Abstract
Background
For many tumors, radiomics provided a relevant prognostic contribution. This study tested whether the computed tomography (CT)-based textural features of intrahepatic cholangiocarcinoma (ICC) and peritumoral tissue improve the prediction of survival after resection compared with the standard clinical indices.
Methods
All consecutive patients affected by ICC who underwent hepatectomy at six high-volume centers (2009–2019) were considered for the study. The arterial and portal phases of CT performed fewer than 60 days before surgery were analyzed. A manual segmentation of the tumor was performed (Tumor-VOI). A 5-mm volume expansion then was applied to identify the peritumoral tissue (Margin-VOI).
Results
The study enrolled 215 patients. After a median follow-up period of 28 months, the overall survival (OS) rate was 57.0%, and the progression-free survival (PFS) rate was 34.9% at 3 years. The clinical predictive model of OS had a C-index of 0.681. The addition of radiomic features led to a progressive improvement of performances (C-index of 0.71, including the portal Tumor-VOI, C-index of 0.752 including the portal Tumor- and Margin-VOI, C-index of 0.764, including all VOIs of the portal and arterial phases). The latter model combined clinical variables (CA19-9 and tumor pattern), tumor indices (density, homogeneity), margin data (kurtosis, compacity, shape), and GLRLM indices. The model had performance equivalent to that of the postoperative clinical model including the pathology data (C-index of 0.765). The same results were observed for PFS.
Conclusions
The radiomics of ICC and peritumoral tissue extracted from preoperative CT improves the prediction of survival. Both the portal and arterial phases should be considered. Radiomic and clinical data are complementary and achieve a preoperative estimation of prognosis equivalent to that achieved in the postoperative setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumor, but its incidence is rapidly increasing.1,2 Systemic therapies are evolving significantly, passing from the limited effectiveness of standard chemotherapy to the promising results of new targeted treatments (anti-FGFR2 and anti-IDH1 drugs).3,4 To date, the standard treatment is liver resection, which is associated with 5-year survival rates ranging between 25 and 40%.5,6
Currently, the most relevant prognostic factors are tumor size, number and distribution of lesions, CA19-9 value, N and R status, and vascular invasion.2,7,8,9 New biomarkers in the peritumoral tissue, such as the immune infiltrate, have been outlined.10,11,12 However, available prognosticators do not adequately fulfil clinicians’ needs for at least two reasons. First, most cannot be appropriately estimated by standard imaging methods and can be assessed a posteriori only at the final pathology evaluation. Second, they do not allow a reliable selection of candidates for surgery, especially among patients with high tumor burden.
In recent years, advanced imaging techniques and quantitative analysis have gained traction in clinical research. Among the different approaches, radiomics has been one of the most studied, thanks to its easy application and high reproducibility.13,14,15 It has been associated with pathology data and the prognosis of several tumors.14,16,17,18
With regard to ICC, some data, almost exclusively reported by Eastern centers, are already available. The textural features extracted from both computed tomography (CT) and magnetic resonance imaging (MRI) can predict overall survival (OS) and progression-free survival (PFS) with high accuracy, improving the performances of standard clinical models.18,19,20,21,22 However, most studies have adopted radiomic signatures that optimize prediction but compromise the reproducibility and interpretability of data. In addition, robust confirmations by Western centers are lacking.
This multicentric study aimed to build a prognostic model combining the radiomic indices of the tumor and peritumoral tissue extracted from preoperative CT together with the preoperative clinical variables (model development).
Material and Methods
The study was conducted according to the Declaration of Helsinki and its later amendments. The local ethics committee of each center approved the study protocol (coordinating center approval: protocol no. 142/21-17/03/2021). No informed consent was required because of the retrospective study design.
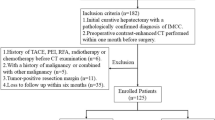
All consecutive patients affected by mass-forming ICC who underwent hepatic resection between January 2009 and December 2019 at six high-volume hepatobiliary centers were considered for the study. The participating centers are listed in Table S1.
The study was a retrospective analysis based on prospectively maintained clinical databases. The inclusion criteria specified age of 18 years or older, preoperative contrast-enhanced CT imaging available for analysis, and tumor larger than 10 mm. The exclusion criteria ruled out mixed hepatocellular carcinoma-ICC, imaging performed more than 60 days before surgery, inadequate CT (inadequate phases, movements, or artifacts), and locoregional treatment of ICC before liver resection.
If the patients had preoperative chemotherapy, the imaging after the end of treatment was analyzed. If a preoperative portal vein embolization was performed, the CT performed before embolization was analyzed.
The primary end points of the study were to identify the radiomic indices of the tumor and peritumoral tissue extracted from the portal and arterial phases of the CT that were associated with overall survival (OS), to build a prognostic model combining radiomic and preoperative clinical variables, and to compare the performance of the combined clinical-radiomic model with that of a pure preoperative clinical model. The secondary end points were to analyze the contribution of radiomic features to prediction of PFS, to elucidate the contribution of radiomics to a clinical model based on the pre- and postoperative variables, and to evaluate the stability of the prognostic role of radiomic features across the participating centers. The study followed the TRIPOD guidelines (Table S2).
Segmentation and Extraction of Radiomic Features
First, the portal phase of the selected CT was analyzed. For patients with multiple ICCs, only the largest lesion was considered. The technique of tumor and peritumoral tissue segmentation has been previously reported.23 In brief, an expert radiologist performed a manual tumor segmentation in each center to generate the first volume of interest (Tumor-VOI). The segmentation was performed using the LifeX software (CEA, Paris, France) in all centers. The peritumoral tissue was segmented by applying an automatic 5-mm expansion to the Tumor-VOI (Margin-VOI). The radiologist checked the Margin-VOI and manually removed any portions of tissue other than liver parenchyma. The VOIs then were copied into the arterial phase, and their positioning was manually adjusted if needed. The segmentation technique was standardized across centers as follows: (1) the authors shared criteria and technical features during meetings held before the data collection, and (2) the tumor segmentation of the first two included patients was performed by the radiologist of each center under remote tutoring by the coordinating center.
The radiomic features were automatically extracted by the LifeX software.24 The indices considered in the analyses are summarized in Table S3.
Statistical Analyses
Table S4 reports the analyzed pre- and postoperative variables. The ICC distribution pattern was classified according to Baheti et al.25 The eighth edition of the American Joint Committee on Cancer (AJCC) TNM classification was used for all the patients.26
The data from different centers were managed as follows: (1) after merging of the databases, queries were sent to participating centers about any outliers; (2) the patients with incomplete radiomic analyses (missing indices) or incomplete follow-up data (status [dead/alive], recurrence [yes/no], OS, PFS) were excluded; (3) for any variables with a proportion of missing data less than 15% of cases, multiple imputation was performed using the Python Miceforest program (MIT, Cambridge, US) with the ImputationKernel function.
Both OS and PFS were computed with the Kaplan-Meier method and compared using the log-rank test. Follow-up data were updated to 31 March 2021. A Cox proportional hazard model was used to identify independent prognosticators. The following clinical variables were selected for inclusion in the model: categorical clinical variables with a p value lower than 0.10 in the univariate analysis, all continuous clinical variables, and the prognosticators reported in the literature.2,7,8,9
Radiomic features were selected through a correlation analysis. When two features had a correlation greater than 0.85, one of the two was removed. Tumor-VOI and Margin-VOI radiomic indices underwent selection separately.
The textural features were standardized and included in the model as continuous variables. A stepwise algorithm was used to select the variables to be retained in the final model. The concordance index (C-index) and its corresponding standard error (SE) were computed for each model. First, the best model without consideration of the enrolling center was found (i.e., patients were considered independently of the enrolling center). Then, the center effect was analyzed by fitting a shared frailty model.27 The variables in the model were those identified for the best Cox model. The Commongens–Andersen test for heterogeneity was used to verify the presence of the center effect.
The p value was considered significant if it was lower than 0.05. The authors used the STATA (StataCorp LLC, College Station, US), R (R core team (2023), Vienna , Austria), and Python software.
Predictive Models
For each outcome (OS/PFS), we considered eight different predictive models. Four models were based on the following preoperative clinical data: (1) clinical data alone (Model OS/PFS_pre#1), (2) clinical data plus the portal-phase Tumor-VOI radiomics (Model OS/PFS_pre#2), (3) clinical data plus the portal-phase Tumor- and Margin-VOI radiomics (Model_OS/PFS_pre#3), and (4) clinical data plus the Tumor- and Margin-VOI radiomics extracted from both the portal and arterial phases of the CT (Model OS/PFS_pre#4). Four analogous models were built, including the postoperative and pathology data (Model OS/PFS_post#1 to 4).
Results
The study enrolled 215 of the 266 patients selected from the six centers (median, 37 patients; interquartile range [IQR] 29–74 patients; Fig. 1). Table 1 summarizes the patients’ characteristics. After a median follow-up period of 28 months (range 1–146 months), 105 (48.8%) of the patients were alive, and 54 patients (25.1% of the total series) were without recurrence. At 3 years, the OS rate was 57.0% (median OS, 47.4 months), and the PFS rate was 34.9% (median PFS, 13.7 months). The association of the clinical variables with OS and PFS is summarized in Table S5.
Preoperative Predictive Models of OS
The prognostic model based on the preoperative clinical variables (Model_OS_pre#1) had a C-index of 0.681 (SE, 0.029) and retained the four following variables: age, CA19-9, tumor pattern, and major hepatectomy (Table S6). Addition of the radiomic features to the clinical model led to a performance improvement (Fig. 2), but the same four clinical variables were retained in all the combined clinical-radiomic models. The model including the Tumor-VOI radiomic features extracted from the portal phase (Model_OS_pre#2) had a C-index of 0.71 (SE, 0.028). Two radiomic features were retained (shape compacity and GLRLM_SRHGE; Table S6). The model based on the clinical data and the portal-phase Tumor- and Margin-VOI radiomics (Model_OS_pre#3) had a C-index of 0.752 (SE, 0.026). It included three Tumor-VOI features (GLRLM_SRHGE, GLCM ContrastVariance, and Humin), and three Margin-VOI features (shape_compacity, skewness, and GLRLM_SRHGE) (Fig. 3a). The last model combined the clinical variables and the radiomic analysis of Tumor- and Margin-VOI in both the portal and the arterial phases (Model_OS_pre#4). It had a C-index of 0.764 (SE, 0.026) and included four clinical variables, six portal-phase radiomic indices (four of the Tumor-VOI and two of the Margin-VOI), and four arterial phase features (two and two, respectively) (Fig. 3b).
Predictive model of overall survival. a Model based on preoperative clinical data and radiomics extracted from the Tumor-VOI and Margin-VOI outlined in the portal phases of the preoperative computed tomography (CT). b Model based on preoperative clinical data and radiomics extracted from the arterial and portal phases (Tumor-VOI and Margin-VOI)
Preoperative Predictive Models of PFS
The prognostic model based on the preoperative clinical variables (Model_PFS_pre#1) had a C-index of 0.657 (SE, 0.025) and retained the following variables: CA 19-9, tumor number, and tumor size (Table S7). The inclusion of the radiomic indices led to a performance improvement (Fig. 2). Two clinical variables (CA 19-9 value and the number of tumors) were retained in all the models. The model combining the clinical variables and the portal-phase Tumor-VOI indices (Model_PFS_pre#2) had a C-index of 0.669 (SE, 0.025). Five radiomic features (two Hounsfield units-related features, entropy, NGLDM_Busyness, and GLZLM_LGZE) were retained (Table S7). The model that also included the portal-phase Margin-VOI radiomic indices (Model_PFS_pre#3) had a C-index of 0.700 (SE, 0.022). It retained five Tumor-VOI features (Humin, GLCM_ContrastVariance, GLRLM_LGRE, NGLDM_Busyness, and GLZLM_LZE) and three Margin-VOI features (two GLZLM features and Entropy) (Fig. 4a). The model including the clinical variables as well as the Tumor- and Margin-VOI radiomic indices extracted from both the portal and arterial phases (Model_PFS_pre#4) had a C-index of 0.719 (SE, 0.023) and included five portal-phase radiomic features (three of the Tumor-VOI and two of the Margin-VOI features), and two arterial-phase radiomic features (both of the Margin-VOI; Fig. 4b).
Predictive model of progression-free survival. a Model based on preoperative clinical data and radiomics extracted from the Tumor-VOI and Margin-VOI outlined in the portal phase of the preoperative computed tomography (CT). b Model based on preoperative clinical data and radiomics extracted from the arterial and portal phases (Tumor-VOI and Margin-VOI)
Postoperative Predictive Models
Considering OS, the clinical model including pre- and postoperative data (Model_OS_post#1) had a C-index of 0.765 (SE, 0.023). It had the same performance as the model that combined the preoperative clinical data and radiomic features (C-index, 0.764). When the radiomic features were added to the postoperative model, the performance progressively improved. The models including the portal-phase Tumor-VOI radiomics, the portal-phase Tumor- and Margin-VOI radiomics, and the arterial- and portal-phase Tumor- and Margin-VOI radiomics (Model_OS_post#2-4) had a C-index of 0.777 (SE, 0.022), 0.789 (SE, 0.024), and 0.803 (SE, 0.023), respectively.
Considering PFS, the clinical model including pre- and postoperative data (Model_PFS_post#1) had a C-index of 0.681 (SE, 0.026). Its performance was slightly inferior to the one based on preoperative clinical data and radiomic features (C-index, 0.719). When the radiomic features were added to the postoperative model, the performance progressively improved. The models including the portal-phase Tumor-VOI radiomics, the portal-phase Tumor- and Margin-VOI radiomics, and the arterial- and portal-phase Tumor- and Margin-VOI radiomics (Model_PFS_post#2-4) had C-indices of 0.702 (SE, 0.023), 0.749 (SE, 0.020), and 0.750 (SE, 0.021), respectively. Tables S8 and S9 summarize the models, and Fig. 2 summarizes their performances.
Stability of the Models Across Centers
The performance of the models was tested by introducing the variable “center.” The p values of the test (p = 0.654 for OS; p = 0.865 for RFS) demonstrated no statistical evidence of heterogeneity across centers. Moreover, the estimated coefficients including the variable “center” were equal to those obtained with the Cox model. For the sake of simplicity and parsimony, the Cox model without the enrolling centers was considered the definitive one.
Discussion
This study demonstrated that the CT-based radiomic features extracted from ICC and its peri-tumoral tissue improve estimation of the prognosis for patients undergoing hepatectomy. Analyzing both the portal and the arterial phases contributes to prognostication. Radiomic features and clinical data are complementary, and their combination allows a preoperative non-invasive prediction of survival equivalent to the one achieved in the postoperative setting when the pathology data are available.
For ICC patients, an accurate estimation of the tumor biology would be crucial for an adequate selection of candidates for surgery, but it is a clinically unmet need for several reasons: the pathology data, which are major determinants of prognosis, are not predictable by standard preoperative imaging;18 standard chemotherapy has limited disease control that precludes its adoption as a selection tool for resectable patients;1,3 and genetic data are not part of the clinical practice to date.
Radiomic features extracted from different imaging methods proved a tight association, with pathology data and prognosis for several tumors. Regarding ICC, preliminary studies not only demonstrated that the textural features of the tumor are associated with both OS and PFS, but also that they may lead to a better outcome prediction than standard clinical data.18,19,20,21,22 Similar data were observed in the current series, with clinical-radiomic models performing better than clinical models.
A further result was as evident in our analysis as in the literature: all models retained standard clinical predictors (e.g., tumor pattern and CA19-9) together with the radiomic indices, reaffirming that the latter carries information complementary to clinical data. However, the available literature has some major limitations that might reduce the reproducibility of results and clinical applicability of radiomics: most studies have been conducted by Eastern authors, have used in-house software for radiomics extraction, and have adopted signatures or scores (mathematical combinations of radiomic features) to optimize prediction.
The current analysis was a large multicenter analysis encompassing six Western referral hepato-pancreato-biliary (HPB) centers and collecting a large number of patients (n = 215). Radiomic features, extracted by a free online software, were separately included in the model, leading to complete replicability of the data.
The interpretability of radiomic data remains an issue, but some hypotheses can be advanced. Higher tumor homogeneity and lower intensity in the portal phase corresponded to a more favorable prognosis. In a standard radiologic evaluation, both characteristics (homogeneity and delayed enhancement) were associated with a less aggressive tumor.28 Margin compacity had a protective effect. This parameter could depict the tumor growth pattern. As observed for colorectal liver metastases,29,30 an infiltrative tumor profile is expected to be associated with a more aggressive disease. Finally, in both the arterial and portal phases, the GLZLM and GLRLM indices were clinically relevant. Again, they may reflect tumor heterogeneity, vascularization, and necrosis.
Three further results deserve consideration. Regarding the first result, both the tumor and the peritumoral tissue contributed to the survival prediction. At least two papers reported similar data, one analyzing magnetic resonance imaging20 and another analyzing positron emission tomography (PET)-CT.31 The liver-tumor interface is the niche of relevant biomarkers that can be unveiled by radiomics, as suggested by the association between the textural features and peritumoral immune infiltrate reported by Yugawa et al.32 Concerning the second result, the current study demonstrated that radiomics extracted from the arterial phase improves the performance of the predictive models. This reflects the radiologic presentation of ICC: its arterial enhancement pattern (peripheral, partial, or complete) has a prognostic relevance.27,33,34,35,36 Finally, the prediction of survival achieved by the preoperative clinical-radiomic model was non-inferior to that achieved by the postoperative clinical model based on the pathology data. As observed for other liver diseases,14,16,17 radiomics not only optimizes but also anticipates the assessment of tumor biology and the prediction of survival.
The current data are therefore clinically relevant. First, clinicians may have an accurate preoperative prediction of post-surgical survival using easy-to-apply and standardized software. This could be the basis for a more effective selection of candidates for surgery and an indication for preoperative systemic therapy. In the near future, radiomics could identify patients who, even if technically deemed resectable, do not benefit from surgery, and, as previously reported for colorectal metastases,16,37 could help in deciding the systemic treatment regimen according to the estimated efficacy. Of course, such decisions must rely on robust evidence and certified protocols.38 Second, even when the pathology data are available, radiomic features may refine the prediction of the outcome. Indications for adjuvant treatment and follow-up schedules could be adapted accordingly. Finally, even if clinicians still are uncertain, the tight association of radiomic data with tumor biology paves the way to their adoption as biomarkers and corroborates the hypothesis of a role in tumor heterogeneity assessment.
Some limitations of this study could be argued. First, the models were not externally validated, even if the homogeneity of results among centers anticipated the reproducibility of data. Second, an easy-to-apply user-friendly application still is lacking. However, in the near future, artificial intelligence protocols will enable easy inclusion of radiomic features into clinical practice. Third, although a significant number of patients were collected, some subgroups were not adequately represented and require further explorations. This may be not an easy task to accomplish because we had to merge the series of six high-volume HPB referral centers across 10 years to collect 215 patients with a rare disease and high-quality preoperative CT suitable for radiomic analyses. Fourth, the late phase of CT also should be considered for possible informative value. Finally, a prospective design with a standardized CT protocol would provide more reliable evidence. Nevertheless, this explorative retrospective analysis supports a clinically relevant concept (i.e., the usability of radiomics in a real-life setting).
Future studies are crucial to fill the gap between preliminary evidence on radiomics and its integration into clinical practice. Multicenter international trials merging Eastern and Western series are needed to collect “big data” and elucidate any geographic heterogeneity. At the same time, prospective trials with standardized protocols for imaging acquisition are essential to unveil the true potential of radiomics. The sample size remains an issue, but the new statistical approaches developed for artificial intelligence-based analyses offer promising solutions.39,40
A further research focus should be the combined analysis of radiomics and genetics. Radiogenomics analyzes the associations between radiomic and genetic data,41 but we believe that the two may have a complementary role: genetics elucidates the mechanisms underlying tumor biology, whereas radiomics provides a broader overview of the neoplasm, capturing its heterogeneity.42 Exploring further the combination of an extended range of “omics” features, including radiomics, genomics, pathomics, and even surgomics, should be considered to maximize our ability to predict patients’ survival, optimize treatment allocation, and design personalized treatment strategies.43
In conclusion, the radiomic analysis of ICC and peritumoral tissue refines the prediction of long-term results for patients undergoing liver resection. Radiomics should be considered part of the standard preoperative assessment of ICC patients. It is a further step toward precision medical and surgical oncology, refining the choice of the treatment protocol and surgical indications.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353–63.
Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6:803–15.
Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364–77.
Torzilli G, Vigano L, Fontana A, et al. Oncological outcome of R1 vascular margin for mass-forming cholangiocarcinoma: a single-center observational cohort analysis. HPB Oxf. 2020;22:570–7.
Doussot A, Gonen M, Wiggers JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223:493–505.
de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–5.
Conci S, Ruzzenente A, Vigano L, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25:3719–27.
Job S, Rapoud D, Dos Santos A, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–81.
Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2021;73(Suppl 1):75–85.
Vigano L, Soldani C, Franceschini B, et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. Impact on prognosis after complete surgery. J Gastrointest Surg. 2019;23:2216–24.
Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Sollini M, Antunovic L, Chiti A, Kirienko M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging. 2019;46:2656–72.
Zwanenburg A, Vallieres M, Abdalah MA, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38.
Fiz F, Vigano L, Gennaro N, et al. Radiomics of liver metastases: a systematic review. Cancers. 2020;12:2881.
Wakabayashi T, Ouhmich F, Gonzalez-Cabrera C, et al. Radiomics in hepatocellular carcinoma: a quantitative review. Hepatol Int. 2019;13:546–59.
Fiz F, Jayakody Arachchige VS, Gionso M, et al. Radiomics of biliary tumors: a systematic review of current evidence. Diagnostics. 2022;12:826.
Mosconi C, Cucchetti A, Bruno A, et al. Radiomics of cholangiocarcinoma on pretreatment CT can identify patients who would best respond to radioembolisation. Eur Radiol. 2020;30:4534–44.
Xu L, Wan Y, Luo C, et al. Integrating intratumoral and peritumoral features to predict tumor recurrence in intrahepatic cholangiocarcinoma. Phys Med Biol. 2021;66:125001.
Park HJ, Park B, Park SY, et al. Preoperative prediction of postsurgical outcomes in mass-forming intrahepatic cholangiocarcinoma based on clinical, radiologic, and radiomics features. Eur Radiol. 2021;31:8638–48.
Yang Y, Zou X, Zhou W, et al. Multiparametric MRI-based radiomic signature for preoperative evaluation of overall survival in intrahepatic cholangiocarcinoma after partial hepatectomy. J Magn Reson Imaging. 2022;56:739–51.
Fiz F, Rossi N, Langella S, et al. Radiomic analysis of intrahepatic cholangiocarcinoma: non-invasive prediction of pathology data: a multicenter study to develop a clinical-radiomic model. Cancers. 2023;15:4204.
Nioche C, Orlhac F, Boughdad S, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78:4786–9.
Baheti AD, Tirumani SH, Shinagare AB, et al. Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdom Imaging. 2014;39:1193–201.
Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. vol 1024, Berlin: Springer; 2017.
Wienke A. Frailty models in survival analysis. 1st edn. London: Chapman and Hall/CRC; 2010.
Jin KP, Sheng RF, Yang C, Zeng MS. Combined arterial and delayed enhancement patterns of MRI assist in prognostic prediction for intrahepatic mass-forming cholangiocarcinoma (IMCC). Abdom Radiol. 2022;47:640–50.
Vigano L, Branciforte B, Laurenti V, et al. The histopathological growth pattern of colorectal liver metastases impacts local recurrence risk and the adequate width of the surgical margin. Ann Surg Oncol. 2022;29:5515–24.
Fernández Moro C, Bozóky B, Gerling M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol. 2018;5:e000217.
Fiz F, Masci C, Costa G, et al. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur J Nucl Med Mol Imaging. 2022;49:3387–400.
Yugawa K, Itoh S, Iseda N, et al. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci Rep. 2021;11:5845.
Min JH, Kim YK, Choi SY, et al. Intrahepatic mass-forming cholangiocarcinoma: arterial enhancement patterns at MRI and prognosis. Radiology. 2019;290:691–9.
Jiao CY, Zhang H, Ji GW, et al. CT-based clinico-radiological nomograms for prognosis prediction in patients with intrahepatic mass-forming cholangiocarcinoma: a multi-institutional study. Eur Radiol. 2022;32:8326–38.
Fujita N, Asayama Y, Nishie A, et al. Mass-forming intrahepatic cholangiocarcinoma: enhancement patterns in the arterial phase of dynamic hepatic CT: correlation with clinicopathological findings. Eur Radiol. 2017;27:498–506.
Viganò L, Lleo A, Muglia R, et al. Intrahepatic cholangiocellular carcinoma with radiological enhancement patterns mimicking hepatocellular carcinoma. Updates Surg. 2020;72:413–21.
Nakanishi R, Oki E, Hasuda H, et al. Radiomics texture analysis for the identification of colorectal liver metastases sensitive to first-line oxaliplatin-based chemotherapy. Ann Surg Oncol. 2021;28:2975–85.
Viganò L, Ammirabile A, Zwanenburg A. Radiomics in liver surgery: defining the path toward clinical application. Updates Surg. 2023;75:1387–90.
Goldenholz DM, Sun H, Ganglberger W, Westover MB. Sample size analysis for machine learning clinical validation studies. Biomedicines. 2023;11:685.
Rajput D, Wang WJ, Chen CC. Evaluation of a decided sample size in machine learning applications. BMC Bioinform. 2023;24:48.
Bodalal Z, Trebeschi S, Nguyen-Kim TDL, Schats W, Beets-Tan R. Radiogenomics: bridging imaging and genomics. Abdom Radiol. 2019;44:1960–84.
Costa G, Cavinato L, Fiz F, et al. Mapping tumor heterogeneity via local entropy assessment: making biomarkers visible. J Digit Imaging. 2023;36:1038–48.
Gumbs AA, Croner R, Abu-Hilal M, et al. Surgomics and the artificial intelligence, radiomics, genomics, oncopathomics and surgomics (AiRGOS) project. Artif Intell Surg. 2023;3:180–5.
Acknowledgment
The current study was funded by the AIRC (Italian Association for Cancer Research) grant no. 2019−23822 (PI: Prof. Luca Viganò). The funders had no role in the design of the study, the collection, the analyses, the interpretation of data, the writing of the manuscript, or the decision to publish the results.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Francesco Fiz, Serena Langella, Simone Conci, Matteo Serenari, Francesco Ardito, Alessandro Cucchetti, Teresa Gallo, Giulia A. Zamboni, Cristina Mosconi, Luca Boldrini, Mariateresa Mirarchi, Stefano Cirillo, Andrea Ruzzenente, Ilaria Pecorella, Nadia Russolillo, Martina Borzi, Giulio Vara, and Caterina Mele. Data analysis was performed by Francesco Fiz, Noemi Rossi, Ilaria Pecorella, Francesca Ieva, and Luca Viganò. The first draft of the manuscript was written by Luca Viganò, Francesca Ieva, and Arturo Chiti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
Arturo Chiti received speaker’s honoraria from Advanced Accelerator Applications, General Electric Healthcare, Sirtex Medical Europe and AmGen Europe and travel grants from General Electric Healthcare and Sirtex Medical Europe. He is a member of Blue Earth Diagnostics’ and Advanced Accelerator Applications’ advisory boards and received scientific support in terms of a 3-year PhD fellowship from the Sanofi Genzyme. Luca Viganò received speaker’s honoraria from Johnson & Johnson. The remaining authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiz, F., Rossi, N., Langella, S. et al. Radiomics of Intrahepatic Cholangiocarcinoma and Peritumoral Tissue Predicts Postoperative Survival: Development of a CT-Based Clinical-Radiomic Model. Ann Surg Oncol 31, 5604–5614 (2024). https://doi.org/10.1245/s10434-024-15457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15457-9