Abstract
Introduction
Having a better understanding of predictors of cancer-related fatigue makes it easier to early identify patients at risk of suffering from long-term fatigue. The aim of this longitudinal study was to identify factors that predict long-term fatigue 6 months after discharge from a rehabilitation clinic using a multidimensional conceptualization.
Method
A mixed sample of cancer survivors (N = 948) were recruited while in-patient at a rehabilitation clinic. The follow-up survey was administered 6 months after they were discharged from the clinic. Fatigue was assessed with the EORTC QLQ-FA12. Predictive values were estimated using hierarchical multiple linear regression analyses.
Results
Mean fatigue scores were 20.7 (cognitive fatigue), 30.9 (emotional fatigue), and 53.2 (physical fatigue) at baseline and significantly lower at follow-up (Cohen’s d 0.12–0.31). Baseline levels of fatigue and depression were identified as important predictors of all dimensions of fatigue. Partnership and time since diagnosis predicted only the levels of physical fatigue. The regression models explained between 36% and 45% of variance in fatigue.
Conclusion
Levels of fatigue in early stages as well as psychosocial issues could enable clinicians to identify patients with elevated long-term fatigue and thus to provide optimal care for improving patients’ quality of life. Findings of different associates of individual dimensions of fatigue support the multidimensional concept of fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-related fatigue (CRF) is defined “as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [1]. CRF is highly prevalent in patients both receiving and recovering from cancer treatment, with rates reaching up to 90% [1, 2]. Longitudinal studies indicate that fatigue often decreases after completing treatment. However, approximately 30% of patients suffer from persistent or chronic fatigue [3, 4]. It has profound effects on quality of life, even in patients who have otherwise been successfully treated for cancer [3, 5]. Knowing more about factors that predict long-term fatigue both helps care providers better identify patients at risk of suffering from it already during treatment and increases their effectiveness at planning individual screenings and intervention programs. Furthermore, offering early intervention programs may reduce fatigue in the long-term and thereby prevent the detrimental impact of fatigue on quality of life [3, 5], work ability [6, 7], and compliance [8, 9].
Previous research on predictors of long-term fatigue is limited. Results indicate that pathogenesis is multifactorial, including physiological/biochemical, psychological, behavioral, and subjective correlates [10,11,12], but it is not clear if these factors cause fatigue or occur as a result of fatigue. Longitudinal studies have revealed that patients who experience fatigue at the beginning of treatment are more likely to suffer from fatigue further along their illness/recovery trajectory [13,14,15]. Other predictors reported in the literature are depressed mood [13, 16], anxiety [17], younger age [14, 18], female sex [18], chemotherapy treatment [18], occupational status [13], and physical limitations [13, 15]. However, research regarding sociodemographic, clinical, and psychological factors, has produced conflicting findings. For example, some studies that examined the factor depression found statistically significant effects [13, 15] while others did not [19,20,21]. Such discrepancies may be explained by differences in definitions and measurements of fatigue, heterogeneous statistical analyses and the time points of assessing predicting factors. Multidimensionality of fatigue is underlined by research about manifestations and causes of fatigue [10, 11] and about interventions of fatigue, which generally showed that combined interventions, e.g., physical activity and psychosocial interventions, had the highest effects in reducing fatigue [22]. Despite these findings, most relevant studies did not consider the multidimensional conceptualization of fatigue, and used a general factor or a sum score. We instead consider it necessary to identify specific predictors for the physical, emotional, and cognitive domains of fatigue.
The aim of this longitudinal study was a unidimensional and multidimensional examination of predictors of fatigue in cancer patients who have completed acute treatment and rehabilitation. We use the term predictor to identify factors which could help estimate the risk to suffer from long-term fatigue based on relationships between different baseline variables and long-term fatigue over a 6-month period, but not to identify causes of fatigue. Referring to the multifactorial concept of the perpetuation of CRF, we tested the impact of sociodemographic, clinical, physical, and psychological factors on the total score and the different dimensions of fatigue when controlling for baseline fatigue levels. For a better interpretation of the results, we additionally provide data about the severity and the change of fatigue levels across the measurement points.
Methods
Study design
We conducted a longitudinal study to examine the associations of predictors of the development of long-term CRF over a 6-month period. The selection of predictors was based on previous research findings which suggest a multifactorial nature of CRF [12]. We included sociodemographic variables (age, sex (male/female), living together with a partner (no/yes), occupational status (unemployed/employed), parent of children under 14 years (no/yes)), clinical variables (chemotherapy (no/yes), radiotherapy (no/yes), time since diagnosis (in months)), and psychological variables (depression, anxiety, sleep difficulties, self-efficacy).
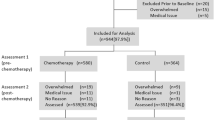
The study took place between October 2016 and April 2017. A group of 1547 patients were consecutively recruited in a standard oncological rehabilitation clinic. In Germany, all cancer patients are entitled to participate in a 3–4 week medical rehabilitation program in order to receive treatment concerning a broad spectrum of physical and psychosocial impairments. However, the treatment is not focused on fatigue. Inclusion criteria were minimum age of 18 years, absence of severe cognitive impairment, and sufficient knowledge of the German language. Participants completed the questionnaires during their stay (t1) and 6 months after rehabilitation clinic discharge (t2). Patients were informed about and asked to take part in the study. Experienced clinicians introduced in the study and gave answers to patients’ questions. At t2, participants received a letter with a pre-stamped envelope to fill in the follow-up questionnaires. All study participants provided written informed consent. The study was approved by the Ethics Committee of the Leipzig University.
Instruments
Fatigue was assessed using the EORTC QLQ-FA12 (provided by the European Organization for Research and Treatment of Cancer, [23]). The EORTC QLQ-FA12 consists of ten items on symptomatology and two additional criteria items, each scored on a four-point Likert scale (higher values represent higher levels). The items cover three dimensions of fatigue, i.e., the physical, emotional, and cognitive domain. Further two criteria items measure the interference of fatigue with daily activities and social life. Scores are transformed to a range of 0 to 100. We also calculated the total score for the EORTC QLQ-FA12 as recommended by a previous study [24], Cronbach’s α of subscales and total scale ranged from 0.79 and 0.93 in studies including German samples [23, 24].
Depression was measured with the Patient Health Questionnaire-9 (PHQ-9; [25]), a 9-item self-report measure for depressive symptoms based on the DSM-IV diagnostic criteria for major depressive disorder. Each item is scored on a four-point Likert scale resulting in a sum score ranging from 0 to 27. The PHQ-9 is reported to be a reliable instrument in a German sample of cancer patients (Cronbach’s α = 0.84) [26].
Anxiety was examined using the Generalized Anxiety Disorder scale-2 (GAD-2), which is based on the ICD-10 diagnostic criteria. It consists of two items that result in a sum score between 0 and 6 (items range from 0 to 3) and showed good measurement properties (Cronbach’s α = 0.75) [27, 28].
We also used the 4-item Jenkins Sleep Scale (JSS) to assess sleep difficulties [29]. Each of its items is rated on a 6-point Likert scale (0–5) based on the frequency of sleep problems. The JSS sum score ranges between 0 and 20, with higher scores reflecting worse sleep problems. Acceptable Cronbach’s α in a German-speaking population had been reported (0.77) [30].
The General Self-Efficacy Scale (GSES, [31]) is a 10-item scale that was designed to assess optimistic attitudes towards coping with a variety of difficult demands in life. It was used in German cancer populations [32], and normative values are available [33]. Items are rated on a 4-point Likert scale (1–4). Scores range from 10 to 40; higher values indicate higher levels of perceived self-efficacy.
Socio-demographic (age, sex, partnership, number and age of children, occupational status) and clinical data (tumor location, time since diagnosis), were obtained from the questionnaires and the medical records.
Statistical analyses
Patients’ characteristics and mean scores of fatigue for each time point were analyzed using descriptive statistics (means and percentages). Effect sizes (Cohen’s d) were calculated for comparisons between fatigue levels at baseline and follow-up; the statistical significance was indicated with confidence intervals. Drop-out analyses were conducted using t tests (continuous data) or chi-squared tests (nominal data).
Hierarchical multiple linear regression analyses were conducted to evaluate the associations of the potential predictor variables at baseline on the total score and the dimensions of fatigue at follow-up. The scores of all fatigue dimensions (subscales of EORTC QLQ-FA12) as well as the sum score were used as dependent variables. Thus, four separate regression models were analyzed. Dummy variables were created for categorical treatment-related and sociodemographic variables. Independent variables were assessed at baseline and entered blockwise into the regression analyses, with Block 1 containing sociodemographic variables, Block 2 containing clinical variables, Block 3 representing psychological variables, and Block 4 containing baseline fatigue (physical, emotional, or cognitive fatigue according to the respective dependent variable). Multicollinearity between the independent variables, especially fatigue, depression, and sleep was tested with correlation analyses and variance inflation factors (VIF). Correlation coefficients ranging from low to moderate and the low VIFs (variance inflation factors < 10) indicate no relevant multicollinearity. p values below .05 were considered statistically significant. All statistical procedures were executed using IBM SPSS Statistics 24.0.
Results
Patients’ characteristics
A total of 1225 patients agreed to participate in the study and provided data for t1. Of those, 986 (80.5%) sent back the t2 questionnaire. A further 38 patients (3.9%) had to be excluded due to incomplete data for variables examined in the regression analyses. Thus, the sample consists of a total of 948 patients. Of these, 496 (52.3%) were female, 738 (77.8%) were living with a partner, and 465 (49.1%) were employed. The mean age was 57 (SD = 15) years. Further sociodemographic and disease-related data are presented in Table 1.
The drop-out analysis showed that patients who participated at both time points were older (57 vs. 52 years; p ≤ 0.001), had lower fatigue levels at baseline (total score, 39.0 vs. 45.4; p ≤ 0.001), and were more likely to be retired than drop-outs (40.2% versus 30.7%; p ≤ 0.001). No significant differences were found regarding education levels and sex.
Levels of fatigue at baseline and follow-up
Table 2 shows that fatigue mean scores were 20.7 (cognitive fatigue), 30.9 (emotional fatigue), and 53.2 (physical fatigue) at baseline; the corresponding follow-up values were 17.7, 25.6, and 44.8. Statistically significant decreases in fatigue severity were observed in all three dimensions, the greatest of which occurred in the physical dimension (d = 0.31).
Predictors at baseline on fatigue levels at follow-up
Across the four outcomes (physical, emotional, cognitive, and overall fatigue), variables entered in Block 1 (sociodemographic variables) and Block 2 (disease- and treatment-related variables) explained between 4% and 6% of the variance (Tables 3, 4, 5, and 6). After psychological variables (Block 3) had been included, these entered variables explained between 26% and 32% of the variance. Once the Block 4 variable (baseline fatigue) had been added, the explained variance increased to values between 36% and 45%. Almost all changes of explained variance as compared to the previous models were statistically significant, except for when clinical variables were added to the emotional and cognitive fatigue models.
In Blocks 1 and 2, female sex, older age, not having a partner, having received chemotherapy, and time since diagnosis were significant predictors of fatigue (Tables 3, 4, 5, and 6). In Block 3, depression was a highly significant factor in all of the fatigue dimensions. Anxiety, sleep difficulties, and lower self-efficacy were statistically significant in models for physical and emotional fatigue. In the full model (i.e., after including Block 4), baseline fatigue emerged as the strongest predictor of fatigue for all outcomes. After entering baseline fatigue, sleep lost its predictive effect on physical fatigue. To the contrary, the predictive effect on partnership and time since diagnosis on fatigue levels substantially increased after entering baseline fatigue levels. Depression remained a significant factor in all fatigue dimensions as it had been before entering baseline fatigue levels. Furthermore, partnership and time since diagnosis only contributed significant proportions of variance in the physical fatigue dimension, and only after the baseline fatigue level was included in the calculations.
Analyses of collinearity between sociodemographic variables showed low to moderate correlation coefficients (.001 to .641). Correlations coefficients of fatigue baseline levels, depression, anxiety, and sleep ranged between .039 and .448. Observed VIFs (variance inflation factors) were between 1.03 and 3.56.
Discussion
The aim of the study was to identify predictors of long-term fatigue in a mixed sample of cancer patients who had completed a post-treatment rehabilitation program. Besides identification of predictors, we also analyzed the severity and temporal course at the two measurement points. Hereby, the differences in fatigue levels across the domains indicate that physical fatigue was rated more severe by the participants than emotional and cognitive fatigue. Nevertheless, this difference could also be explained otherwise: while the items assessing physical fatigue are closely related to the experience of “normal” tiredness (e.g., “Did you lack energy?”), items that operationalize cognitive (e.g., “Did you feel confused?”) or emotional fatigue (e.g., “Did you feel helpless?”) are less likely to be reported by people who do not have fatigue. Because normative values of our assessment instrument for specific clinical samples have not been established yet, it was not possible to assess the degree to which fatigue in our sample was rated in relation to other cancer patients. Comparing fatigue levels in our sample with those in the general population, the levels of fatigue are higher in our sample of cancer patients [34]. Kecke et al. [24] investigated a sample of 354 female cancer patients during their hospital stay and 3 months later using the EORTC QLQ-FA12. They found similar levels of emotional fatigue, but higher levels of physical and cognitive fatigue, a fact which may have to do with characteristics specific to patients who are in rehabilitation programs. Results from studies that used the fatigue subscale of the EORTC QLQ-C30 also indicated lower levels of physical fatigue in cancer survivors and cancer patients attending a rehabilitation program than in our sample [14, 35, 36]. Furthermore, we found significant changes in fatigue at follow-up. Fatigue levels were significantly lower at the 6-month follow-up measurement than they were at baseline, especially in the physical dimension (d = 0.31).
Baseline fatigue level was the strongest predictive factor of long-term fatigue, a finding that is in line with those of several other studies [13, 14, 16, 37]. Nevertheless, this association was stronger in our sample than in those from other studies (e.g., beta = 0.39–0.50 in our study versus beta = 0.31 in [13]). This could be due to differences in the study design: in our study, participants’ fatigue levels were measured while they were taking part in a rehabilitation program, i.e., at a point when most patients had already completed acute treatment. In contrast, other studies used fatigue baseline levels assessed during acute treatment, where fatigue is much more related to temporary treatment side effects. Furthermore higher depression scores, living without a partner and longer time since diagnosis were associated with increased levels of fatigue 6 months after discharge from the rehabilitation clinic.
Our results are in line with those of van Muijen et al., affirming the additional impact of depression on all dimensions of fatigue (beta 0.13–0.19) [13], but in contrast of other studies which observed that baseline fatigue neutralized the variance proportion of depression when measuring long-term fatigue [19, 21]. Therefore, depression appears to have an impact on the trajectory of fatigue development. This means that key symptoms of depression that are not common symptoms of fatigue, for example, capacity for enjoyment, lack of interests, low self-confidence, and feelings of worthlessness, can perpetuate or intensify long-term fatigue.
Partnership status had a substantial impact on physical fatigue and total fatigue scores, thus confirming the assumption that partner (spouse or live-in partner) support, such as attention, concern, and help with everyday tasks can positively affect the quality of life of the patients [13, 18]. We did not, however, observe a similar decreasing effect on emotional or cognitive fatigue.
Furthermore, this study indicates that longer time since diagnosis corresponds with increased physical fatigue, a finding that may be due to response shift or the increase of responsibilities. Cancer survivors may be more likely to rate their fatigue levels more highly when they begin resuming their everyday tasks, caretaking responsibilities, and work duties they had before falling ill and thereby begin noticing the impacts of fatigue more acutely at that point than when they were in treatment or rehabilitation.
The positive association between fatigue levels and sleep disturbances has often been described in the literature [20, 36, 37]. Nevertheless, in our study, this association did not remain statistically significant after adding baseline fatigue. Thus, sleep disturbances are not associated with changes in fatigue, a result which is in line with only a few studies (e.g. [21]).
Although the proportion of explained variance in sociodemographic and clinical variables is small (< 5%), the changes in R2 were significant with one notable exception regarding clinical variables. Clinical variables only appeared to have a significant impact on physical fatigue. Thus, we assume that clinical factors are more related to physical fatigue than they are to other dimensions of fatigue. Considering the fact that across all dimensions of fatigue, the highest increase in R2 was found when adding psychological factors (0.22–0.31), we think that psychological factors are associated with long-term fatigue to a great extent, even in the physical manifestation of fatigue [38]. For the planning of multimodal interventions, it seems to be important to take into account psychological interventions for all manifestations of fatigue.
Importantly, we showed that number and nature of predictors differed with respect to the fatigue dimensions, a fact that reinforces the understanding of fatigue as a multidimensional concept. Especially sociodemographic and treatment-related variables showed higher associations to physical fatigue than in other dimensions of fatigue as stated above. In the case of cognitive fatigue, the results suggest that there are other crucial factors that we have yet to identify. Except of depression and baseline fatigue, the associations between further variables (e.g., partnership) and long-term cognitive fatigue are much weaker than in other dimensions of fatigue. Thus, we think that cognitive fatigue differs from physical and emotional fatigue. Comparing the unidimensional and multidimensional examination of predictors, there are also differences. The significant effect of time since diagnosis on physical fatigue disappears using a total score of fatigue. Vice versa, the effect of partnership on emotional and in particular on cognitive fatigue can be overestimated, a fact which is important for planning interventions of fatigue. On the other hand, depression is strongly associated with fatigue regardless of the dimension of fatigue. In this case, the application of a total score is reasonable. Thus, we conclude that both the use of uni- or multidimensional assessments and analyses of fatigue are justified depending on the research question.
This study has some limitations, including the heterogeneity of the sample with respect to cancer entities and reliance on self-report data for treatment variables. Furthermore, there may exist a selection bias, as only one third of cancer patients in Germany are treated in a rehabilitation clinic; higher age, care dependency, and palliative disease status could be reasons for low utilization, but also patients with higher family and work commitments more seldom use rehabilitation care. To the contrary, patients suffering from treatment side effects are more likely to be found in rehabilitation settings. However, a study which compared inpatient, outpatient, or rehabilitation settings regarding the symptom burden of cancer patients found similar fatigue mean scores for all settings [39]. Furthermore, according to the dropout analyses, participants who remained in the study until t2 were older, retired, and had lower baseline fatigue levels, i.e., the group of patients who are supposed to have fewer professional demands. Another limitation relates to the fatigue instrument we used. Very few reference data currently is available for this measure. Furthermore, we only used two measurement time points, which limits information about the exact course of fatigue. For example, we cannot decide whether fatigue levels at the 6-month follow-up measurement indicate the presence of chronic persistent fatigue or if the fatigue seen at that point is a more recently developed phenomenon. Even though we tested for a large variety of associating factors according to multifactorial persistence of fatigue, we did not assess biochemical factors. Due to a lack of prospective data, we could not supply information about causality of factors which are associated with fatigue.
The main strength of the study is that we could analyze predictors of fatigue in a large sample of cancer patients using a longitudinal design. The results confirmed that it is important to describe predictors of cognitive, emotional, and physical fatigue separately. As far as implications for clinical practice are concerned, our results support the assumption that cancer survivors benefit when care providers assess fatigue as early as possible, but also depressive symptomatology, sleep difficulties, and self-efficacy when administering cancer treatment in order to better plan the follow-up care. Psychological variables emerged as being relevant to all manifestations of fatigue even over longer periods of time and thus play an important role in cancer patients’ and survivors’ quality of life. We showed here that it is possible to identify factors that help predict who is more likely to suffer from fatigue 6 months after completing post-cancer rehabilitation and therefore to extract those patients early enough who should be screened more closely in aftercare and to encourage such patients to participate in fatigue prevention interventions. Furthermore, the results underline the importance of psychosocial components of care plans for preventing or managing long-term fatigue.
References
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C, National comprehensive cancer network (2015) Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw 13:1012–1039
Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, Cleeland CS (2014) Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 120:425–432. https://doi.org/10.1002/cncr.28434
Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS (2011) Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society’s studies of cancer survivors. Cancer 117:2779–2790. https://doi.org/10.1002/cncr.26146.
Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAH, Rovers MM, Knoop H (2016) Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 27:965–974. https://doi.org/10.1093/annonc/mdw099
Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, McCready DR, Wong RKS, Jiang H, Howell D (2016) Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv 10:51–61. https://doi.org/10.1007/s11764-015-0450-2
Kim YA, Yun YH, Chang YJ, Lee J, Kim MS, Lee H-S, Zo JI, Kim J, Choi YS, Shim YM, Yoon SJ (2014) Employment status and work-related difficulties in lung cancer survivors compared with the general population. Ann Surg 259:569–575. https://doi.org/10.1097/SLA.0b013e318291db9d
Islam T, Dahlui M, Majid HA, Nahar AM, Mohd Taib NA, Su TT (2014) Factors associated with return to work of breast cancer survivors: a systematic review. BMC Public Health 14(Suppl 3):S8. https://doi.org/10.1186/1471-2458-14-S3-S8
Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S (2002) Fatigue associated with cancer and its treatment. Support Care Cancer 10:389–398. https://doi.org/10.1007/s005200100293
Berger AM, Gerber LH, Mayer DK (2012) Cancer-related fatigue: implications for breast cancer survivors. Cancer 118:2261–2269. https://doi.org/10.1002/cncr.27475
Piper BF, Lindsey AM, Dodd MJ (1987) Fatigue mechanisms in cancer patients: developing nursing theory. Oncol Nurs Forum 14:17–23
Glaus A, Crow R, Hammond S (1996) A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer 4:82–96
Purcell A, Fleming J, Haines T, Bennett S (2009) Cancer-related fatigue: a review and a conceptual framework to guide therapists’ understanding. Br J Occup Ther 72:79–86. https://doi.org/10.1177/030802260907200205
van Muijen P, Duijts SFA, Bonefaas-Groenewoud K, van der Beek AJ, Anema JR (2017) Predictors of fatigue and work ability in cancer survivors. Occup Med (Lond) 67:703–711. https://doi.org/10.1093/occmed/kqx165
Kreissl S, Mueller H, Goergen H, Mayer A, Brillant C, Behringer K, Halbsguth TV, Hitz F, Soekler M, Shonukan O, Rueffer JU, Flechtner HH, Fuchs M, Diehl V, Engert A, Borchmann P (2016) Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: a longitudinal study of the German Hodgkin study group. Lancet Oncol 17:1453–1462. https://doi.org/10.1016/S1470-2045(16)30093-6
Fisch MJ, Zhao F, O’Mara AM, Wang XS, Cella D, Cleeland CS (2014) Predictors of significant worsening of patient-reported fatigue over a 1-month timeframe in ambulatory patients with common solid tumors. Cancer 120:442–450. https://doi.org/10.1002/cncr.28437
Vardy JL, Dhillon HM, Pond GR, Renton C, Dodd A, Zhang H, Clarke SJ, Tannock IF (2016) Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 27:1761–1767. https://doi.org/10.1093/annonc/mdw252
Servaes P, Gielissen MFM, Verhagen S, Bleijenberg G (2007) The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology 16:787–795. https://doi.org/10.1002/pon.1120
Husson O, Mols F, van de Poll-Franse, de Lonneke VJ, Schep G, Thong MSY (2015) Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Support Care Cancer 23:2165–2174. https://doi.org/10.1007/s00520-014-2577-5
Goldstein D, Bennett BK, Webber K, Boyle F, Souza d, Paul L, Wilcken NRC et al (2012) Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol 30:1805–1812. https://doi.org/10.1200/JCO.2011.34.6148
Goedendorp MM, Gielissen MFM, Bleijenberg G, Verhagen, Constans A H H V M (2013) Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manag 45:213–222. https://doi.org/10.1016/j.jpainsymman.2012.02.009
Pertl MM, Hevey D, Collier S, Lambe K, O’Dwyer A-M (2014) Predictors of fatigue in cancer patients before and after chemotherapy. J Health Psychol 19:699–710. https://doi.org/10.1177/1359105313477675
Goedendorp MM, Gielissen, Marieke F M, Bleijenberg G, Verhagen, Constantijn A H H V M. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009:CD006953. doi:https://doi.org/10.1002/14651858.CD006953.pub2.
Weis J, Tomaszewski KA, Hammerlid E, Ignacio Arraras J, Conroy T, Lanceley A, Schmidt H, Wirtz M, Singer S, Pinto M, Alm el-Din M, Compter I, Holzner B, Hofmeister D, Chie WC, Czeladzki M, Harle A, Jones L, Ritter S, Flechtner HH, Bottomley A, on Behalf of the EORTC Quality of Life Group (2017) International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J Natl Cancer Inst 109 (5). https://doi.org/10.1093/jnci/djw273
Kecke S, Ernst J, Einenkel J, Singer S, Hinz A (2017) Psychometric properties of the fatigue questionnaire EORTC QLQ-FA12 in a sample of female cancer patients. J Pain Symptom Manag 54:922–928. https://doi.org/10.1016/j.jpainsymman.2017.08.007
Löwe B, Gräfe K, Zipfel S, Witte S, Loerch B, Herzog W (2004) Diagnosing ICD-10 depressive episodes: superior criterion validity of the patient health questionnaire. Psychother Psychosom 73:386–390. https://doi.org/10.1159/000080393
Hinz A, Mehnert A, Kocalevent R-D, Brähler E, Forkmann T, Singer S, Schulte T (2016) Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 16:22. https://doi.org/10.1186/s12888-016-0728-6
Löwe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, Schneider A, Brähler E (2010) A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord 122:86–95. https://doi.org/10.1016/j.jad.2009.06.019
Plummer F, Manea L, Trepel D, McMillan D (2016) Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry 39:24–31. https://doi.org/10.1016/j.genhosppsych.2015.11.005
Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM (1988) A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 41:313–321
Kudielka BM, von Känel R, Gander M-L, Fischer JE (2004) Effort-reward imbalance, overcommitment and sleep in a working population. Work Stress 18:167–178. https://doi.org/10.1080/02678370410001731785
Schwarzer RJM (1995) Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M (eds) Measures in health psychology: a user’s portfolio. NFER-Nelson, Windsor, pp 35–37
Thieme M, Einenkel J, Zenger M, Hinz A (2017) Optimism, pessimism and self-efficacy in female cancer patients. Jpn J Clin Oncol 47:849–855. https://doi.org/10.1093/jjco/hyx079
Hinz A, Schumacher J, Albani C, Schmid G, Brähler E. Bevölkerungsrepräsentative Normierung der Skala zur Allgemeinen Selbstwirksamkeitserwartung. Diagnostica 2006;52:26–32. doi:https://doi.org/10.1026/0012-1924.52.1.26.
Hinz A, Weis J, Brähler E, Mehnert A (2018) Fatigue in the general population: German normative values of the EORTC QLQ-FA12. Qual Life Res 27:2681–2689. https://doi.org/10.1007/s11136-018-1918-0
Arndt V, Stegmaier C, Ziegler H, Brenner H (2006) A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 107:2496–2503. https://doi.org/10.1002/cncr.22274
Schultz SL, Dalton SO, Christensen J, Carlsen K, Ross L, Johansen C (2011) Factors correlated with fatigue in breast cancer survivors undergoing a rehabilitation course, Denmark, 2002-2005. Psychooncology 20:352–360. https://doi.org/10.1002/pon.1739
Peoples AR, Roscoe JA, Block RC, Heckler CE, Ryan JL, Mustian KM, Janelsins MC, Peppone LJ, Moore DF, Coles C, Hoelzer KL, Morrow GR, Dozier AM (2017) Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: a multicenter NCORP study. Support Care Cancer 25:1271–1278. https://doi.org/10.1007/s00520-016-3520-8
Kuhnt S, Ernst J, Singer S, Rüffer JU, Kortmann R-D, Stolzenburg J-U, Schwarz R (2009) Fatigue in cancer survivors-prevalence and correlates. Onkologie 32:312–317. https://doi.org/10.1159/000215943
Hinz A, Weis J, Faller H, Brähler E, Härter M, Keller M, Schulz H, Wegscheider K, Koch U, Geue K, Götze H, Mehnert A (2018) Quality of life in cancer patients-a comparison of inpatient, outpatient, and rehabilitation settings. Support Care Cancer 26:3533–3541. https://doi.org/10.1007/s00520-018-4211-4
Funding
This study was supported by the German Cancer Aid under Grant (No: 7011 2267).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kuhnt Susanne and Friedrich Michael are equally contributing first authors.
Rights and permissions
About this article
Cite this article
Susanne, K., Michael, F., Thomas, S. et al. Predictors of fatigue in cancer patients: a longitudinal study. Support Care Cancer 27, 3463–3471 (2019). https://doi.org/10.1007/s00520-019-4660-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-4660-4