Abstract
Background
Cancer-related fatigue (CRF) is the most distressing symptom in the overall cancer population. For patients with esophageal cancer, CRF may even be harder to predict and control due to its complicated and prolonged treatment. Moreover, communication difficulties due to disease progression or treatment may further diminish esophageal cancer patients’ ability to communicate about CRF. However, little research has addressed the trajectory and associating factors of CRF in this population, especially during the active treatment phase. The purpose of this study was (1) to evaluate and compare the level of CRF at three time points, namely before treatment, a month after concurrent chemoradiotherapy (CCRT), and a week after surgery, and (2) to identify associated factors of CRF.
Methods
This prospective cohort study used a questionnaire to evaluate esophageal cancer patients’ CRF at three time points. Repeated measures ANOVA and linear regression were used to analyze the data.
Results
This study included 73 participants. The severity of all CRF aspects intensified significantly over the course of treatment, reaching the highest level after surgery (P < 0.001). Worries of physician invalidation at baseline (P < 0.05) and marital status associated with CRF after CCRT and after surgery.
Conclusions
This is the first study to demonstrate the relationship between CRF and physician invalidation. Clinicians must be aware of the intensifying trend of CRF and provide timely intervention when caring for patients with esophageal cancer during cancer treatment. Reducing the worries of physician invalidation may alleviate CRF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cancer-related fatigue (CRF) is a multifaceted concept with a complicated and unidentified mechanism. The 10th version of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) has included CRF as an official diagnosis and defined it as a personal perception or experience that is affected by cognitive, physical, psychological, behavioral, and emotional factors. The manifestations of CRF include fatigue, inattention, sleep disorders, functional impairment, and feelings of powerlessness. CRF is different from fatigue in that it is difficult to relieve CRF simply via resting or sleeping.1 The National Comprehensive Cancer Network (NCCN) has pointed out that CRF is a chronic and continuous feeling of fatigue caused by cancer or cancer treatment.
CRF has long been identified as one of the most distressing and poorly controlled symptoms experienced by patients with cancer.2 CRF cause profound and longer-lasting impacts on cancer patients’ health-related quality of life due to its long-term, complicated, and large-scale influences.3,4,5 Studies have proposed that several demographic and clinical factors, including age, partnership status, body weight, hemoglobin, and dietary intake, affect fatigue levels.6,7,8,9,10,11,12 Although not specifically targeting CRF, healthcare provider–patient communication has been widely suggested as an important factor in symptom management. A systematic review concluded that the interactions between healthcare providers and patients are associated with cancer patients’ ability to cope.13 Researchers further proposed that negative doctor–patient communication, especially physician invalidation, plays a significant role in deteriorating symptoms.14,15 While previous studies investigated the impacts of health communication on overall symptom management, very few studies addressed the direct link between specific communication elements and particular symptoms.
While the challenges of CRF vary across different types of cancers, a previous study demonstrated that more than 60% of patients with esophageal cancer experience CRF, which significantly affects their survival and prognosis.16 The lengthy and intense treatment process of esophageal cancer may be one of the greatest challenges in managing CRF in this population. While concurrent chemoradiotherapy (CCRT) followed by surgery remain the first-line treatment option for patients with esophageal cancer, this trimodality treatment affects patients’ head and neck, thoracic, and abdominal systems and typically requires patients to be hospitalized for months.17 In addition to the complicated therapy, patients with esophageal cancer may have a greater risk of severe CRF due to their compromised intake ability, which is closely linked to CRF.12,18 Although research has highlighted the severity and poor control of CRF in the esophageal cancer population and called for immediate attention, there is scant literature on how CRF develops during the active phase of treatment. Thus, the goal of our study was to examine the trajectory and associating factors of CRF during and after trimodality treatment in patients with esophageal cancer. The specific goals were 1) to evaluate and compare the level of CRF at three time points: before treatment, a month after CCRT, and a week after surgery, and (2) to identify associating factors of CRF.
Methods
This prospective cohort study used a questionnaire to evaluate esophageal cancer patients’ CRF at three time points: at baseline, a month after CCRT, and a week after surgery. Demographic variables and disease information were collected at baseline. The study was approved by the Research Ethics Committee of the National Taiwan University Hospital. All participants were asked to provide their written, informed consent.
Participants and Setting
Patients who (1) were diagnosed with stage II or III esophageal cancer, (2) were aged 20 years or older, and (3) planned to receive trimodality therapy were approached when they were admitted to the surgical floors of a medical center in Northern Taiwan. Patients who had impaired cognitive functioning and were diagnosed with multiple cancers or recurrent cancer were excluded. Along with the participants’ treatment, they were required to complete a fatigue and communication questionnaire at baseline, a month after CCRT, and a week after surgery. Demographic data also were collected.
Instruments
The Taiwan cancer-related fatigue cognition questionnaire (TCRFCQ V1.0) contains six dimensions and was used to evaluate multiple aspects of CRF and related issues, including the communication problem of physician incalidation.5 Specifically, the TCRFCQ V1.0 measures three aspects of CRF (i.e., physical, psychological, and cognitive aspect) by dimensions of “life power (3 items),” “treatment helplessness (5 items),” and “unfocused life (6 items),” respectively. Example questions are “I have difficulty paying attention (unfocused life),” “I have no confidence in the completion of treatment (treatment helplessness),” and “I still feel tired even after waking up (life power).”
The other three dimensions covered associated issues, including “fatigue attribution,” “help expectation,” and “worries of physician invalidation.” The “worries of physician invalidation” dimension measures the extent to which patients worry about a lack of understanding or acceptance from their physicians. An example question is “I worried that the issue I would raise would upset doctors.” TCRFCQ V1.0 contains a total of 28 items and employs a five-point Likert scale. For the different dimensions of CRF, namely, “life power,” “treatment helplessness,” and “unfocused life,” higher scores represent worse CRF and range from 14 to 70. For the associated issues, higher scores on the “fatigue attribution” and “help expectation” dimensions mean higher awareness of potential CRF causes or the need for help. Higher scores on the “worries of physician invalidation” dimension suggest that more physician invalidation is expected and range from 3 to 15. The TCRFCQ V1.0 has been tested in the cancer population in Taiwan and demonstrated good validity and reliability (Cronbach’s α = 0.889).5 The Cronbach’s α of the current study was 0.887. Due to the limited scope of the study, we only reported the data of relevant dimensions, namely the three CRF aspects and worries of physician invalidation.
Data Analysis
All data were collected and managed using the Research Electronic Data Capture (REDCap). In addition to the descriptive analysis, Mauchly’s sphericity test was first used for validation; if the variances were equal among time points, repeated measures ANOVA was applied to examine the mean differences in CRF among time points. If the variances were unequal, the Greenhouse–Geisser test was applied to adjust for the lack of sphericity. CRF was determined to be the sum score of the “life power,” “treatment helplessness,” and “unfocused life” dimensions of TCRFCQ V1.0. Bonferroni correction was then performed to clarify the direction of the changes in CRF over time. Linear regression was employed to identify associating factors of CRF. The independent variables that could be potential associating factors of CRF were identified through a literature review, including demographic variables (e.g., age, sex, and marital status), disease-related factors (e.g., hemoglobin and level of dysphagia), and physician invalidation. Cases with the missing data were omitted in the analysis. The minimum sample size required was 43 (f = 0.25, α = 0.05, β = 0.95). The P value was set at <0.05. G*power 3.1.9.4. was used to estimate sample size and IBM SPSS 24.0 was used to conduct the analysis.
Results
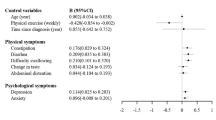
Among the approached 104 participants, 31 were excluded based on exclusion criteria, and the remaining 73 agreed to participate in the study. At the second time point, a month after CCRT, 65 participants (89.04%) completing the questionnaire. At the final time point, 62 participants (84.93%) completed the questionnaires a week after surgery. Figure 1 shows the flow diagram of study participation. Most participants were male (n = 70, 98.9%) and married (n = 57, 74%). Their age ranged from 34 to 87 (mean 59.1 ± 10.4) years. The mean body mass index (BMI) was 22.6 ± 3.1; 13.7% were underweight, and 32.9% were overweight. The majority of the participants were diagnosed with squamous cell carcinoma (n = 67, 91.8%) and underwent tri-incision and lymph node dissection (n = 47, 75.8%). Only 9 (12.3%) of them did not report any eating barriers, whereas 19 (26%) participants were only able to consume a soft diet, 22 (30.1%) could only drink liquids, and 23 (31.5%) reported complete dysphasia. Table 1 presents the detailed demographic information of the participants. Their CRF ratings ranged from 22 to 54, 31 to 59, and 32 to 66 at baseline, post-CCRT, and post-surgery, respectively. The ratings of physician invalidation ranged from 4 to 13, 6 to 14, and 6 to 13 at baseline, post-CCRT, and post-surgery, respectively.
Changes in CRF Over Time
Data are presented as the mean ± standard deviation, unless otherwise stated. The mean CRF score was 35.86 ± 5.14 at baseline and increased to 45.39 ± 6.30 after CCRT. After surgery, the mean CRF score increased even higher to 48 ± 7.13. A one-way repeated measures ANOVA was conducted to determine whether there was a statistically significant difference in CRF over the course of the trimodality therapy. The assumption of sphericity was met, as assessed by Mauchly’s test of sphericity, χ2 (2) = 4.10, P = 0.13. There were statistically significant changes in CRF along with the process of trimodality therapy, F (2, 112) = 97.49, P < 0.0005, partial η2 = 0.75. Post-hoc analysis with a Bonferroni correction revealed that CRF statistically significantly increased from baseline to post-CCRT (9.53 [95% CI, 7.56-11.50], P < 0.0005), from post-CCRT to post-surgery (2.61 [95% CI, 0.11-5.11], P < 0.0005), and from baseline to post-surgery (12.14 [95% CI, 9.87-14.41] ). All CRF dimensions, except for the dimension of treatment helplessness, demonstrated similar trends. While there was a significant increase in treatment helplessness from baseline to post-CCRT, there was a slight, nonsignificant decrease in treatment helplessness from post-CCRT to post-surgery (Table 2). Figure 2 shows the CRF trends over time.
Associating Factors of CRF
A linear regression established that baseline worries of physician invalidation was the only significant associating factor of post-CCRT CRF, F(1, 65) = 12.95, P = 0.001. Baseline worries of physician invalidation accounted for 17% of the explained variability in post-CCRT CRF. For post-surgery CRF, the baseline worries of physician invalidation remained a significant associating factor. Baseline worries of physician invalidation and marital status significantly associated with post-surgery CRF, F(2, 60) = 4.12, P = 0.021, and together they explained 12% of variability in post-surgery CRF. However, marital status was a nonsignificant factor (P = 0.13). Table 3 presents the regression models with CRF at different time points.
Discussion
This study examined the trends and associating factors of CRF before, during, and after the trimodality treatment in patients with esophageal cancer. Regarding the first goal of the study, the results showed that the severity of overall CRF and most CRF aspects intensified significantly over the course of the trimodality treatment. Regarding the second goal of the study, worries of physician invalidation before the trimodality treatment and marital status significantly associated with CRF both during and a week after the treatment.
The significant increase in CRF over time was similar to the trend evident in previous research. Researchers found that patients with cancer reported the highest levels of CRF at the end of chemotherapy.19,20 However, many patients with esophageal cancer received various types of treatment in addition to chemotherapy. Several studies and a meta-analysis reported that the severity of CRF among patients with esophageal cancer sustained and increased months to years after treatment.21,22,23 Yet, these studies examined CRF at the subacute phase (i.e., 1 to 3 months after treatment) or years into survivorship. Our study is one of the few to closely monitor CRF during the active treatment phase in this population and confirmed that CRF increased significantly from the beginning to the end of the trimodality treatment. While relatively few patients with esophageal cancer are discharged shortly after surgery, this result is particularly informative for those caring for patients with esophageal cancer during the active treatment phase. The results also supported that it is not just a single cancer treatment that can cause CRF, and a combination of treatments is actually a risk factor for CRF.23,24
More importantly, the findings of this study demonstrate that multiple aspects of CRF worsen over time. As clinicians have already designed different interventions addressing specific CRF aspects (e.g., psychological interventions for psychological aspects and activity-based interventions for physical aspects), knowing the changes in each CRF aspect is necessary to select tailored interventions.25,26 For example, as esophageal cancer patients reported increased CRF in many aspects during the acute treatment phase, they may benefit from a combination of interventions at this specific phase. Future studies are needed to examine whether selecting interventions based on the evaluation of CRF aspects can be most effective.
The results of this study showed that worries of physician invalidation before the treatment may affect subsequent CRF. Patients who worried more about physician invalidation before treatment reported severe CRF after CCRT and surgery. Similarly, Greville-Harris et al. (2015) reported that the perception of doctor invalidation may worsen symptoms. Another study also found that physicians’ low validation and high invalidation contributed to greater pain interference.15 While this evidence did not precisely address CRF, to the best of our knowledge, our study was the first to explore and show a significant relationship between physician invalidation and CRF. This finding is particularly important for patients with esophageal cancer, because they often experience unique communication barriers due to disease or treatment.27,28 If healthcare providers can intervene early to reduce the worries of physician invalidation, it may alleviate the severity of CRF during and after the treatment. While more research is needed to confirm the underlying mechanism, this relationship may be explained by the impacts of “worries of physician invalidation” on patients’ “communication self-efficacy.” One study revealed that self-efficacy for symptom communication is positively linked to better health-related quality of life.29 Future work is needed to untangle the relationship among symptom experience and various communication variables.
Marital status also was included in the association model for CRF shortly after the trimodality treatment. Specifically, patients who were single were more likely to have severe CRF. Although a couple of studies yielded similar results, showing that living with a partner is a significant protective variable of CRF, marital status was a nonsignificant factor in our study.8,24 The extent to which partnership status affects CRF may differ according to sex and cultural background. For example, most patients with esophageal cancer in Taiwan lived with their families if they were single, which may be very different from the situation experienced by patients who are single and live alone. The nonsignificant relationship between marital status and CRF also may be influenced by the small sample size of this study. Other than marital status, as in another study,30 we did not find any significant relationship between demographic variables and CRF, including variables that were proposed by other studies to predict CRF, such as age.6,7,31 Nevertheless, none of these studies focused on the esophageal cancer population. Moreover, contrary to previous research, correlations were not found between body mass index, hemoglobin, and oral intake ability.10,12,18 However, these previous studies did not specifically target patients with esophageal cancer and only self-reported ability of food intake was analyzed in this study. More studies are necessary to clarify the relationship between nutrition variables and CRF in different cancer populations using more comprehensive measurements.
This study had some limitations. While it is established that symptoms correlate with each other, we only focused on the impacts of selected demographic and clinical variables on CRF and may ignore the impacts of other symptoms. Another limitation relates to subject retention. Because some participants dropped out of the study due to deteriorating physical condition, the results may be biased as their data were excluded from the analysis. However, our retention rate was within an acceptable range (>80%).
Conclusions
The results of this study demonstrated significant worsening of CRF along with the active trimodality treatment. Notably, a novel finding indicated that more worries of physician invalidation associated with more intense CRF during and a week after trimodality treatment. Clinicians must be aware of the intensifying trend of CRF and provide relevant education and timely intervention when caring for patients with esophageal cancer undergoing trimodality treatment. Patients with esophageal cancer struggle to regain control and ownership of their life as they adjust to the disease, symptoms, and treatment,32 and providing a clear picture of the CRF trajectory can help them learn what to expect and thus empower them. Because worries of physician invalidation are significantly linked to CRF, there is an urgent need for healthcare providers to address this issue early, especially in esophageal cancer patients who experience special challenges in communication. Future studies are needed to clarify the role and mechanism of the impact of communication on CRF.
References
Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park, NY). 1998;12(11A):369–77.
Corbett T, Walsh JC, Groarke A, Moss-Morris R, Morrissey E, McGuire BE. Cancer-related fatigue in post-treatment cancer survivors: theory-based development of a web-based intervention. JMIR Cancer. 2017;3(2):e6987.
Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist. 2007;12:11–21.
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12:4–10.
Lai S-C, Lin W-C, Chen C-H, Wu S-Y. Development of a Taiwan cancer-related fatigue cognition questionnaire: reliability and validity. Oncotarget. 2017;8(17):28880.
Stobäus N, Müller MJ, Küpferling S, Schulzke J-D, Norman K. Low recent protein intake predicts cancer-related fatigue and increased mortality in patients with advanced tumor disease undergoing chemotherapy. Nutr Cancer. 2015;67(5):818–24.
Kreissl S, Mueller H, Goergen H, et al. Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol. 2016;17(10):1453–62.
Susanne K, Michael F, Thomas S, Peter E, Andreas H. Predictors of fatigue in cancer patients: a longitudinal study. Support Care Cancer. 2019;27(9):3463–71.
Inglis JE, Kleckner AS, Lin P-J, et al. Excess body weight and cancer-related fatigue, systemic inflammation, and serum lipids in breast cancer survivors. Nutr Cancer. 2020;6:1–11.
Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Sympt Management. 2003;26(1):604–14.
Van Der Weijst L, Surmont V, Schrauwen W, Lievens Y. Predictors for cancer-related fatigue among patients with advanced lung cancer attending palliative care department: a prospective observational study.
Inglis JE, Lin P-J, Kerns SL, et al. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr Cancer. 2019;71(1):21–40.
Prip A, Møller KA, Nielsen DL, Jarden M, Olsen M-H, Danielsen AK. The patient–healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs. 2018;41(5):E11.
Greville-Harris M, Dieppe P. Bad is more powerful than good: the nocebo response in medical consultations. Am J Med. 2015;128(2):126–9.
Edlund SM, Wurm M, Holländare F, Linton SJ, Fruzzetti AE, Tillfors M. Pain patients’ experiences of validation and invalidation from physicians before and after multimodal pain rehabilitation: associations with pain, negative affectivity, and treatment outcome. Scand J Pain. 2017;17(1):77–86.
Stauder M, Romero Y, Kabat B, et al. Overall survival and self-reported fatigue in patients with esophageal cancer. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2013;21(2):511–9.
Qureshi HA, Abouyared M, Barber B, Houlton JJ. Surgical options for locally advanced oropharyngeal cancer. Curr Treat Options Oncol. 2019;20(5):1–11.
Han-Markey TL. Examination of the association of diet and persistent cancer-related fatigue: a pilot study. Paper presented at: Oncology nursing forum 2013.
Miller M, Maguire R, Kearney N. Patterns of fatigue during a course of chemotherapy: results from a multi-centre study. Eur J Oncol Nurs. 2007;11(2):126–32.
Berger AM, Lockhart K, Agrawal S. Variability of patterns of fatigue and quality of life over time based on different breast cancer adjuvant chemotherapy regimens. Oncol Nurs Forum. 2009;5:49.
Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30(14):1615–9.
Jacobs M, Macefield R, Elbers R, et al. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res. 2014;23(4):1097–115.
Hurmuzlu M, Aarstad H, Aarstad A, Hjermstad M, Viste A. Health-related quality of life in long-term survivors after high-dose chemoradiotherapy followed by surgery in esophageal cancer. Dis Esophagus. 2011;24(1):39–47.
Yang S, Chu S, Gao Y, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019;8(7):738.
Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660.
Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. BioMed Res Int. 2015;2:15.
Hagi T, Makino T, Yamasaki M, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26(13):4754–64.
Chen S-C, Yu P-J, Hong M-Y, et al. Communication dysfunction, body image, and symptom severity in postoperative head and neck cancer patients: factors associated with the amount of speaking after treatment. Support Care Cancer. 2015;23(8):2375–82.
Edmond SN, Shelby RA, Kimmick GG, Marcom PK, Peppercorn JM, Keefe FJ. Symptom communication in breast cancer: relationships of holding back and self-efficacy for communication to symptoms and adjustment. J Psychosoc Oncol. 2013;31(6):698–711.
Goldstein D, Bennett BK, Webber K, et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol. 2012;30(15):1805–12.
Ruiz-Casado A, Álvarez-Bustos A, de Pedro CG, Méndez-Otero M, Romero-Elías M. Cancer-related fatigue in breast cancer survivors: a review. Clin Breast Cancer. 2020;6:108.
Larsen MK, Schultz H, Mortensen MB, Birkelund R. Patients’ experiences with illness, treatment, and decision-making for esophageal cancer: a qualitative study in a danish hospital setting. Global Qual Nurs Res. 2020;7:2333393620935098.
Acknowledgments
This work was supported by the National Taiwan University Hospital [grant number: 108-S4131]. The authors thank department of thoracic surgery of National Taiwan University Hospital in helping with patient recruitment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsou, YL., Lee, JM. & Tang, CC. The Trajectory of Cancer-Related Fatigue and Its Associating Factors in Patients with Esophageal Cancer Receiving Treatments: A Prospective Longitudinal Study. Ann Surg Oncol 29, 2784–2790 (2022). https://doi.org/10.1245/s10434-021-11294-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11294-2