y. Park, Sci. Rep. 8, 7330 (2018).
H. Lee, S. Yoon, J. Jo, B. Jeon, T. Hyeon, K. An, J.Y. Park, Faraday Discuss. 214, 353 (2019).
H. Lee, J. Lim, C. Lee, S. Back, K. An, J.W. Shin, R. Ryoo, Y. Jung, J.Y. Park, Nat. Commun. 9, 2235 (2018).
J.R. Renzas, G.A. Somorjai, J. Phys. Chem. C 114, 17660 (2010).
S.W. Lee, W. Park, H. Lee, H.C. Song, Y. Jung, J.Y. Park, ACS Catal. 9, 8424 (2019).
H. Lee, I.I. Nedrygailov, S.W. Lee, J.Y. Park, Top. Catal. 61, 915 (2018).
H. Lee, I.I. Nedrygailov, Y.K. Lee, C. Lee, H. Choi, J.S. Choi, C.-G. Choi, J.Y. Park, Nano Lett. 16, 1650 (2016).
I.I. Nedrygailov, C. Lee, S.Y. Moon, H. Lee, J.Y. Park, Angew. Chem. Int. Ed. Engl. 55, 10859 (2016).
F. Zaera, Chem. Rev. 112, 2920 (2012).
I.I. Nedrygailov, C. Lee, S.Y. Moon, H. Lee, J.Y. Park, Angew. Chem. Int. Ed. Engl. 128, 11017 (2016).
I.I. Nedrygailov, C. Lee, S.Y. Moon, H. Lee, J.Y. Park, Rev. Sci. Instrum. 87 114101 (2016).
S.H. Lee, I.I. Nedrygailov, S. Oh, J.Y. Park, Catal. Today 303, 282 (2018).
J.M. Campos-Martin, G. Blanco-Brieva, J.L.G. Fierro, Angew. Chem. Int. Ed. Engl. 45, 6962 (2006).
N.M. Wilson, D.W. Flaherty, J. Am. Chem. Soc. 138, 574 (2015).
M.W. Knight, H. Sobhani, P. Nordlander, N.J. Halas, Science 332, 702 (2011).
Y.K. Lee, H. Lee, C. Lee, E. Hwang, J.Y. Park, J. Phys. Condens. Matter 28, 254006 (2016).
Y.K. Lee, C.H. Jung, J. Park, H. Seo, G.A. Somorjai, J.Y. Park, Nano Lett. 11 (10), 4251 (2011).
Y. Park, J. Choi, C. Lee, A.-N. Cho, D.W. Cho, N.-G. Park, H. Ihee, J.Y. Park, Nano Lett. 19, 5489 (2019).
S.Y. Moon, H.C. Song, E.H. Gwag, I.I. Nedrygailov, C. Lee, J.J. Kim, W.H. Doh, J.Y. Park, Nanoscale 10, 22180 (2018).
S. Linic, P. Christopher, D.B. Ingram, Nat. Mater. 10, 911 (2011).
N.J. Halas, S. Lal, W.-S. Chang, S. Link, P. Nordlander, Chem. Rev. 111, 3913 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.Y., Somorjai, G.A. Nanodiode-based hot electrons: Influence on surface chemistry and catalytic reactions. MRS Bulletin 45, 26–31 (2020). https://doi.org/10.1557/mrs.2019.295
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2019.295