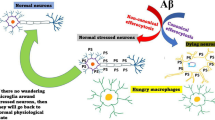
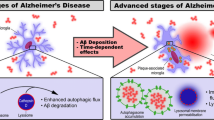
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive cognitive decline, amyloid-β (Aβ) plaques and the formation of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. Increasing evidence has demonstrated that the damage of cell plays an important role in AD. Cell death is a critical phenomenon for physiological functions, which promotes AD pathogenesis. Programmed cell death, including necroptosis, pyroptosis, autophagy, and ferroptosis, have been discovered that have unique biological functions and pathophysiological characteristics. Here, we review the available evidence detailing the mechanisms of programmed microglial death, including pyroptosis, autophagy, and ferroptosis. We also highlight the role of programmed death of microglia during the process of AD and focus on the connection between the disease and cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AD is a neurological condition characterised by progressive decline in cognition, with concomitant functional decline. The main pathological hallmarks of Alzheimer’s disease are the aggregation of beta amyloid peptides into extracellular plaques and hyperphosphorylated tau proteins into intracellular neurogenic fiber tangles, accompanied by neuroinflammation, gliosis and neurodegeneration (1). Based on 2020 Alzheimer’s Disease Facts and Figures (2), more than 5.8 million Americans have AD dementia today, which is the most common form of dementia worldwide (2). Despite remarkable advances in unraveling the biological underpinnings of AD during the last 25 years, no drugs have been found that can slow the progression of AD while promising preclinical trials have repeatedly ended in failure to translate into treatments for AD patients (3).
Cell death is a critical phenomenon for physiological functions such as immunity, development, and tissue homeostasis. Programmed cell death is a distinct biochemical pathway in which cells die to elicit various physiological outcomes and functions as a defense mechanism against various infections, diseases, and microorganisms (4). Programmed cell death is well known as a type of cell death during the earlier period. Recently, regulated pathways of cell death, including necroptosis, pyroptosis, autophagy, and ferroptosis (5), have been discovered that have unique biological functions and pathophysiological characteristics (6, 7). Cell death is inseparable from the progression of inflammation in AD. Regardless of the types of death, they all promote the occurrence and development of inflammation. Hyperphosphorylated tau protein aggregates into NFTs, which is a major pathological hallmark of AD. It is proved that hyperphosphorylation of tau may result in dysfunction of autophagy, ferroptosis and pyroptosis (8–10). On the contrary, the damage of cell, including autophagy, ferroptosis and pyroptosis, can exacerbate the progression of tau hyperphosphorylation, which aggravates the inflammatory state of AD.
Microglia are immune cells in the brain that participate in maintaining immune defense of the nervous system (11, 12). Microglia are modified macrophages that compose approximately 10%–12% of total cells (13) and are considered instigators of damage and guardians of brain homeostasis, playing vital roles in both neuroprotection and neurodegeneration (14). However, there is no systematic description of the effect of programmed microglial death on the nervous system.
In this review, we evaluate the association between programmed microglial death and AD, and we discuss the role of NFTs and tau in explaining how cell death might contribute to AD. The findings are contextually framed by evidence that activation of the innate immune system alters central nervous system mechanisms and increases cell damage, possibly driving onset and progression of AD. Inhibiting the progressive of cell death could be targets for the prevention of AD.
Autophagy
Three types of autophagy have been reported: macroautophagy, micro autophagy, and chaperone-mediated autophagy. Among them, macro autophagy is the most widely studied and well described of the three types (15). Some studies have shown that macro autophagy is the main pathway of intracellular degradation (16). Autophagy, which acts as a protective mechanism, maintains normal cellular function and homeostasis by degrading engulfed substances (17). Autophagy is involved in the degradation and elimination of damaged, denatured, aged or dysfunctional organelles, denatured proteins, and other biological macromolecules through a process that provides the energy necessary for cell survival and repair (18). Autophagy is a cellular catabolic process involving the sequestration of misfolded proteins and damaged cytoplasmic organelles by a double-membrane structure known as an autophagosome and the degradation of the engulfed contents via fusion with a lysosome (12). Therefore, this autophagy-lysosomal pathway plays an important role in maintaining normal cellular function and intracellular homeostasis. Accumulating evidence indicates that autophagy is closely associated with inflammasomes, and these factors mutually influence each other. In neurodegenerative diseases, microglial autophagy is impaired and downregulated (19). The transformation from the M1 to the M2 phenotype can protect the body from excessive inflammatory injury, and one of the mechanisms affecting macrophage polarization is autophagy (20). The phosphatidylinositol 3-kinase (PI3K)-mTOR pathway is important in regulating macrophage polarization. Activation of the
PI3K-mTOR pathway can increase M2 polarization while inhibition of PI3K or mTOR exerts the opposite effect (21). However, microglial autophagy transforms microglia from a proinflammatory state to a favorable anti-inflammatory state and inhibits NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome-mediated inflammatory responses, acting as negative regulators (22). Ma’ (23) results indicate that the overactivation of autophagy is responsible for the M1 polarization of microglia and promotes microglial apoptosis. Emerging evidence indicates that overactivation of the NLRP3 inflammasome in microglia is a driving factor that exacerbates pathology and ultimately accelerates neuronal death and the progression of neurodegenerative diseases (24, 25). In mammalian cells, microglial autophagy has been demonstrated to be critical for microglial activation in vitro, and the inhibition of microglial autophagy results in the upregulation of proinflammatory cytokines, causing increased M1 microglial activation (26–28), (Figure 1).
Autophagy pathway
The energy sensors mTORC1 and AMPK control autophagy activation via the ULK1 complex. Various stress conditions stimulate ULK1 complex phosphorylation of the VPS34-Beclin-1 complex. Following activation, the ULK1 and PI3KC3 complex regulate the formation of the autophagosomes. The resulting LC3-I is activated and binds on the autophagosome and is esterified to form LC3-II. Finally, the mature autophagosome fuses with lysosomes to autolysosomes, and regulates the polarity of microglia.
Cognitive Decline
One of the reasons causes cognitive decline of AD is the sequential functional modifications of the mitochondrial dysfunction, including inflammation, impaired energy metabolism, oxidative stress and synaptic dysfunction (29, 30). The significance of autophagy dysfunction in AD pathophysiology is now appreciated due to evidence reporting dysfunctional autophagy in the AD leading to neuronal degeneration (31–33).
In autophagy-deficient AD mice, Nilsson et al, reported an important reduction associates with intraneuronal Aβ accumulation increasing in Aβ metabolism, which further enhances neurodegeneration and memory impairment (34). Abubakar’s research revealed that improved memory function, demonstrated via Morris water maze test, was associated with reduced Aβ levels and related pathology (35). Glatigny et al. showed that the induction of novel stimuli increases autophagy by fostering synaptic plasticity in hippocampal neurons. In addition, they showed that restoring autophagy levels in old hippocampi reverses memory deficits (36).
Given the extensive observations showing autophagic alterations in the brain of AD patients or in different mouse models of AD, it is now well accepted that improving of the autophagic flux ameliorates cognitive impairment in AD mouse models (37–39). Restoration of neuronal mitophagy was found sufficient to ameliorate the cognitive decline in an AD mouse model by preventing synaptic failure (40). Thus, the cognitive decline of AD is related to the autophagy closely, which may inhibit the progress of cognitive decline, in brain.
NFTs and tau
Extracellular amyloid plaque deposits, composed of Aβ peptides, as well as intracellular accumulation of NFTs, consisting of hyperphosphorylated tau (p-tau) protein are regarded as the characters of AD (41). Major pathological hallmarks of AD include intracellular deposition of neurofibrillary tangles (NFTs), which were associated with paired helical filaments (PHF), p-tau, and extracellular accumulation of Aβ peptide in the senile plaques (42, 43). Data show that Aβ peptides and tau protein accumulation, the principal hallmarks of AD, can be influenced due to autophagy dysregulation (44). Studies suggest that dysfunction of the autophagy-lysosome system promotes the formation of tau oligomers and accumulation of insoluble tau species (9).
It has been reported that tau secretion is promoted by autophagy inducers and knockdown of beclin-1 (45). Besides, the accumulation of hippocampal phosphorylated tau is responsible for abnormal mitophagy function, mitochondrial dynamics hippocampal-based learning and memory impairments in tau mice (46). It has been reported that the phosphorylated tau can also interact with VDAC1 and Drp1, likely leading to mitochondrial dysfunction and abnormal mitophagy, ultimately possibly leading to damage and cognitive decline (47, 48).
Aβ
Autophagy is closely related to the metabolism of Aβ, and autophagy is an important regulator of its production and clearance. The precursor protein of Aβ, APP, and the γ-secretase that cleaves APP are highly enriched in autophagosomes. Many studies have shown that Aβ secretion is significantly reduced in autophagy-deficient mouse models (49). This result was also verified in AD patients, where patients with severely impaired autophagy showed a significant decrease in learning ability and cognitive function (50). Induction of autophagy recovery accelerates Aβ clearance and restores cognitive function. Autophagy can influence Aβ clearance by affecting multiple stages of Aβ (51). It was shown that healthy microglia can wrap and degrade Aβ through autophagosomes, but the expression of autophagy-related proteins NBR1 and ATG7 was reduced in microglia of 5XAD mice, and the degradation of Aβ was impaired in 5xFAD microglia, further suggesting the necessity of autophagy in microglia for the degradation of Aβ (52).
Ferroptosis
Ferroptosis, which mainly occurs in the brain (53, 54), is defined as an iron-dependent form of regulated cell death that occurs through the lethal accumulation of lipid-based reactive oxygen species (ROS) when glutathione (GSH)-dependent lipid peroxide repair systems are compromised (55). Cellular accumulation of lipid peroxidation products and lethal ROS are characteristics of ferroptosis and ultimately result in oxidative stress and cell death. The peroxidation of proteins, nucleic acids, and lipids is promoted when intracellular iron accumulation generates ROS and causes oxidative stress via the Fenton reaction, which is the key process that propagates ferroptosis (56–58). Morphologically, ferroptosis is mainly characterized by shrunken mitochondria with increased membrane density and normal-sized nuclei, as well as diminished or vanished mitochondrial cristae and ruptured outer membranes (59). A wealth of evidence underscores the tight link between ferroptosis and the nervous system. There are three hallmarks of ferroptosis that were highlighted by Dixon and Stockwell: the loss of lipid peroxide repair capacity by the phospholipid hydro peroxidase GPX4, which was identified as a key regulator of ferroptosis in cancer cells (60); the availability of redox-active iron; and the oxidation of PUFA-containing phospholipids (61). Among them, it is accepted that the fundamental characteristic of ferroptosis is lipid peroxidation (62). As GPX4 is a central protector against the formation of lipid hydroperoxides (63), and its degradation is a mandatory signaling event in the execution of ferroptotis cell death (60, 64, 65). Ferroptosis widely exists in various parts of the central nervous system, such as the cerebral cortex, hippocampus, striatum, and spinal cord (59). Ferroptosis has been implicated in several neurological diseases, such as neurodegenerative diseases, hemorrhagic stroke and ischemic stroke (66, 67).
Recent studies have shown that iron accumulation in microglia also contributes to microglial dysfunction and Aβ accumulation (68). (Figure 2)
Ferroptosis
System xc- transports intracellular Glu to the extracellular space and extracellular Cys2 into the cell, which is then transformed into Cys for GSH synthesis. Excess irons are the basis for ferroptosis execution. Circulated iron was combined with transferrin in the form of Fe3+, and then it entered into cells by TFR1. Iron in Fe3+ form was deoxidized to iron in Fe2+ by iron oxide reductase STEAP3. Ultimately, Fe2+ was released into a labile iron pool in the cytoplasm from the endosome mediated.
Cognitive Decline
Several studies have observed the excess brain iron accumulation was associated with accelerated cognitive decline in AD patients (69). Iron rises in the brain with aging and may be pathological because it is associated with cognitive decline prior to disease (70, 71). The increasing of iron occurs as early as the mild cognitive impairment stage of AD and contributes to longitudinal outcomes (72, 73). It was found out that there is a large amount of activated iron-containing microglia around Aβ plaques (74, 75). The brain iron burden is elevated in AD patients, combined with Aβ positron emission tomography (PET), which indicates that brain iron load is positively associated with Aβ deposition-related cognitive decline, suggesting that cognitive function damage may exacerbate due to iron combine with Aβ. The pathologic mechanism could be that iron promotes the production of free radicals and oxidative stress and possibly also involves ferroptosis (76, 77).
The maintenance of glutathione GSH is a key antioxidant element in brain redox homeostasis (78). In the AD model, N-acetyl cysteine (NAC) can protect neurons’ function and improving learning and memory deficits via increasing GSH levels (79). A recent study indicated that deficiency of ferroportein (Fpn) in principal neurons in neocortex increased iron levels and induced AD-like hippocampal atrophy and memory deficits, while restoring Fpn expression could effectively ameliorate the memory loss and ferroptosis in AD model mice (80).
NFTs and tau
Aging and changes in iron metabolism are associated with the development of Aβ plaques and NFTs (81). Iron and ferritin are found within plaques, NFTs, and blood vessels in AD (82). Svobodová et al. (83) demonstrated in an APP/PS1 transgenic mice model that free iron and ferritin accumulation follows amyloid plaque formation in the cerebral cortex area. Actually, iron deposition has been involved in the misfolding process of the Aβ plaques and NFTs (8). Additionally, iron is related to the development of tau protein, which is present through the induction and regulation of tau phosphorylation (8, 84). It is possible that this surface trafficking of APP may be impaired by the hyperphosphorylation and aggregation of tau (thus lowering the soluble fraction of tau) during AD pathogenesis (8, 85). Tau loss preceded iron accumulation, and APP treatment lowered iron (86). The evidence suggests that iron interacts with tau to cause neurodegeneration in AD and related conditions; conversely, tau maintains cellular iron homeostasis, however a putative role of an iron-tau interaction in ferroptotis stress needs further investigation.
Aβ
In AD, neuroinflammation exacerbates Aβ deposition. Lipid metabolites in iron death trigger inflammation, which in turn mutually promotes iron death by increasing iron deposition. Iron is involved in Aβ plaque deposition, and iron accumulation accelerates Aβ plaque deposition (69). Iron dysregulation increases the production of ROS and is one of the prominent manifestations of AD pathology. Excessive increase in ROS promotes the development of Aβ. As reported by Kristin et al. Aβ induces strong ROS production in BV2 microglia via NADPH oxidase. Intracellular iron depletion inhibits Aβ-induced ROS. Aβ plaques are surrounded by microglia overexpressing HO-1 (87). Fernández-Mendívil’s study showed that (88) overexpression of HO-1 in microglia under inflammatory conditions leads to toxic accumulation of iron leading to iron death. In addition, mitochondrial contraction serves as one of the unique markers of iron death, and mitochondrial dysfunction is associated with Aβ accumulation. The level of iron in the brain is closely linked to both translation and processing of APP.
Pyroptosis
Pyroptosis is a proinflammatory form of programmed cell death that triggers an inflammatory response upon infection or other stimuli (89). Pyroptosis features rapid plasma membrane rupture and the release of proinflammatory intracellular contents such as interleukin 1β (IL-1β) and interleukin 18 (IL-18) (90). Pyroptosis is regarded as a critical host defense mechanism against intracellular pathogenic bacteria by releasing these organisms into the extracellular environment, where they can be killed by neutrophils (91–93). Pyroptosis is dependent on inflammatory caspases (caspase-1 and caspase-4/5/11) and is accompanied by inflammation. Canonical pyroptosis is executed by cleaved caspase-1, which not only causes cell lysis but also mediates the proteolytic cleavage and release of IL-1β and IL-18 (94). Chang’s (89) data demonstrated that intensive pyroptosis and increased caspase-1 activity indeed occurred in activated microglia after cardiac arrest, along with elevated levels of IL-1β and IL-18. This finding shows the inseparable connection between pyroptosis, caspase-1, IL-1β, and IL-18. Furthermore, recent studies have reported that gasdermin D (GSDMD) is the executioner of pyroptosis (95). After being cleaved by caspase-1, the GSDMD N-terminal domain (GSDMD-N) can form pores in the plasma membrane. Holes in the cell membrane cause the loss of cell integrity. IL-1β and IL-18 are released through these pores, thereby perpetuating the inflammatory response.
In addition, ATP acts as a canonical activator that induces NLRP3 inflammasome activation in macrophages, leading to caspase-1/GSDMD-mediated pyroptosis. Zeng’s (93) study showed that ATP was able to induce alternative pyroptosis in macrophages in which NLRP3-mediated rapid pyroptosis was blocked, highlighting another interplay between the pyroptosis and apoptosis pathways. When the oxygen level decreases, mitochondria produce a large amount of ROS in response to hypoxia, which is a key stimulus that promotes the activation of NLRP3 inflammasomes. NLRP3 inflammasome activation can also activate caspase-1, resulting in pyroptosis. Poh et al. (96) provided evidence to support that the inflammatory response induced by inflammasome activation through proinflammatory mediators such as both IL-1β and IL-18, damage-associated molecular patterns (DAMPs) (i.e., HMGB1 and IL-1α) and inflammasome components released into the extracellular environment caused pyroptosis in microglial cells (Figure 3).
Pyroptosis
In the canonical model of pyroptosis, inflammasome sensor proteins recognize cellular stressors, including those from bacteria, viruses, toxins etc. These stressors activate inflammasome sensors indirectly, such as NLRP3. NLRP3 subsequently activates caspase-1 via the adaptor protein ASC. Caspase-1 processes and activates IL-1β and IL-18, and also cleaves GSDMD to release the membrane pore-forming GSDMD-N domain. GSDMD-N pores promote the release of activated IL-1β and IL-18. Cytosolic LPS binds Caspase-4/5/11 to trigger their cleavage of GSDMD, but not IL-1β and IL-18.
Cognitive Decline
In the AD brain and AD transgenic mice, the expression of several pyroptosis-related proteins, including NLRP1, NLRP3 and caspase-1, is increased, and inhibiting pyroptosis alleviated the recognition dysfunction of APP/PS1 mice (97, 98). NLRP3 deficiency in AD model results in the rescue of memory deficits and a decrease of Aβ deposition (99). So, NLRP3 inflammasome-mediated pyroptosis may provide a progressive memory loss of AD. It has been reported that NLRP3 inflammasome activation is caused by the action of cathepsin B released from the lysosome rather than the direct actions of Aβ (100). These findings were confirmed by a separate study showing that cathepsin B inhibitors improve the memory deficit in transgenic AD mice (101).
Recently, studies have shown that neuroinflammation inhibition can regulate the cognitive function of AD (102). Several studies have demonstrated that pyroptosis plays an important role in mediating the occurrence of neuroinflammation (103). To evaluate whether disturbing pyroptosis might affect cognitive function, Li et al. assessed spontaneous alternation in the Y-maze as a measure of spatial memory, and a significant improvement in spatial memory disorder was shown in the caspase inhibitor treated models (10).
NFTs and tau
Numerous studies have pointed out that overexpression of IL-1β aggravates AD pathogenesis, owing to tau hyperphosphorylation (104), which inhibits long-term potentiation and affects synaptic plasticity (105). IL-1β can increase the phosphorylation of tau proteins (106). IL-18 shares structural similarities with proteins in the IL-1 family, which can also modulate the hyperphosphorylation of tau protein by increasing the expression of kinases involved in tau phosphorylation (10). Recently, a study by Ising and co-workers showed that NLRP3 acted as a link between Aβ plaques and NFTs formation (25). These authors demonstrated that the injection of Aβ-containing APP/PS1 brain homogenates induced tau hyperphosphorylation in tau22 mice, but not in tau22 mice deficient for NLRP3, indicating that NLRP3 was an important mediator of Aβ-induced tau pathology. What’s more, li’s study also discovered that suppressing the activity of caspase resulted in a remarkable reduction of pyroptosis-related proteins and significantly inhibited the hyperphosphorylation of tau proteins (10).
Aβ
A previous study described that Aβ accumulates in AD brain to form characteristic plaques, which then activate NLRP3 inflammatory vesicles that trigger cellular scorching via the NLRP3/caspase-1/GSDMD signaling pathway, ultimately leading to elevated levels of inflammatory factors IL-1β and IL-18 (107). In addition, Aβ secretion of IL-1β is dependent on NLRP3, ASC and caspase-1 activity and requires release of histone B from damaged lysosomes (108). In addition, aberrant activation of microglia-specific NLRP3 leads to microglia Aβ phagocytosis dysfunction during the pathology of AD. In a mouse model of AD, intracellular activation of NLRP3 inflammasomes was observed to promote M1 phenotype microglia activation, leading to Aβ accumulation and higher cognitive impairment[109]. Inhibition of NLRP3 inflammatory vesicle activation reduces Aβ-induced neuroinflammation in microglia and thus treats AD (110).
Conclusion and Perspectives
Microglial death is a complex process involving physiological and pathological activities in the body and is regulated by a variety of factors. And AD, as one of the most commonly diseases around the world, has bothered human all the time. We are just beginning to understand how microglia function in health and are altered in AD. The way of microglial death perhaps plays a significant role in the proceeding of AD. In the early stage of the disease, the excess brain iron accumulation is occurred with accelerated cognitive decline in AD. During the development of AD, microglial autophagy plays an important role in the removal of misfolded protein aggregates, the clearance of damaged mitochondria and their resultant ROS, and the degradation of the NLRP3 inflammasome or its components. Additionally, NLRP3 inflammasome-mediated pyroptosis may also provide a progressive memory loss of AD. Studies have indicated that the targeted inhibition of NLRP3 inflammasome overactivation at different levels by NLRP3 inflammasome inhibitors or autophagy inducers could inhibit the occurrence or development of cognitive decline caused by AD. Intracellular accumulation of neurofibrillary tangles (NFTs), consisting of p-tau protein are regarded as the characters of AD. It is reported that iron deposition has been involved in the misfolding process of the Aβ plaques and NFTs. The accumulation of phosphorylated tau is responsible for abnormal mitophagy function, mitochondrial dynamics hippocampal-based learning and memory impairments in tau mice. The overexpression of IL-1β aggravates AD pathogenesis, owing to tau hyperphosphorylation. On the contrary, IL-1β can increase the phosphorylation of tau proteins, which would increase the severity of AD. Studies also discovered that suppressing the activity of inflammation resulted in a remarkable reduction of pyroptosis-related proteins and significantly inhibited the hyperphosphorylation of tau proteins. These microglial death modes are inextricably linked with the phenotypic conversion of microglia, energy metabolism, and the occurrence and development of neurological diseases, like AD.
The modes mentioned in this review of cell death attract widespread attention. To sum up, programmed cell death might be a risk factor for AD. Programmed cell death may be a biologically plausible mechanism for AD pathogenesis, and the effects of cell damage about AD provide a framework with which to understand how microglia might contribute to the early, and possibly the later, course of AD. Therefore, further understanding of microglial death can provide more reliable evidence for the clinical treatment of AD.
References
DeTure, M.A. and D.W. Dickson, The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 2019. 14(1): p. 32. doi:https://doi.org/10.1186/s13024-019-0333-5
2020 Alzheimer’s disease facts and figures. Alzheimers Dement, 2020. doi:https://doi.org/10.1002/alz.12068
Mullane, K. and M. Williams, Alzheimer’s disease beyond amyloid: Can the repetitive failures of amyloid-targeted therapeutics inform future approaches to dementia drug discovery? Biochem Pharmacol, 2020. 177: p. 113945. doi:https://doi.org/10.1016/j.bcp.2020.113945
Sharma, I., et al., Exploring the Focal Role of Pyroptosis in Diabetes Mellitus. Biointerface Research in Applied Chemistry, 2021. 11(5): p. 13557–13572. doi:https://doi.org/10.33263/briac115.1355713572
Vanden Berghe, T., et al., Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol, 2014. 15(2): p. 135–47. doi:https://doi.org/10.1038/nrm3737
Yang, W.S. and B.R. Stockwell, Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol, 2016. 26(3): p. 165–176. doi:https://doi.org/10.1016/j.tcb.2015.10.014
D’Arcy, M.S., Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int, 2019. 43(6): p. 582–592. doi:https://doi.org/10.1002/cbin.11137
Yan, N. and J. Zhang, Iron Metabolism, Ferroptosis, and the Links With Alzheimer’s Disease. Front Neurosci, 2019. 13: p. 1443. doi:https://doi.org/10.3389/fnins.2019.01443
Li, Q., Y. Liu, and M. Sun, Autophagy and Alzheimer’s Disease. Cell Mol Neurobiol, 2017. 37(3): p. 377–388. doi:https://doi.org/10.1007/s10571-016-0386-8
Li, Y., et al., Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomed Pharmacother, 2020. 121: p. 109618. doi:https://doi.org/10.1016/j.biopha.2019.109618
Louveau, A. [Cerebral lymphatic drainage: implication in the brain immune privilege]. Med Sci (Paris), 2015. 31(11): p. 953–6. doi:https://doi.org/10.1051/medsci/20153111005
Sevenich, L., Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol, 2018. 9: p. 697. doi:https://doi.org/10.3389/fimmu.2018.00697
Singh, V., et al., Isolation and Characterization of Microglia from Adult Mouse Brain: Selected Applications for ex Vivo Evaluation of Immunotoxicological Alterations Following in Vivo Xenobiotic Exposure. Chemical Research in Toxicology, 2014. 27(5): p. 895–903. doi:https://doi.org/10.1021/tx500046k
Amor, S., et al., Inflammation in neurodegenerative diseases. Immunology, 2010. 129(2): p. 154–69. doi:https://doi.org/10.1111/j.1365-2567.2009.03225.x
Zhang, J., et al., Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy. Life Sci, 2021. 276: p. 118957. doi:https://doi.org/10.1016/j.lfs.2020.118957
Russell, R.C., H.X. Yuan, and K.L. Guan, Autophagy regulation by nutrient signaling. Cell Res, 2014. 24(1): p. 42–57. doi:https://doi.org/10.1038/cr.2013.166
Jung, S., H. Jeong, and S.W. Yu, Autophagy as a decisive process for cell death. Exp Mol Med, 2020. 52(6): p. 921–930. doi:https://doi.org/10.1038/s12276-020-0455-4
Wang, Y. and W.D. Le, Autophagy and Ubiquitin-Proteasome System. Adv Exp Med Biol, 2019. 1206: p. 527–550. doi:https://doi.org/10.1007/978-981-15-0602-4_25
Li, M., et al., Autophagy in the HTR-8/SVneo Cell Oxidative Stress Model Is Associated with the NLRP1 Inflammasome. Oxid Med Cell Longev, 2021. 2021: p. 2353504. doi:https://doi.org/10.1155/2021/2353504
Ip, W.K.E., et al., Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science, 2017. 356(6337): p. 513–519. doi:https://doi.org/10.1126/science.aal3535
Qiu, P., Y. Liu, and J. Zhang, Review: the Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation, 2019. 42(1): p. 6–19. doi:https://doi.org/10.1007/s10753-018-0890-8
Wu, A.G., et al., Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev, 2021. 65: p. 101202. doi:https://doi.org/10.1016/j.arr.2020.101202
Ma, K., et al., Toll-Like Receptor 2-Mediated Autophagy Promotes Microglial Cell Death by Modulating the Microglial M1/M2 Phenotype. Inflammation, 2020. 43(2): p. 701–711. doi:https://doi.org/10.1007/s10753-019-01152-5
Gordon, R., et al., Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med, 2018. 10(465). doi:https://doi.org/10.1126/sdtranslmed.aah4066
Ising, C., et al., NLRP3 inflammasome activation drives tau pathology. Nature, 2019. 575(7784): p. 669–673. doi:https://doi.org/10.1038/s41586-019-1769-z
Han, H.E., et al., Activation of Autophagy Pathway Suppresses the Expression of iNOS, IL6 and Cell Death of LPS-Stimulated Microglia Cells. Biomol Ther (Seoul), 2013. 21(1): p. 21–8. doi:https://doi.org/10.4062/biomolther.2012.089
Cho, M.H., et al., Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy, 2014. 10(10): p. 1761–75. doi:https://doi.org/10.4161/auto.29647
Su, P., et al., The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience, 2016. 319: p. 155–67. doi:https://doi.org/10.1016/j.neuroscience.2016.01.035
Reddy, P.H. and D.M. Oliver, Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells, 2019. 8(5). doi:https://doi.org/10.3390/cells8050488
Reddy, P.H., Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem, 2006. 96(1): p. 1–13. doi:https://doi.org/10.1111/j.1471-4159.2005.03530.x
Thellung, S., et al., Pharmacological activation of autophagy favors the clearing of intracellular aggregates of misfolded prion protein peptide to prevent neuronal death. Cell Death Dis, 2018. 9(2): p. 166. doi:https://doi.org/10.1038/s41419-017-0252-8
Tsvetkov, A.S., et al., A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci U S A, 2010. 107(39): p. 16982–7. doi:https://doi.org/10.1073/pnas.1004498107
Kovács, T., et al., The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci Rep, 2017. 7: p. 42014. doi:https://doi.org/10.1038/srep42014
Nilsson, P. and T.C. Saido, Dual roles for autophagy: degradation and secretion of Alzheimer’s disease Aβ peptide. Bioessays, 2014. 36(6): p. 570–8. doi:https://doi.org/10.1002/bies.201400002
Wani, A., et al., Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy, 2021: p. 1–20. doi:https://doi.org/10.1080/15548627.2021.1872187
Glatigny, M., et al., Autophagy Is Required for Memory Formation and Reverses Age-Related Memory Decline. Curr Biol, 2019. 29(3): p. 435–448.e8. doi:https://doi.org/10.1016/j.cub.2018.12.021
Majumder, S., et al., Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One, 2011. 6(9): p. e25416. doi:https://doi.org/10.1371/journal.pone.0025416
Spilman, P., et al., Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One, 2010. 5(4): p. e9979. doi:https://doi.org/10.1371/journal.pone.0009979
Zhu, Z., et al., Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci, 2013. 33(32): p. 13138–49. doi:https://doi.org/10.1523/jneurosci.4790-12.2013
Lonskaya, I., et al., Diminished parkin solubility and co-localization with intraneuronal amyloid-β are associated with autophagic defects in Alzheimer’s disease. J Alzheimers Dis, 2013. 33(1): p. 231–47. doi:https://doi.org/10.3233/jad-2012-121141
Cai, Q. and Y.Y. Jeong, Mitophagy in Alzheimer’s Disease and Other Age-Related Neurodegenerative Diseases. Cells, 2020. 9(1). doi:https://doi.org/10.3390/cells9010150
Pradeepkiran, J.A., A.P. Reddy, and P.H. Reddy, Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer’s disease. Drug Discov Today, 2019. 24(2): p. 616–623. doi:https://doi.org/10.1016/j.drudis.2018.11.005
Oliver, D. and P.H. Reddy, Dynamics of Dynamin-Related Protein 1 in Alzheimer’s Disease and Other Neurodegenerative Diseases. Cells, 2019. 8(9). doi:https://doi.org/10.3390/cells8090961
Menzies, F.M., et al., Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron, 2017. 93(5): p. 1015–1034. doi:https://doi.org/10.1016/j.neuron.2017.01.022
Kang, S., et al., Autophagy-Mediated Secretory Pathway is Responsible for Both Normal and Pathological Tau in Neurons. J Alzheimers Dis, 2019. 70(3): p. 667–680. doi:https://doi.org/10.3233/jad-190180
Kandimalla, R., et al., Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum Mol Genet, 2018. 27(1): p. 30–40. doi:https://doi.org/10.1093/hmg/ddx381
Manczak, M. and P.H. Reddy, Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet, 2012. 21(11): p. 2538–47. doi:https://doi.org/10.1093/hmg/dds072
Manczak, M. and P.H. Reddy, Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum Mol Genet, 2012. 21(23): p. 5131–46. doi:https://doi.org/10.1093/hmg/dds360
Di Meco, A., et al., Autophagy Dysfunction in Alzheimer’s Disease: Mechanistic Insights and New Therapeutic Opportunities. Biol Psychiatry, 2020. 87(9): p. 797–807. doi:https://doi.org/10.1016/j.biopsych.2019.05.008
Collier, J.J., et al., Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N Engl J Med, 2021. 384(25): p. 2406–2417. doi:https://doi.org/10.1056/NEJMoa1915722
Zhang, Z., et al., Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res Rev, 2021. 72: p. 101464. doi:https://doi.org/10.1016/j.arr.2021.101464
Estfanous, S., et al., Elevated Expression of MiR-17 in Microglia of Alzheimer’s Disease Patients Abrogates Autophagy-Mediated Amyloid-β Degradation. Front Immunol, 2021. 12: p. 705581. doi:https://doi.org/10.3389/fimmu.2021.705581
Dixon, S.J., et al., Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 2012. 149(5): p. 1060–72. doi:https://doi.org/10.1016/j.cell.2012.03.042
Gao, M., et al., Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell, 2015. 59(2): p. 298–308. doi:https://doi.org/10.1016/j.molcel.2015.06.011
Stockwell, B.R., et al., Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell, 2017. 171(2): p. 273–285. doi:https://doi.org/10.1016/j.cell.2017.09.021
Cao, J.Y. and S.J. Dixon, Mechanisms of ferroptosis. Cell Mol Life Sci, 2016. 73(11–12): p. 2195–209. doi:https://doi.org/10.1007/s00018-016-2194-1
Fanzani, A. and M. Poli, Iron, Oxidative Damage and Ferroptosis in Rhabdomyosarcoma. Int J Mol Sci, 2017. 18(8). doi:https://doi.org/10.3390/ijms18081718
Youssef, L.A., et al., Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood, 2018. 131(23): p. 2581–2593. doi:https://doi.org/10.1182/blood-2017-12-822619
Yao, M.Y., et al., Role of ferroptosis in neurological diseases. Neurosci Lett, 2021. 747: p. 135614. doi:https://doi.org/10.1016/j.neulet.2020.135614
Yang, W.S., et al., Regulation of ferroptotic cancer cell death by GPX4. Cell, 2014. 156(1–2): p. 317–331. doi:https://doi.org/10.1016/j.cell.2013.12.010
Dixon, S.J. and B.R. Stockwell, The Hallmarks of Ferroptosis. Annual Review of Cancer Biology, 2019. 3(1): p. 35–54. doi:https://doi.org/10.1146/annurevcancerbio-030518-055844
Luo, X., et al., Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ, 2021. 28(6): p. 1971–1989. doi:https://doi.org/10.1038/s41418-020-00719-2
Seibt, T.M., B. Proneth, and M. Conrad, Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med, 2019. 133: p. 144–152. doi:https://doi.org/10.1016/j.freeradbiomed.2018.09.014
Kagan, V.E., et al., Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol, 2017. 13(1): p. 81–90. doi:https://doi.org/10.1038/nchembio.2238
Dar, H.H., et al., A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(») sabotage of theftferroptosis. Redox Biol, 2021. 45: p. 102045. doi:https://doi.org/10.1016/j.redox.2021.102045
Weiland, A., et al., Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol, 2019. 56(7): p. 4880–4893. doi:https://doi.org/10.1007/s12035-018-1403-3
Wu, J.R., Q.Z. Tuo, and P. Lei, Ferroptosis, a Recent Defined Form of Critical Cell Death in Neurological Disorders. J Mol Neurosci, 2018. 66(2): p. 197–206. doi:https://doi.org/10.1007/s12031-018-1155-6
McIntosh, A., et al., Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol, 2019. 29(5): p. 606–621. doi:https://doi.org/10.1111/bpa.12704
Ayton, S., et al., Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry, 2020. 25(11): p. 2932–2941. doi:https://doi.org/10.1038/s41380-019-0375-7
Ghadery, C., et al., R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging, 2015. 36(2): p. 925–32. doi:https://doi.org/10.1016/j.neurobiolaging.2014.09.013
Acosta-Cabronero, J., et al., In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan. J Neurosci, 2016. 36(2): p. 364–74. doi:https://doi.org/10.1523/jneurosci.1907-15.2016
Smith, M.A., et al., Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis, 2010. 19(1): p. 363–72. doi:https://doi.org/10.3233/jad-2010-1239
Ayton, S., N.G. Faux, and A.I. Bush, Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun, 2015. 6: p. 6760. doi:https://doi.org/10.1038/ncomms7760
Kroner, A., et al., TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron, 2014. 83(5): p. 1098–116. doi:https://doi.org/10.1016/j.neuron.2014.07.027
van Duijn, S., et al., Cortical Iron Reflects Severity of Alzheimer’s Disease. J Alzheimers Dis, 2017. 60(4): p. 1533–1545. doi:https://doi.org/10.3233/jad-161143
Ayton, S., et al., Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain, 2017. 140(8): p. 2112–2119. doi:https://doi.org/10.1093/brain/awx137
van Bergen, J.M.G., et al., Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. Neuroimage, 2018. 174: p. 308–316. doi:https://doi.org/10.1016/j.neuroimage.2018.03.021
Yan, H.F., et al., Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther, 2021. 6(1): p. 49. doi:https://doi.org/10.1038/s41392-020-00428-9
Fu, A.L., Z.H. Dong, and M.J. Sun, Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res, 2006. 1109(1): p. 201–6. doi:https://doi.org/10.1016/j.brainres.2006.06.042
Bao, W.D., et al., Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ, 2021. 28(5): p. 1548–1562. doi:https://doi.org/10.1038/s41418-020-00685-9
Reichert, C.O., et al., Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int J Mol Sci, 2020. 21(22). doi:https://doi.org/10.3390/ijms21228765
Smith, M.A., et al., Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A, 1997. 94(18): p. 9866–8. doi:https://doi.org/10.1073/pnas.94.18.9866
Svobodová, H., et al., Elevated age-related cortical iron, ferritin and amyloid plaques in APP(swe)/PS1(deltaE9) transgenic mouse model of Alzheimer’s disease. Physiol Res, 2019. 68(Suppl 4): p. S445–S451. doi:https://doi.org/10.33549/physiolres.934383
Nikseresht, S., A.I. Bush, and S. Ayton, Treating Alzheimer’s disease by targeting iron. Br J Pharmacol, 2019. 176(18): p. 3622–3635. doi:https://doi.org/10.1111/bph.14567
Wong, B.X., et al., β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One, 2014. 9(12): p. e114174. doi:https://doi.org/10.1371/journal.pone.0114174
Tuo, Q.Z., et al., Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry, 2017. 22(11): p. 1520–1530. doi:https://doi.org/10.1038/mp.2017.171
Long, H.Z., et al., The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front Aging Neurosci, 2022. 14: p. 888989. doi:https://doi.org/10.3389/fnagi.2022.888989
Fernández-Mendívil, C., et al., Aging and Progression of Beta-Amyloid Pathology in Alzheimer’s Disease Correlates with Microglial Heme-Oxygenase-1 Overexpression. Antioxidants (Basel), 2020. 9(7). doi:https://doi.org/10.3390/antiox9070644
Chang, Y., et al., NLRP3 inflammasome-mediated microglial pyroptosis is critically involved in the development of post-cardiac arrest brain injury. J Neuroinflammation, 2020. 17(1): p. 219. doi:https://doi.org/10.1186/s12974-020-01879-1
Man, S.M., R. Karki, and T.D. Kanneganti, Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev, 2017. 277(1): p. 61–75. doi:https://doi.org/10.1111/imr.12534
Jorgensen, I. and E.A. Miao, Pyroptotic cell death defends against intracellular pathogens. Immunol Rev, 2015. 265(1): p. 130–42. doi:https://doi.org/10.1111/imr.12287
Shi, J., W. Gao, and F. Shao, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci, 2017. 42(4): p. 245–254. doi:https://doi.org/10.1016/j.tibs.2016.10.004
Zeng, C.Y., et al., ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis, 2019. 24(9–10): p. 703–717. doi:https://doi.org/10.1007/s10495-019-01551-x
Bergsbaken, T., S.L. Fink, and B.T. Cookson, Pyroptosis: host cell death and inflammation. Nat Rev Microbiol, 2009. 7(2): p. 99–109. doi:https://doi.org/10.1038/nrmicro2070
Ding, H.G., et al., Hypercapnia promotes microglial pyroptosis via inhibiting mitophagy in hypoxemic adult rats. CNS Neurosci Ther, 2020. 26(11): p. 1134–1146. doi:https://doi.org/10.1111/cns.13435
Poh, L., et al., Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun, 2019. 75: p. 34–47. doi:https://doi.org/10.1016/j.bbi.2018.09.001
Tan, M.S., et al., Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis, 2014. 5(8): p. e1382. doi:https://doi.org/10.1038/cddis.2014.348
Ross, J., et al., A selective, non-peptide caspase-1 inhibitor, VRT-018858, markedly reduces brain damage induced by transient ischemia in the rat. Neuropharmacology, 2007. 53(5): p. 638–42. doi:https://doi.org/10.1016/j.neuropharm.2007.07.015
Heneka, M.T., et al., NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature, 2013. 493(7434): p. 674–8. doi:https://doi.org/10.1038/nature11729
Halle, A., et al., The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol, 2008. 9(8): p. 857–65. doi:https://doi.org/10.1038/ni.1636
Hook, V.Y., M. Kindy, and G. Hook, Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem, 2008. 283(12): p. 7745–53. doi:https://doi.org/10.1074/jbc.M708362200
Chen, H., et al., Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett, 2016. 246: p. 7–16. doi:https://doi.org/10.1016/j.toxlet.2016.01.014
Shen, H., et al., Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav, 2021. 11(4): p. e02063. doi:https://doi.org/10.1002/brb3.2063
Griffin, W.S., et al., Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation, 2006. 3: p. 5. doi:https://doi.org/10.1186/1742-2094-3-5
Biundo, F., et al., A role for tau in learning, memory and synaptic plasticity. Sci Rep, 2018. 8(1): p. 3184. doi:https://doi.org/10.1038/s41598-018-21596-3
Wagner, L.K., et al., Immunoproteasome deficiency alters microglial cytokine response and improves cognitive deficits in Alzheimer’s disease-like APPPS1 mice. Acta Neuropathol Commun, 2017. 5(1): p. 52. doi:https://doi.org/10.1186/s40478-017-0453-5
Sita, G., et al., NLRP3 and Infections: β-Amyloid in Inflammasome beyond Neurodegeneration. Int J Mol Sci, 2021. 22(13). doi:https://doi.org/10.3390/ijms22136984
Litwiniuk, A., et al., Contribution of Mitochondrial Dysfunction Combined with NLRP3 Inflammasome Activation in Selected Neurodegenerative Diseases. Pharmaceuticals (Basel), 2021. 14(12). doi:https://doi.org/10.3390/ph14121221
Sharma, D. and T.D. Kanneganti, The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol, 2016. 213(6): p. 617–29. doi:https://doi.org/10.1083/jcb.201602089
Sharma, B., et al., Role of NLRP3 Inflammasome and Its Inhibitors as Emerging Therapeutic Drug Candidate for Alzheimer’s Disease: a Review of Mechanism of Activation, Regulation, and Inhibition. Inflammation, 2022: p. 1–32. doi:https://doi.org/10.1007/s10753-022-01730-0
Acknowledgements
This work was supported by the National Natural Science Foundation of China [82001473].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Zhongshan Hospital of Fudan university.
Additional information
Conflict of interest
All authors claim that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Qiu, Z., Zhang, H., Xia, M. et al. Programmed Death of Microglia in Alzheimer’s Disease: Autophagy, Ferroptosis, and Pyroptosis. J Prev Alzheimers Dis 10, 95–103 (2023). https://doi.org/10.14283/jpad.2023.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.3