Abstract
Purpose
Intrahepatic cholangiocarcinoma (IHC) is an aggressive disease with few reliable preoperative biomarkers. This study aims to elucidate if radiomics extracted from preoperative [18F]FDG PET/CT may grant a non-invasive biological characterization of IHC and predict outcome after complete resection of the tumor.
Methods
All patients preoperatively imaged by [18F]FDG PET/CT who underwent hepatectomy for mass-forming IHC in the period 2010–2019 were retrospectively evaluated. On PET images, manual slice-by-slice segmentation of IHC was performed (Tumor-VOI). A 5-mm margin region was semi-automatically generated around the tumor (Margin-VOI). Textural analysis was performed using the LifeX software. Analyzed outcomes included tumor grading (G3 vs. G1-2), microvascular invasion (MVI), overall survival (OS), and progression-free survival (PFS). The performances of the combined clinical-radiomic models were compared with those of standard clinical models.
Results
Overall, 74 patients (40 females, median age 68 years) were included. Considering tumor grading and MVI, the models combining the clinical data and radiomics of the Tumor-VOI had better performances than the clinical ones (AUC = 0.78 vs. 0.72 for grading; 0.87 vs. 0.78 for MVI). The inclusion into the models of radiomics of the Margin-VOI further improved the prediction of grading (AUC = 0.83), but not of MVI. Considering OS and PFS, the models including the preoperative clinical data and radiomics of the Tumor-VOI and Margin-VOI had better performances than the pure clinical ones (C-index = 0.81 vs. 0.76 for OS; 0.81 vs. 0.72 for PFS) and similar to the models including the pathology and postoperative data (C-index = 0.81 for OS; 0.79 for PFS). No model retained the standard SUV measures.
Conclusion
The PET-based radiomics of IHC can predict pathology data and allow a reliable preoperative evaluation of prognosis. The radiomics of both the tumoral and peritumoral areas had clinical relevance. The combined clinical-radiomic models outperformed the pure preoperative clinical ones and achieved performances non-inferior to the postoperative models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (IHC) is the second commonest liver malignancy, with increasing incidence over the last decades [1, 2]. It has aggressive biology that leads to a low resectability rate at diagnosis, poor response to systemic therapies, and unfavorable prognosis [1, 3, 4]. Surgery is the only potentially curative treatment, with a 5-year survival rate of about 25–40%, but is affected by a non-negligible recurrence risk [4,5,6,7]. Few preoperative biomarkers are available (e.g., tumor number and size, Ca 19–9 value, and suspicion of lymph node metastases); the strongest predictors of the outcome are those derived a posteriori from the surgical specimen [5, 8,9,10,11]. Emerging biomarkers have been identified in the peritumoral tissue, at the liver-tumor interface, but, again, they can be assessed only after resection [12,13,14]. Reliable non-invasive preoperative assessment of both tumor biology and prognosis is still an unmet need.
In the most recent years, non-invasive image-based tissue analyses have gained traction, being radiomics the commonest approach [15, 16]. Specific patterns of voxels have shown some associations with the tumor biology of several cancers [17,18,19]. Considering IHC, some reports demonstrated that textural features extracted from preoperative computed tomography (CT) and magnetic resonance imaging (MRI) predict pathological data and patients’ outcomes with high accuracy, outperforming traditional clinical models [20,21,22]. 18F-fluorodeoxyglucose (FDG) uptake of IHC at positron emission tomography (PET)/CT has been associated with tumor aggressiveness and prognosis [23,24,25], but no data about PET-based radiomics have been reported.
The present study aims to test the predictive and prognostic roles of PET-based radiomics in patients with surgically treated mass-forming IHC. We analyzed the textural features of both the tumor and the peritumoral tissue.
Material and methods
Population
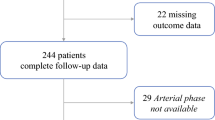
All consecutive patients undergoing a first liver resection for mass-forming IHC between January 2010 and December 2019 at the authors’ institution were considered. Inclusion criteria were diagnosis of IHC confirmed at final pathology, IHC ≥ 10 mm, complete resection (R0/R1), and availability of preoperative [18F]FDG PET/CT images for radiomic analysis. Patients with IHC < 10 mm were excluded because the minimal number of voxels for radiomics could have not been attained, considering the irregular shape of tumors. Moreover, we excluded patients with recurrent IHC after previous liver resection, those having local recurrence after a loco-regional treatment (ablation or transarterial embolization), and patients with mixed hepatocellular-cholangiocellular carcinoma. Patients undergoing liver resection after preoperative portal vein embolization were included only if the PET/CT was performed before the embolization. Additional exclusion criteria were PET/CT imaging performed > 60 days before surgery, poor PET/CT image quality, attenuation correction performed with methods other than CT-µmaps, and metal artifacts precluding an adequate liver assessment at PET/CT.
This study was performed in line with the principles of the Declaration of Helsinki. The local review board approved the study (protocol number 5/21). Because of the retrospective nature of the analysis, the need for informed consent was waived.
Image acquisition
[18F]FDG PET/CT image acquisition was performed according to versions 1.0 [26] and 2.0 [27] of the European Association of Nuclear Medicine (EANM) guidelines before and since February 2015, respectively. Briefly, fasting > 4 h before 18F-FDG injection and blood glucose levels < 200 mg/dl were requested. Images were acquired 60 ± 5 min after injection of 350–550 MBq of 18F-FDG, using either a Siemens Biograph 6 LSO (Siemens, Erlangen, Germany) or a General Electric Discovery 690 (General Electric Healthcare, Waukesha, WI, USA) PET/CT scanner. The acquisition range comprised the region between the orbital plane and the mid-thigh. All PET images were reconstructed via an iterative algorithm and corrected for attenuation using the CT dataset. The X-ray computed tomography was acquired using a “low dose” setting (voltage 130–140 kV, current: 95–140 mA). No contrast medium was injected. Details about PET/CT acquisition and reconstruction are provided in Supplementary Table 1.
Image analysis
Two authors (FF and GC) performed a manual slice-by-slice tumor segmentation on PET images defining the Tumor-VOI (volume of interest). In patients with a multinodular disease, only the largest tumor was segmented. The co-registered CT images were used as a reference to identify IHC in case of low FDG uptake and not to include activity spilled onto non-tumor tissue. A VOI of peritumoral tissue (Margin-VOI) was then automatically generated by capturing a 5-mm-thick rim surrounding the Tumor-VOI. The Margin-VOI was manually reviewed to remove any portion of tissue other than liver parenchyma (e.g., lung parenchyma, gallbladder, large vessels, and colon) and, in case of multinodular disease, to exclude any portion of contiguous tumors.
The radiomic analyses were then performed with the LifeX 5.1 software (LITO, CEA, Inserm, CNRS, Univ. Paris-Sud, Université Paris Saclay, France; www.lifexsoft.org) [28]. We extracted radiomic features from both VOIs (Tumor-VOI and Margin-VOI) separately. Features related to the CT, the technical descriptors, and the “rim” variables were excluded. A total of 48 indexes were considered for the analyses: seven conventional descriptors of the uptake intensity (SUVmean, SUVmin, SUVmax, SUVstd, and the SUV tertiles), six first-order features, three shape features, seven gray-level co-occurrence matrices (GLCM), 11 gray-level run-length matrices (GLRLM), three neighboring gray-level difference matrices (NGLDM), and 11 gray-level zone length matrices (GLZLM).
Supplementary Table 1 provides an overview of the image acquisition and processing parameters, according to the international initiative for biomarker standardization (IBSI).
Patients’ management
Management of patients with IHC was performed according to good clinical practice as previously reported [5, 29, 30]. In brief, a multidisciplinary team defined the treatment planning after complete staging, i.e., thoracoabdominal CT, hepatic MRI, and [18F]FDG PET/CT. Only patients amenable to complete resection were considered for surgery. Preoperative chemotherapy was scheduled in case of upfront unresectable disease and for selected patients with severe tumor burden. Since 2019, adjuvant chemotherapy is systematically planned for all patients; before 2019, it was considered on a case-by-case basis.
Clinical and pathological definitions
The tumor pattern was defined according to Baheti et al.: pattern type 1 if solitary tumor; pattern type 2 if solitary tumor with satellite nodules or multiple tumors into a single segment; and pattern type 3 if multifocal disease involving multiple segments [31]. The residual tumor (R) status was defined according to the final pathology (R0 if margin ≥ 1 mm; R1 if < 1 mm) [5]. The nodal (N) status was defined as follows: cN status according to the evidence of lymph node metastases at imaging (cN + /cN −); pN status according to the nodal status at final pathology (pN + /pN −).
Endpoints
The primary endpoints of the study were as follows: (1) to analyze the contribution of the radiomic features of the IHC to the preoperative prediction of two pathology parameters: tumor grading (G, G3 vs. G1–2) and microvascular invasion (MVI, present vs. absent); (2) to analyze the contribution of the radiomic features of the IHC to the preoperative prediction of overall survival (OS) and progression-free survival (PFS) after complete resection. The secondary endpoints were (1) to analyze the contribution of the radiomic features extracted from the peritumoral tissue (see details in the “Image analysis” section) to the predictive models of the two pathology parameters (grading and MVI) and survival (OS and PFS) and (2) to compare the performances of the prognostic models based on the preoperative clinical data + radiomics with those of the postoperative models (i.e., the current standard of care) including pathology data.
Statistical analyses
The data were retrospectively analyzed from a prospectively maintained registry. Categorical variables were summarized with the use of frequencies and proportions, while continuous variables with the use of means and standard deviations or medians and ranges. Categorical variables were compared with the chi-square or Fisher’s exact tests, as appropriate. Continuous variables were assessed graphically to determine distribution normality and were compared with parametric (unpaired T-test) or non-parametric (Mann–Whitney U-test) tests, accordingly. Continuous variables were included as continuous predictors to preserve and maximize their predictive contribution.
A multivariate logistic regression model was performed to estimate the adjusted association between each candidate predictor and (1) the tumor grading (G3 vs. G1–2) and (2) the MVI. A backward stepwise regression approach was used. The clinical variables were selected according to a priori knowledge and the results of univariate analysis. All radiomic features were initially considered. For their selection and inclusion into the model, a two-step approach was used. First, the correlation between features was evaluated, and if > 0.85 one of the two features was removed. Whenever possible (if the stepwise selection method resulted to be convergent), a stepwise selection preserving the original variables was applied. If not possible, a principal component analysis (PCA) of the two groups of variables (Tumor-VOI and Margin-VOI) was performed, leading to Tumor and Margin fingerprints (named Tumor-f and Margin-f in the text). For each of them, we retained components of the PCA that explain ≥ 90% of the original features’ variability. The retained fingerprints have neither clinical nor biological interpretation, but related estimates (odds ratio and confidence intervals) may be interpreted as usual. The final predictive model underwent internal cross-validation by splitting the series into a training set (90% of the population) and a validation set (10%). The validation procedure was repeated 100 times over 100 different samples. Results are reported in terms of mean (Std Dev) accuracy. Other machine learning methods were tested (e.g., CART, Random Forest) but the ratio between the sample size and the number of available features did not allow for stability of the results.
Survival probabilities were estimated with the Kaplan–Meier method and compared with the log-rank test. OS was computed from the date of surgery to the date of death or the last follow-up contact; PFS was computed to the date of the first recurrence or the last follow-up contact. Survival data were updated to December 2020, the 31st. A multivariate Cox regression model was used to assess the adjusted association between each candidate predictor and OS and PFS. The clinical variables were selected according to a priori knowledge and the results of univariate analysis. All radiomic features were included and were managed as previously reported.
A p-value < 0.05 was considered significant for all tests. The analyses were carried out using SPSS V. 24 for Mac (IBM, Armonk NY, USA) and STATA for Windows (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Analyzed models
We considered two different scenarios. First, we analyzed a preoperative scenario (Preop). Only the clinical data available before surgery were included. For the N status, the lymph node metastases evident at preoperative imaging were considered (cN status). For each outcome (G, MVI, OS, and PFS), three models were considered: clinical (Model1), clinical + radiomics of the tumor (Model2), and clinical + radiomics of the tumor + radiomics of the peritumoral tissue (Model3). Second, we analyzed a postoperative scenario (Postop). The same three models were compared. In the postoperative scenario, the preoperative, postoperative, and pathology data were included (pN status instead of cN one). In the manuscript, the models will be identified by a name indicating the scenario (Preop/Postop), the outcome (G/MVI/OS/PFS), and the covariates (Model1/Model2/Model3).
Results
In the study period, 109 consecutive patients underwent resection for IHC. Of those, 74 patients were selected for the analyses according to the above-mentioned criteria (Supplementary Fig. 1). Table 1 summarizes the patients’ characteristics. IHC had a type 1 pattern in two-thirds of patients (n = 49), and the median tumor size in the whole series was 56 mm (range, 15–190 mm). At final pathology, the tumor grading was G3 in 23 (31%) patients, and MVI was evident in 34 (46%). After a mean follow-up of 30.3 months (38.8 months for survivors, range 3–112 months, no patient was lost to follow-up), 34 patients (45%) were alive, 14 without disease and 20 with recurrence. OS was 48.1% and 40.0% at 3 and 5 years after surgery, respectively; PFS was 25.5% and 8.2%.
Prediction of tumor grading
Table 2 and Supplementary Table 2 summarize the results of the univariate and multivariate analyses. The clinical model (Preop_G_Model1) had an AUC of 0.718 (Fig. 1a). When the radiomics of the Tumor-VOI was considered (Preop_G_Model 2), the tumor pattern and four radiomic features (Kurtosis, GLCM_Energy, GLRLM_LRLGE, and GLZLM_LGZE) were retained in the model. The AUC increased to 0.783 (Fig. 1b). When the textural features of both the Tumor-VOI and Margin-VOI were considered (Preop_G_Model 3), the patients’ age, the tumor pattern, and five radiomic fingerprints (two from the Tumor-VOI and three from the Margin-VOI) were retained in the model. The AUC of the model further increased to 0.834 (Fig. 1c). The model had 0.743 accuracy, 0.652 sensitivity, and 0.787 specificity. In the cross-validation, the accuracy of the Preop_G_Model 3 was 0.723 (Std Dev 0.172). SUV measures were not associated with the tumor grading. Table 3 and Supplementary Table 3 summarize the performances of the different models.
Prediction of microvascular invasion
Table 4 and Supplementary Table 4 summarize the results of the univariate and multivariate analyses. The clinical model (Preop_MVI_Model1) had an AUC of 0.773 (Fig. 2a). When the radiomics of the Tumor-VOI was considered (Preop_MVI_Model2), twelve variables were retained in the model. Of those, age and six textural features were independent predictors of MVI (Histo_IQR, Kurtosis, GLCM_Homogeneity, GLRLM_LRE, NGLDM_Coarseness, and GLZLM_LZE). The AUC of the combined model increased to 0.871 (Fig. 2b). When the radiomics of both the Tumor-VOI and Margin-VOI was considered (Preop_MVI_Model3), one clinical variable (age) and nine textural indexes were independent predictors of MVI, including six from the Tumor-VOI (GLCM_Energy, GLCM_Correlation, GLRLM_LRE, NGLDM_Coarseness, GLZLM_LZE, and GLZLM_LGZE) and three from the Margin-VOI (NGLDM_Coarseness, NGLDM_Busyness, and GLZLM_ZLNU). The AUC of the model was 0.881 (Fig. 2c). The model had 0.819 accuracy, 0.853 sensitivity, and 0.789 specificity. In the internal validation, the accuracy of the model was 0.681 (Std Dev 0.180). SUV-based parameters were not associated with MVI. Table 3 and Supplementary Table 3 summarize the performances of the different models.
Prediction of overall survival
The results of the univariate and multivariate analyses are summarized in Supplementary Table 5 and Fig. 3, respectively. The performances of the different prognostic models are summarized in Table 3. The clinical model (Preop_OS_Model1) identified three predictors of OS (age, tumor pattern, and cN status) and had a C-index of 0.76 (Fig. 3a). When the radiomics of the Tumor-VOI was considered (Preop_OS_Model2), the same clinical variables were confirmed together with the NGLDM_Coarseness (Fig. 3b). The C-index was 0.77. When the textural features of both the Tumor-VOI and Margin-VOI were considered (Preop_OS_Model3), the age, tumor pattern, and cN status were independent prognosticators together with the NGLDM_Coarseness of the Margin-VOI (Fig. 3c). The C-index of the model was 0.81. SUV measures were not predictors of overall survival.
In the postoperative scenario, the three models (Post_OS_Model1—clinical, Post_OS_Model2—clinical + radiomics of the Tumor-VOI, and Post_OS_Model3—clinical + radiomics of the Tumor-VOI + radiomics of the Margin-VOI) had similar performances (C-index 0.81, 0.82, and 0.80, respectively, Table 3). The models are detailed in Supplementary Table 6.
Prediction of progression-free survival
Supplementary Table 5 summarizes the results of the univariate analysis. Figure 4 and Table 3 summarize the results of the multivariate analyses. The clinical model (Preop_PFS_Model1) identified three independent predictors of PFS (age, tumor pattern, and cN status, Fig. 4a) and had a C-index of 0.72. When the radiomics of the Tumor-VOI was considered (Preop_PFS_Model2), the tumor pattern and three textural features (GLCM_Homogeneity, GLRLM_LRE, and NGLDM_Contrast) were independent prognosticators (Fig. 4b). The C-index of the model increased to 0.76. When the radiomics of both the Tumor-VOI and Margin-VOI was considered (Preop_PFS_Model3), the following predictors of PFS were identified: the tumor pattern, two radiomic features of the Tumor-VOI (GLRLM_GLNU and GLCM_Energy), and two radiomic features of the Margin-VOI (GLCM_Contrast and GLZLM_SZE) (Fig. 4c). The C-index further increased to 0.81. SUV measures were not predictors of PFS.
Including in the model the postoperative and pathology data, the pure clinical model (Postop_PFS_Model1) had a C-index of 0.79; the model combining clinical data and radiomics of the Tumor-VOI (Postop_PFS_Model2) had a C-index of 0.81; the model including also the radiomics of the Margin-VOI (Postop_PFS_Model3) had a C-index of 0.86 (Table 3). The models are detailed in Supplementary Table 7.
Discussion
The present study demonstrated the predictive and prognostic role of the textural features extracted from [18F]FDG PET in mass-forming IHC. These findings, compared to other clinical settings, are even more decisive since IHC is an aggressive tumor at increasing incidence with few reliable biomarkers [2,3,4]. Indeed, tumor aggressiveness, mainly depicted by the tumor grading and MVI, can be established only a posteriori on the surgical specimen [5, 10, 11]. To date, treatment planning relies on morphological criteria (e.g., the tumor pattern and size) and CA 19–9 value, which are poor surrogates of tumor biology [4, 5, 32].
In the most recent years, some authors explored the contribution of CT- and MRI-based radiomics extracted from IHC [20,21,22, 33,34,35,36]. They reported that advanced imaging analysis can predict tumor grading, MVI, and survival with good performances (AUC = 0.730–0.890 for grading; AUC = 0.700–0.950 for MVI; C-index ≥ 0.70 for OS) [21, 22, 33,34,35,36]. Unfortunately, most published studies [21, 33,34,35,36] adopted radiomic signatures (a single value summarizing multiple textural features), precluding any evaluation of the single features and limiting the reproducibility of their models. To the best of our knowledge, textural features extracted from [18F]FDG PET have not been explored in IHC before. We demonstrated that they can contribute to a reliable prediction of the pathology data and survival. Furthermore, as previously reported for CT and MRI [21, 22], radiomics improved the predictive/prognostic performances of clinical variables. Specifically, our findings showed that the information provided by the radiomic features were complementary to the one provided by the clinical and radiological parameters: the standard biomarkers (e.g., the tumor pattern and CA 19–9 value) remained, and their combination with radiomics achieved the best prediction of pathology data and survival. Notably, all prognostic models that combined preoperative clinical and radiomic variables had performances even similar to the clinical postoperative ones, which include pathology details (OS, C-index 0.81 for the model based on preoperative data + radiomics vs. 0.81 for the model based on pre- and postoperative data; PFS, C-index 0.81 vs. 0.79, respectively).
We observed that low-uptake linear patterns were associated with less aggressive tumors (G1-2 or MVI-), while indexes of augmented dispersion of uptake values (e.g., kurtosis) and large intratumoral areas of reduced uptake were associated with more aggressive ones. The latter features could correspond to the heterogeneous tumors with intratumoral necrotic areas at standard imaging modalities. Considering prognosis, tumor homogeneity indexes (e.g., co-localization matrices energy) predicted a better survival, while margin heterogeneity indexes (“gray-level” non-uniformity, interquartile range, and small zone emphasis) predicted a worse one. In the present analyses, the SUV and its related indexes were the great absentees. They have never been retained in the final predictive/prognostic models. Some authors reported a prognostic role of the SUV measures in IHC patients [23,24,25], but, as previously reported for colorectal liver metastases [37], the radiomic features, in particular the second-order matrices, outperform the mere uptake semi-quantification.
Our data also proved that radiomic features of both tumoral and peritumoral areas had clinical relevance. Specifically, the radiomic analysis of the peritumoral area — the liver-tumor interface — gave an independent complementary contribution, being retained in all models (Model3). We have already demonstrated that texture analysis identifies the peritumoral microenvironment as a separate entity, different from tumor and normal liver parenchyma [38]. In modern oncology, the peritumoral area is the object of major research and contains new emerging biomarkers, first and foremost the immune infiltrates [12,13,14]. Yugawa et al. provided preliminary evidence of the association between the standard SUV measures and immune infiltrate in IHC [39]. Even if we demonstrated that the inclusion of the textural features extracted from the Margin-VOI optimized the prediction of tumor grading and survival, further analyses are needed to elucidate the association between radiomics of the peritumoral area and pathology data.
The present study is clinically relevant for several reasons. First, it unveils new non-invasive preoperative biomarkers for a disease at increasing incidence and poor prognosis. Second, it opens the way to a reliable preoperative estimation of tumor biology, which is mandatory to achieve a better selection of candidates for surgery and a precision medicine approach. The model combining preoperative and radiomic data achieved prognostic performances similar to the model including postoperative and pathology data. This confirms the capability of radiomics to anticipate prognosis assessment. Of note, the full model combining radiomics with pre- and postoperative data achieved the best performances for PFS prediction. It could be useful in the postoperative setting to select candidates for adjuvant treatment and refine follow-up schedules. Finally, radiomics strengthens the current role of PET/CT in the preoperative staging of IHC [40], highlighting a new potential contribution. Some future perspectives can be also outlined. The analysis of the underlying low-dose CT could provide additional information. [18F]FDG PET/CT is the current standard, but new tracers, such as 68 Ga-FAPI, could provide stronger data and open the way to theragnostic applications [41]. In combination with standard clinical and radiological data, radiomics could improve the evaluation of the tumor response to systemic therapies. As observed for colorectal liver metastases, textural features could even provide a reliable prediction of the therapy effectiveness since diagnosis [42, 43]. Several trials concerning target therapies and immunotherapies for IHC patients are ongoing. Radiomics could identify new biomarkers to select candidates for different treatments.
Some limitations should be mentioned. First, the present study is a single-center retrospective analysis collecting a limited number of patients (n = 74). We provided a preliminary evaluation, needing further confirmations. Second, external validation of data is lacking. Nevertheless, evidence in favor of radiomics was consistent through different analyses and confirmed at internal validation. Third, a custom-made algorithm could have yielded a higher number of textural indexes but would have decreased the explainability and reproducibility of the models.
In conclusion, the texture analysis of [18F]FDG PET/CT in mass-forming IHC patients led to a reliable preoperative assessment of tumor aggressiveness and prognosis. The combined analysis of the textural features from the tumor and its margin maximized the accuracy of prediction and brought to the preoperative setting predictions that are usually reached only after the resection when the pathology data are available. Radiomics represents an innovative non-invasive biomarker that could contribute to a precision medicine approach for patients affected by IHC.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111.
Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594–9.
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80.
Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364–77.
Torzilli G, Viganò L, Fontana A, Procopio F, Terrone A, Cimino MM, Donadon M, Del Fabbro D. Oncological outcome of R1 vascular margin for mass-forming cholangiocarcinoma. A single center observational cohort analysis. HPB. 2020;22:570–7.
Conci S, Viganò L, Ercolani G, Gonzalez E, Ruzzenente A, Isa G, Salaris C, Fontana A, Bagante F, Pedrazzani C, Campagnaro T, Iacono C, De Santibanes E, Pinna DA, Torzilli G, Guglielmi A. Outcomes of vascular resection associated with curative intent hepatectomy for intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2020;46:1727–33.
Hue JJ, Rocha FG, Ammori JB, Hardacre JM, Rothermel LD, Chavin KD, Winter JM, Ocuin LM. A comparison of surgical resection and liver transplantation in the treatment of intrahepatic cholangiocarcinoma in the era of modern chemotherapy: an analysis of the National Cancer Database. J Surg Oncol. 2021;123:949–56.
Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Pawlik TM. Defining long-term survivors following resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2017;21:1888–97.
Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, Allen PJ, Farges O, Kingham TP, Regimbeau JM, D’Angelica MI, Azoulay D, Jarnagin WR. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223:493–505.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Tsukamoto M, Yamashita YI, Imai K, Umezaki N, Yamao T, Okabe H, Nakagawa S, Hashimoto D, Chikamoto A, Ishiko T, Yoshizumi T, Maehara Y, Baba H. Predictors of cure of intrahepatic cholangiocarcinoma after hepatic resection. Anticancer Res. 2017;37:6971–5.
Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2021;73:75–85.
Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, Elarouci N, Ayadi M, Adam R, Azoulay D, Castaing D, Vibert E, Cherqui D, Samuel D, Sa Cuhna A, Marchio A, Pineau P, Guettier C, de Reyniès A, Faivre J. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–81.
Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A, Donadon M, Roncalli M, Aghemo A, Di Tommaso L, Torzilli G. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. Impact on prognosis after complete surgery. J Gastrointest Surg. 2019;23:2216–24.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Song J, Yin Y, Wang H, Chang Z, Liu Z, Cui L. A review of original articles published in the emerging field of radiomics. Eur J Radiol. 2020;127:108991.
Sollini M, Antunovic L, Chiti A, Kirienko M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging. 2019;46:2656–72.
Fiz F, Viganò L, Gennaro N, Costa G, La Bella L, Boichuk A, Cavinato L, Sollini M, Politi LS, Chiti A, Torzilli G. Radiomics of liver metastases: a systematic review. Cancers. 2020;12:2881.
Wakabayashi T, Ouhmich F, Gonzalez-Cabrera C, Felli E, Saviano A, Agnus V, Savadjiev P, Baumert TF, Pessaux P, Marescaux J, Gallix B. Radiomics in hepatocellular carcinoma: a quantitative review. Hepatol Int. 2019;13:546–59.
Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, Xia YX, Zhang YD, Jiang WJ, Li XC, Wang XH. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29:3725–35.
Park HJ, Park B, Park SY, Choi SH, Rhee H, Park JH, Cho ES, Yeom SK, Park S, Park MS, Lee SS. Preoperative prediction of postsurgical outcomes in mass-forming intrahepatic cholangiocarcinoma based on clinical, radiologic, and radiomics features. Eur Radiol. 2021;31:8638–48.
Zhang J, Wang X, Zhang L, Yao L, Xue X, Zhang S, Li X, Chen Y, Pang P, Sun D, Xu J, Shi Y, Chen F. Radiomics predict postoperative survival of patients with primary liver cancer with different pathological types. Ann Transl Med. 2020;8:820.
Yoh T, Seo S, Morino K, Fuji H, Ikeno Y, Ishii T, Taura K, Nakamoto Y, Higashi T, Kaido T, Uemoto S. Reappraisal of prognostic impact of tumor SUVmax by 18F-FDG-PET/CT in intrahepatic cholangiocarcinoma. World J Surg. 2019;43:1323–31.
Ikeno Y, Seo S, Iwaisako K, Yoh T, Nakamoto Y, Fuji H, Taura K, Okajima H, Kaido T, Sakaguchi S, Uemoto S. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18F-FDG-PET is associated with the KRAS mutation status and prognosis. J Transl Med. 2018;16:95.
Harimoto N, Hoshino K, Muranushi R, Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Tomonaga H, Higuchi T, Tsushima Y, Shirabe K. Impact of metabolic parameters of 18F-fluorodeoxyglucose positron-emission tomography after hepatic resection in patients with intrahepatic cholangiocarcinoma. Anticancer Res. 2019;39:971–7.
Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78:4786–9.
Viganò L, Lleo A, Muglia R, Gennaro N, Samà L, Colapietro F, Roncalli M, Aghemo A, Chiti A, Di Tommaso L, Solbiati L, Colombo M, Torzilli G. Intrahepatic cholangiocellular carcinoma with radiological enhancement patterns mimicking hepatocellular carcinoma. Updates Surg. 2020;72:413–21.
Torzilli G. Ultrasound-guided liver surgery. 1st ed. Springer; 2014.
Baheti AD, Tirumani SH, Shinagare AB, Rosenthal MH, Hornick JL, Ramaiya NH, Wolpin BM. Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdom Imaging. 2014;39:1193–201.
Conci S, Ruzzenente A, Viganò L, Ercolani G, Fontana A, Bagante F, Bertuzzo F, Dore A, Pinna AD, Torzilli G, Iacono C, Guglielmi A. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25:3719–27.
Yang C, Huang M, Li S, Chen J, Yang Y, Qin N, Huang D, Shu J. Radiomics model of magnetic resonance imaging for predicting pathological grading and lymph node metastases of extrahepatic cholangiocarcinoma. Cancer Lett. 2020;470:1–7.
Yao X, Huang X, Yang C, Hu A, Zhou G, Lei J, Shu J. A novel approach to assessing differentiation degree and lymph node metastasis of extrahepatic cholangiocarcinoma: prediction using a radiomics-based particle swarm optimization and support vector machine Model. JMIR Med Inform. 2020;8:e23578.
Zhou Y, Zhou G, Zhang J, Xu C, Wang X, Xu P. Radiomics signature on dynamic contrast-enhanced MR images: a potential imaging biomarker for prediction of microvascular invasion in mass-forming intrahepatic cholangiocarcinoma. Eur Radiol. 2021;31:6846–55.
Xu L, Yang P, Liang W, Liu W, Wang W, Luo C, Wang J, Peng Z, Xing L, Huang M, Zheng S, Niu T. A radiomics approach based on support vector machine using MR images for preoperative lymph node status evaluation in intrahepatic cholangiocarcinoma. Theranostics. 2019;9:5374–85.
Rahmim A, Bak-Fredslund KP, Ashrafinia S, Lu L, Schmidtlein CR, Subramaniam RM, Morsing A, Keiding S, Horsager J, Munk OL. Prognostic modeling for patients with colorectal liver metastases incorporating FDG PET radiomic features. Eur J Radiol. 2019;113:101–9.
Fiz F, Costa G, Gennaro N, la Bella L, Boichuk A, Sollini M, Politi LS, Balzarini L, Torzilli G, Chiti A, Viganò L. Contrast administration impacts CT-based radiomics of colorectal liver metastases and non-tumoral liver parenchyma revealing the “radiological” tumour microenvironment. Diagnostics. 2021;11:1162.
Yugawa K, Itoh S, Iseda N, Kurihara T, Kitamura Y, Toshima T, Harada N, Kohashi K, Baba S, Ishigami K, Oda Y, Yoshizumi T, Mori M. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci Rep. 2021;11:5845.
Lamarca A, Barriuso J, Chander A, McNamara MG, Hubner RA, ÓReilly D, Manoharan P, Valle JW. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol. 2019;71:115–29.
Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:4396–414.
Nakanishi R, Oki E, Hasuda H, Sano E, Miyashita Y, Sakai A, Koga N, Kuriyama N, Nonaka K, Fujimoto Y, Jogo T, Hokonohara K, Hu Q, Hisamatsu Y, Ando K, Kimura Y, Yoshizumi T, Mori M. Radiomics texture analysis for the identification of colorectal liver metastases sensitive to first-Line oxaliplatin-based chemotherapy. Ann Surg Oncol. 2021;28:2975–85.
van Helden EJ, Vacher YJL, van Wieringen WN, van Velden FHP, Verheul HMW, Hoekstra OS, Boellaard R, Menke-van der Houven van Oordt CW. Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur J Nucl Med Mol Imaging. 2018;45:2307–2317.
Funding
The present study was supported by the AIRC (Italian Association for Cancer Research) [grant #2019–23822] (PI: Luca Viganò).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by FF, CM, GC, MS, FI, and LV. The first draft of the manuscript was written by FF, CM, GC, MS, and LV, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Humanitas Clinical and Research Hospital (date 23/02/2021: protocol number 5/21).
Informed consent
Because of the retrospective nature of the analysis, the need for informed consent was waived.
Competing interests
The authors declare no competing interests. Considering the conflicts of interest in general, we state that (1) L.V. received speaker’s honoraria from Johnson & Johnson; (2) A.C. received speaker’s honoraria from Advanced Accelerator Applications, General Electric Healthcare, Sirtex Medical Europe, and AmGen Europe; received travel grants from General Electric Healthcare and Sirtex Medical Europe; is a member of Blue Earth Diagnostics’ and Advanced Accelerator Applications’ advisory boards; and received scientific support, in terms of a 3-year Ph.D. fellowship, from the Sanofi Genzyme; (3) F.F. has been a consultant for the MSD Sharp & Dohme GmbH (LLC).
The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fiz, F., Masci, C., Costa, G. et al. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur J Nucl Med Mol Imaging 49, 3387–3400 (2022). https://doi.org/10.1007/s00259-022-05765-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05765-1