Abstract
Background
Few reports have discussed the association between total tumor volume (TTV) and prognosis in patients with colorectal liver metastases (CRLM). The present study aimed to evaluate the usefulness of TTV for predicting recurrence-free survival and overall survival (OS) in patients receiving initial hepatic resection or chemotherapy, and to investigate the value of TTV as an indicator for optimal treatment selection for patients with CRLM.
Patients and Methods
This retrospective cohort study included patients with CRLM who underwent hepatic resection (n = 93) or chemotherapy (n = 78) at the Kobe University Hospital. TTV was measured using 3D construction software and computed tomography images.
Results
A TTV of 100 cm3 has been previously reported as a significant cut-off value for predicting OS of CRLM patients receiving initial hepatic resection. For patients receiving hepatic resection, the OS for those with a TTV ≥ 100 cm3 was significantly reduced compared with those with a TTV < 100 cm3. For patients receiving initial chemotherapy, there were no significant differences between the groups divided according to TTV cut-offs. Regarding OS of patients with TTV ≥ 100 cm3, there was no significant difference between hepatic resection and chemotherapy (p = 0.160).
Conclusions
TTV can be a predictive factor of OS for hepatic resection, unlike for initial chemotherapy treatment. The lack of significant difference in OS for CRLM patients with TTV ≥ 100 cm3, regardless of initial treatment, suggests that chemotherapeutic intervention preceding hepatic resection may be indicated for such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is responsible for a high incidence of cancer-related mortality worldwide.1 The liver is the most common site of metastatic disease for patients with colorectal cancer, and hepatic resection remains the only curative approach to colorectal liver metastases (CRLM).2,3 However, the definition of resectability is vague and remains controversial. Many studies have reported the predictive factors in patients with resectable CRLM, including tumor–node–metastasis classification, primary tumor site, metastatic tumor site, age, carcinoembryonic antigen (CEA) level, maximal tumor diameter, and intrahepatic tumor number.4,5,6
The concept of total tumor volume (TTV) as a prognostic factor was originally proposed in 2006 by Tsai et al. It can be measured simply by computed tomography (CT) or magnetic resonance imaging (MRI).7 Since then, the usefulness of TTV as a predictor of overall survival (OS) for many types of solid tumors has been reported.8,9,10 Liver tumors are relatively easy to measure, and the significance of TTV for OS and recurrence-free survival (RFS) for hepatocellular carcinoma has been demonstrated in several reports.11,12 However, its usefulness for patients with CRLM has not been studied sufficiently.
The significance of TTV as a predictor of prognosis in patients with resectable CRLM has been previously reported, and a TTV of 100 cm3 has been reported to be a significant cut-off value for predicting OS in patients requiring hepatic resection.13 The significance of TTV for chemotherapy treatment of patients with CRLM, however, remains unclear. The present study therefore aimed to investigate the usefulness of TTV as a prognostic factor for patients who underwent hepatic resection and chemotherapy and to determine the optimal treatment strategy for patients with CRLM.
Patients and Methods
Patient Selection
This retrospective cohort study recruited consecutive patients with CRLM who underwent hepatic resection (n = 98) or chemotherapy (n = 78) without extrahepatic metastases as their initial treatment at Kobe University Hospital between April 2008 and June 2019. However, seven patients who underwent hepatic resection were excluded (details shown in Fig. 1). Finally, a total of 171 patients, including 93 patients with hepatic resection and 78 patients with chemotherapy, participated in the present study.
The eligibility criteria for initial hepatic resection for CRLM were (i) technically resectable tumors, which involved no more than three hepatic segments; (ii) indocyanine green retention rate at 15 min (ICGR15) < 25%; (iii) residual functional volume of the liver > 30% of the standard liver volume; (iv) no apparent main portal vein trunk involvement, and (v) an Eastern Cooperative Oncology Group performance status score of 0–2.14
The eligibility criteria for initial chemotherapy treatment were (i) technically unresectable intrahepatic tumors (considering intrahepatic tumor number, maximal tumor diameter, and timing of metastases) and (ii) respecting individual patient’s choice of treatment. The regimens of first-line treatment included oxaliplatin or irinotecan (modified FOLFOX/XELOX/FOLFILI/FOLFOXILI) and fluoropyrimidines (5-FU/capecitabine). In addition, biological agents (such as panitumumab and bevacizumab) could be added to chemotherapy according to tumor (RAS mutational status, sidedness) and patient characteristics.
Exclusion criteria for patients who underwent hepatic resection were (i) patients who did not undergo initial hepatic resection; (ii) incomplete resection (gross residual tumor); (iii) two-stage hepatectomy; and (iv) hepatic resection after liver transplantation. An exclusion criterion for patients who underwent chemotherapy was a history of hepatic resection before chemotherapy treatment.
Informed consent was obtained using an opt-out form. This study complied with the standards of the Declaration of Helsinki and was approved by the institutional ethics board of Kobe University Hospital in 2021 (approval number B210197).
Assessment and Study Design
The value of the TTV to predict prognosis was estimated by OS after initial hepatic resection or chemotherapy. Patients who were lost to follow-up were censored on the date of the last contact. OS was calculated from the date of the therapeutic intervention (initial hepatic resection or chemotherapy commencement) to the date of death. Patients were followed up until death or June 2022.
TTV was measured in all patients with Ziostation2® (Ziosoft, Tokyo, Japan) software as previously described,13 using 3D images constructed from the original preoperative dynamic contrast-enhanced CT scan.
Statistical Analyses
All statistical analyses were two-tailed, and the threshold for significance was p < 0.05. Descriptive data were presented with medians, ranges, number, and percentages. Categorical variables were compared using the chi-squared test, and continuous variables were compared using the Student’s t-test or the Mann–Whitney U-test. The TTV cut-off value that would best predict OS and RFS were determined using receiver operating characteristic (ROC) curve analysis, and cut-off values for OS and RFS were defined as 100 cm3 and 10 cm3, respectively, based on our previous study.13 Survival data for the treatment groups were analyzed with Kaplan–Meier plots, log-rank tests for equality of survival curves, and Cox proportional hazards regression. To identify the predictors of survival, univariable and multivariable analyses of prognostic factors were performed using the Cox proportional hazards model. All statistical analyses were conducted using JMP®14 software (SAS Institute, Cary, North Carolina, USA).
Results
Patient Characteristics
In total, 171 patients were enrolled in the present study, and their characteristics are presented in Table 1. Comparisons between both groups for patient characteristics showed that the group that underwent initial chemotherapy had a higher proportion of female patients, intrahepatic tumor number ≥ 5, bilobar tumor distribution, CEA levels, carbohydrate antigen (CA)19-9 level, and albumin–bilirubin (ALBI) score. The mean age, location of the primary site, maximal tumor diameter, and proportion of metachronous metastases were similar for the two groups without any significant differences. Preoperative chemotherapy was administered to six patients who underwent hepatic resection.
Determination of Total Tumor Volume Cut-off Values
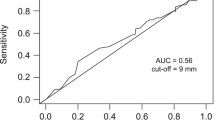
ROC curve analysis was used to investigate the association between TTV and OS in the hepatic resection group. It showed that the TTV cut-off value for OS was defined as 100 cm3 [area under the ROC curve (AUC): 0.762 for OS; sensitivity 89%, specificity 66%] and that of RFS was defined as 10 cm3 (AUC: 0.682 for RFS; sensitivity 71%, specificity 67%). Based on these results and those of our previous study,13 the cut-off values of 100 cm3 for OS and 10 cm3 for RFS were also applied in this study.
Identification of Predictive Factors for Overall Survival
The association of several variables with OS after hepatic resection for CRLM was investigated. In univariable analysis, primary lymph node metastasis, primary tumor location in the right colon, bilobar tumor distribution, and TTV ≥ 100 cm3 were associated with shorter OS. Multivariable analyses indicated that TTV ≥ 100 cm3 was independently associated with poorer OS [hazard ratio (HR) 5.26; 95% confidence interval (CI) 0.94–13.2; p < 0.001].
Overall Survival of the Entire Cohort according to Total Tumor Volume
All patients including those who underwent initial hepatic resection or chemotherapy (n = 171) were divided into three groups according to TTV cut-off values: TTV < 10 cm3 (57 patients), 10 cm3 ≤ TTV < 100 cm3 (71 patients), and TTV ≥ 100 cm3 (43 patients). Figure 2A shows that OS among patients with TTV ≥ 100 cm3 [median survival time (MST): 28 months, 3 year OS: 34%] was significantly reduced compared with that among patients with TTV < 10 cm3 (MST: 86.5 months, 3 year OS: 76%, p < 0.001) or 10 cm3 ≤ TTV < 100 cm3 (MST: 58 months, 3 year OS: 63%, p = 0.004) (patient characteristics are presented in Supplementary Table 1).
Kaplan–Meier analyses of overall survival after therapeutic intervention according to TTV. Patients were divided into three groups based on TTV cut-off values of 10 cm3 and 100 cm3: TTV < 10 cm3 (lowest volume), 10 cm3 ≤ TTV < 100 cm3 (mid volume), and TTV ≥ 100 cm3 (highest volume). A Entire cohort: p = 0.417 (lowest versus mid volume), p = 0.004 (mid versus highest volume), p < 0.001 (lowest versus highest volume). B The group of patients who underwent initial hepatic resection: p = 0.146 (lowest versus mid volume), p = 0.048 (mid versus highest volume), p = 0.002 (lowest versus mid volume). C The group of patients who underwent initial chemotherapy. The patients with TTV < 10 cm3 (lowest volume), 10 cm3 ≤ TTV < 100 cm3 (mid volume), and TTV ≥ 100 cm3 (highest volume) had no significant difference: p = 0.971 (lowest versus mid volume), p = 0.579 (mid versus highest volume), p = 0.271 (lowest versus highest volume) (log-rank test). TTV, total tumor volume
Overall Survival of Patients Undergoing Hepatic Resection according to Total Tumor Volume
Patients initially treated with hepatic resection (n = 93) were divided into three groups: TTV < 10 cm3 (34 patients), 10 cm3 ≤ TTV < 100 cm3 (43 patients), and TTV ≥ 100 cm3 (16 patients). Figure 2B shows that OS among patients with TTV ≥ 100 cm3 (MST: 37 months, 3 year OS: 53%) was significantly reduced compared with that of TTV < 10 cm3 (MST: 112 months, 3 year OS: 56%, p = 0.018) or 10 cm3 ≤ TTV < 100 cm3 (MST: 114 months, 3 year OS: 72%, p = 0.048) (patient characteristics are presented in Supplementary Table 2).
Overall Survival of Patients Undergoing Chemotherapy according to Total Tumor Volume
Patients who underwent initial treatment by chemotherapy (n = 78) were divided into three groups: TTV < 10 cm3 (23 patients), 10 cm3 ≤ TTV < 100 cm3 (28 patients), and TTV ≥ 100 cm3 (27 patients). Figure 2C shows that there were no significant differences in OS (MST: 23 versus 25 versus 28 months, 3 year OS: 43% versus 43% versus 15%) among these three groups (patient characteristics are presented in Supplementary Table 3).
Overall Survival Based on Total Tumor Volume
To identify the appropriate treatment for patients with CRLM, OS was compared between patients who underwent initial hepatic resection or chemotherapy, according to TTV. For patients with TTV < 10 cm3, the OS of the hepatic resection group (n = 34) was significantly greater than that of the chemotherapy group (n = 23) (MST: 112 versus 23 months, HR 0.05, 95% CI 0.02–0.21, p < 0.001) (Fig. 3A) (patient characteristics are presented in Supplementary Table 4).
Comparison of survival after therapeutic intervention according to TTV with different treatment methods. A In the patients with TTV < 10 cm3, Kaplan–Meier estimate of overall survival after hepatic resection versus chemotherapy showed a significant difference (p < 0.001). B In the patients with 10 cm3 ≤ TTV < 100 cm3, similarly, Kaplan–Meier estimate showed a significant difference (p = 0.007). C Contrastingly, in the patients with TTV ≥ 100 cm3, Kaplan–Meier estimate showed no significant difference (p = 0.160) (log-rank test). TTV, total tumor volume
Similarly, for patients with 10 cm3 ≤ TTV < 100 cm3, the OS of those treated initially with hepatic resection (n = 43) was also significantly greater than that of those treated initially with chemotherapy (n = 29) (MST: 114 versus 25 months, HR 0.21, 95% CI 0.08– 0.54, p = 0.007) (Fig. 3B) (patient characteristics are presented in Supplementary Table 5).
Meanwhile, for patients with TTV ≥ 100 cm3, there was no significant difference between the OS of those with initial treatment by hepatic resection (n = 16) or chemotherapy (n = 26) (MST: 37 versus 28 months, HR 0.54, 95% CI 0.22–1.30, p = 0.160) (Fig. 3C). Detailed characteristics of patients with TTV ≥ 100 cm3 for both groups are presented in Table 2. Compared with the patients who underwent hepatic resection, the proportion of females, CEA level, ALBI score, proportion with intrahepatic tumor number ≥ 5, synchronous metastases, and bilobar tumor distribution were significantly higher for those who underwent chemotherapy.
In addition, the OS of patients who underwent initial chemotherapy treatment tended to be higher than that for those who underwent initial hepatic resection until 24 months; however, the OS reduced approximately 24 months after therapeutic intervention (Fig. 3C), and the survival difference between the hepatic resection and chemotherapy became larger thereafter.
Discussion
The present study demonstrated that patients with CRLM and TTV ≥ 100 cm3 who underwent initial hepatic resection had decreased survival compared with those with TTV < 100 cm3. This was also shown in our previous report.13 On the other hand, for patients with CRLM who underwent initial chemotherapy treatment, TTV did not affect OS. Additionally, for patients with CRLM and TTV ≥ 100 cm3, there was no significant difference in OS between patients receiving initial hepatic resection or chemotherapy. This indicates that in patients with CRLM with TTV ≥ 100 cm3, indication for surgical resection may be limited as the initial treatment. To the best of our knowledge, this is the first study comparing the OS of patients with CRLM who underwent initial hepatic resection or chemotherapy, with a focus on the TTV.
Although hepatic resection is the only potentially curative treatment for CRLM,2,3,15 the recurrence rates are high16 and several prognostic factors have been reported that affect the OS after hepatic resection.4,5,6,17,18,19 Above all, maximal tumor diameter and intrahepatic tumor number are the two most reported and well-known factors. When calculating TTV, the maximal tumor diameter was the most important factor because TTV and maximal tumor diameter were strong confounding factors. To avoid a confounding interaction, the present study included only TTV in multivariable analyses. When maximal tumor diameter was used instead of TTV ≥ 100 cm3 in the multivariable analysis, the hazard ratio was lesser than that for TTV (3.83 vs. 5.26, respectively, data not shown), indicating the significance of TTV rather than that of maximal tumor diameter.
TTV may be more useful as a prognostic factor of OS than maximal tumor diameter for the following reasons: An accurate assessment of tumor burden with maximal tumor diameter is difficult because we tend to assume that the tumor is spherical, but not all malignant tumors are spherical. Additionally, TTV directly and precisely represents the tumor burden of each patient, unlike the separate measurements of the other factors, such as tumor diameter and tumor number.
A recent study of patients with CRLM reported the poor OS of patients with a high metabolically active tumor volume measured by fluorodeoxyglucose (FDG)-positron emission tomography (PET)–CT.20 However, we think the tumor burden measurement of their study was underestimated because the analysis relied only on the detection of FDG-PET-positive lesions. Furthermore, measurement of TTV using CT in our study has merit because it is easy to use at any facility, unlike FDG-PET–CT. Therefore, TTV would be a logical surrogate in the preoperative settings for CRLM.
Interestingly, the OS of patients who underwent chemotherapy had no significant differences according to TTV (Fig. 2C), and the individual survival curves were similar in the present study. The advantage of chemotherapy compared with hepatic resection may be its less invasive nature, although there are few reports about risk stratification with OS and chemotherapy for CRLM. The maximal tumor diameter was previously reported to be useful in predicting prognosis for CRLM.21 Another report showed that response evaluation criteria in solid tumors may be a potential biomarker for the early prediction of chemosensitivity in CRLM.22 Accordingly, controversy remains regarding the risk stratification of chemotherapy for CRLM, but the present study suggests that tumor chemosensitivity may have a stronger impact on OS, regardless of TTV in patients with CRLM.
In the present study, survival curves were similar for those with TTV ≥ 100 cm3, regardless of initial treatment, suggesting that surgical indication should be considered cautiously for this patient population. Survival curves did not show significant differences during the first 2 years of therapeutic intervention. These results support our hypothesis that chemotherapeutic intervention can be beneficial for patients with TTV ≥ 100 cm3, because hepatic resection is highly invasive to the patients. Evidence from recent studies has demonstrated that chemotherapy provided a high response rate for colorectal cancer, with the reported objective response rate (ORR) between 57 and 95.5%, and disease control rate (DCR) between 84 and 96%.23,24,25,26,27 Neoadjuvant chemotherapy has recently become the standard treatment for advanced pancreatic cancer, with the reported ORR between 21 and 31.6%, and DCR between 48 and 78%.28,29,30,31,32 Considering the significantly higher ORR and DCR of CRLM compared with those of pancreatic cancer, proceeding upfront with chemotherapy for CRLM may be quite acceptable, and TTV ≥ 100 cm3 can be used to function as a selection criterion for initial chemotherapy treatment for CRLM patients.
There have been some reports that emphasize appropriate chemotherapy regimens that promote tumor downstaging for patients with CRLM and subsequently render unresectable tumors into resectable ones (i.e., conversion chemotherapy).33,34,35 However, how long a patient should stay on downstaging chemotherapy before hepatic resection remains undetermined. Some reports suggest that hepatic resection should be carried out in patients as soon as their disease becomes resectable,36 while others argue that it is better to wait for maximal tumor shrinkage before hepatic resection.34 Our research indicates that a factor of TTV < 100 cm3 could be a useful indicator for consideration of hepatic resection after downstaging chemotherapy, although the present hypothesis cannot be demonstrated due to the small sample size in the present study. In addition, the prognosis of chemotherapy was worse than that of hepatic resection after 2 years of therapeutic intervention. Accordingly, it can be speculated that chemotherapy alone may not provide long-term benefits for CRLM. Further studies assessing the appropriate intervention timing of hepatic resection will be required.
The limitations of this study included its retrospective nature, single-center design, and selection bias regarding treatment. Thus, a definitive conclusion cannot be drawn from the present study, and caution will be mandatory for interpreting these data. Nevertheless, despite these limitations, considering that there are few facilities where such tumors with TTV ≥ 100 cm3 can be resected safety, this study is clinically significant as it provides evidence supporting a potentially effective therapeutic option for managing CRLM. Future prospective, multicenter clinical studies will be necessary to elucidate the value of TTV in patients with CRLM.
In conclusion, the present study showed TTV to be a prognostic marker for patients undergoing initial hepatic resection with CRLM, whereas the prognosis of patients undergoing initial chemotherapy treatment was not affected by TTV. Considering that the OS was similar for patients with TTV ≥ 100 cm3 undergoing either initial chemotherapy or hepatic resection, TTV ≥ 100 cm3 may be a significant indicator of chemotherapeutic intervention preceding hepatic resection in patients with CRLM.
References
Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. Cancer J Clin. 2020;70:145–64.
Yamamoto M, Yoshida M, Furuse J, et al. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci. 2021;28:1–25.
Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810.
Kawaguchi Y, Kopetz S, Tran Cao HS, et al. Contour prognostic model for predicting survival after resection of colorectal liver metastases: development and multicentre validation study using largest diameter and number of metastases with RAS mutation status. Br J Surg. 2021;108:968–75.
Yoshimoto T, Morine Y, Imura S, et al. Maximum diameter and number of tumors as a new prognostic indicator of colorectal liver metastases. In Vivo. 2017;31:419–23.
Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78.
Tsai CH, Lin CM, Hsieh CC, Hsu WH, Wang HW, Wang LS. Tumor volume is a better prognostic factor than greatest tumor diameter in stage Ia non-small cell lung cancer. Thorac Cardiovasc Surg. 2006;54:537–43.
Yang CJ, Kim DY, Lee JH, et al. Prognostic value of total tumor volume in advanced-stage laryngeal and hypopharyngeal carcinoma. J Surg Oncol. 2013;108:509–15.
Tang X, He Q, Sun G, et al. Total tumor volume should be considered as an important prognostic factor for synchronous multiple gastric cancer patients with curative gastrectomy. Pathol Oncol Res. 2020;26:2169–75.
Tarsitano A, Ricotta F, Cercenelli L, et al. Pretreatment tumor volume and tumor sphericity as prognostic factors in patients with oral cavity squamous cell carcinoma. J Craniomaxillofac Surg. 2019;47:510–5.
Lee YH, Hsia CY, Hsu CY, Huang YH, Lin HC, Huo TI. Total tumor volume is a better marker of tumor burden in hepatocellular carcinoma defined by the Milan criteria. World J Surg. 2013;37:1348–55.
Lee YH, Hsu CY, Huang YH, et al. α-fetoprotein-to-total tumor volume ratio predicts post-operative tumor recurrence in hepatocellular carcinoma. J Gastrointest Surg. 2013;17:730–8.
Tai K, Komatsu S, Sofue K, et al. Total tumour volume as a prognostic factor in patients with resectable colorectal cancer liver metastases. BJS Open. 2020;4:456–66.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Bramhall SR, Gur U, Coldham C, et al. Liver resection for colorectal metastases. Ann R Coll Surg Engl. 2003;85:334–9.
Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–26.
Margonis GA, Amini N, Buettner S, et al. The prognostic impact of primary tumor site differs according to the KRAS mutational status: a study by the International Genetic Consortium for Colorectal Liver Metastasis. Ann Surg. 2021;273:1165–72.
Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–26 (discussion 626–617).
Shindoh J, Nishioka Y, Yoshioka R, et al. KRAS mutation status predicts site-specific recurrence and survival after resection of colorectal liver metastases irrespective of location of the primary lesion. Ann Surg Oncol. 2016;23:1890–6. https://doi.org/10.1245/s10434-016-5087-5.
Huang YT, Park J, Chong S, Hugh TJ, Ng WL, Lin M. The prognostic value of fluorodeoxyglucose positron emission tomography metabolic tumor volume in solitary colorectal liver metastasis. Asia Pac J Clin Oncol. 2017;13:e262–70.
He H, Shen W, Chen W, et al. Prognostic factors of patients with unresectable liver metastasis from colorectal cancer after failed conversion chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:1261–7.
Nakanishi R, Oki E, Hasuda H, et al. Radiomics texture analysis for the identification of colorectal liver metastases sensitive to first-line oxaliplatin-based chemotherapy. Ann Surg Oncol. 2021;28:2975–85. https://doi.org/10.1245/s10434-020-09581-5.
Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the Phase II CheckMate 142 study. J Clin Oncol. 2021;2021:Jco2101015.
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124:587–94.
Avallone A, Piccirillo MC, Nasti G, et al. Effect of bevacizumab in combination with standard oxaliplatin-based regimens in patients with metastatic colorectal cancer: a randomized clinical trial. JAMA Netw Open. 2021;4:e2118475.
Hu H, Wang K, Huang M, et al. Modified FOLFOXIRI with or without cetuximab as conversion therapy in patients with RAS/BRAF wild-type unresectable liver metastases colorectal cancer: the FOCULM multicenter phase II trial. Oncologist. 2021;26:e90–8.
Modest DP, Martens UM, Riera-Knorrenschild J, et al. FOLFOXIRI plus panitumumab as first-line treatment of ras wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol. 2019;37:3401–11.
Bockorny B, Macarulla T, Semenisty V, et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the COMBAT/KEYNOTE-202 trial. Clin Cancer Res. 2021;27:5020–7.
Chen J, Hua Q, Wang H, et al. Meta-analysis and indirect treatment comparison of modified FOLFIRINOX and gemcitabine plus nab-paclitaxel as first-line chemotherapy in advanced pancreatic cancer. BMC Cancer. 2021;21:853.
O’Reilly EM, Lee JW, Zalupski M, et al. Randomized, multicenter, phase ii trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol. 2020;38:1378–88.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–35.
Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–71.
Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9.
Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–53. https://doi.org/10.1007/s10434-001-0347-3.
Acknowledgment
The authors would like to thank all investigators, clinicians, and research staff who supported this study, the patients who participated in this study, and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest or financial ties to disclose.
Ethical Approval
The protocol for this research project has been approved by the ethics committee of Kobe University Hospital in 2021 (Approval Number B210197).
Informed Consent
The patients’ consent for participation was obtained through an opt-out method.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimura, Y., Komatsu, S., Nagatani, Y. et al. The Usefulness of Total Tumor Volume as a Prognostic Factor and in Selecting the Optimal Treatment Strategy of Chemotherapeutic Intervention in Patients with Colorectal Liver Metastases. Ann Surg Oncol 30, 6603–6610 (2023). https://doi.org/10.1245/s10434-023-13746-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13746-3