Abstract
Background
Adrenal metastasectomy is associated with increased survival in non-small cell lung cancer (NSCLC) with isolated adrenal metastases. Although clinical use of adrenal metastasectomy has expanded, indications remain poorly defined. The aim of this study was to evaluate the clinical benefit of adrenal metastasectomy for all lung cancer subtypes.
Patients and Methods
We performed a retrospective cohort study of patients who underwent adrenal metastasectomy for metastatic lung cancer at six institutions between 2001 and 2015. The primary outcomes were disease-free survival (DFS) and overall survival (OS). Cox proportional hazards regressions and Kaplan–Meier survival analysis were performed.
Results
For 122 patients, the mean age was 60.5 years and 49.2% were female. Median time to detection of the metastasis was 11 months, and 41.8% were ipsilateral to the primary lung cancer. Median DFS was 40 months (1 year: 64.8%; 5 year: 42.9%). Factors associated with longer DFS included primary tumor resection [hazard ratio (HR): 0.001; p = 0.005], longer time to adrenal metastasis (HR: 0.94; p = 0.005), and ipsilateral metastases (HR: 0.13; p = 0.004). Shorter DFS corresponded with older age (HR: 1.11; p = 0.01), R1 resection (HR: 8.94; p = 0.01), adjuvant radiation (HR: 9.45; p = 0.02), and open adrenal metastasectomy (HR: 10.0; p = 0.03). Median OS was 47 months (1 year: 80.2%; 5 year: 35.2%). Longer OS was associated with ipsilateral metastasis (HR: 0.55; p = 0.02) and adjuvant chemotherapy (HR: 0.35; p = 0.02). Shorter OS was associated with extra-adrenal metastases at adrenalectomy (HR: 3.52; p = 0.007), small cell histology (HR: 15.0; p = 0.04), and lung radiation (HR: 3.37; p = 0.002).
Discussion
Durable survival was observed in patients undergoing adrenal metastasectomy and should be considered for isolated adrenal metastases of NSCLC. Small cell histology and extra-adrenal metastases are relative contraindications to adrenal metastasectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lung cancer continues to be the leading cause of cancer-related deaths globally.1 The adrenal gland is a common site of lung cancer metastasis, affecting approximately 8% of patients with metastatic lung cancer.2 A study of patients with non-small cell lung cancer (NSCLC) reported median survivals of 6.5 months for those with a single adrenal metastasis and 2.8 months for those with multiple metastatic foci in one adrenal gland.3 Prognosis remains poor for patients with adrenal metastases who are treated nonoperatively, with median reported survivals of 6 and 8.5 months for those treated with radiotherapy alone4 or chemotherapy alone5 respectively.
We previously demonstrated that durable survival is observed in patients undergoing adrenal metastasectomy for a mix of primary tumor types.6 Lung cancer continues to be both the most common indication for adrenal metastasectomy and the most studied. Prior investigations have demonstrated a durable survival benefit from adrenalectomy in NSCLC patients with isolated adrenal metastases.7,8,9,10 A systematic review and analysis of 114 patients undergoing adrenalectomy demonstrated a 5-year survival of approximately 25% in patients with NSCLC and metachronous or synchronous adrenal metastases.11 For 8 patients with NSCLC who underwent chemotherapy followed by surgical resection, median survival was as high as 31 months.5 However, these studies were restricted to NSCLC and, therefore, do not represent the full spectrum of disease in lung cancer patients.
Although the use of adrenal metastasectomy for lung cancer is increasing,12 clinical utility across the spectrum of disease has not been thoroughly explored. We therefore sought to investigate the survival outcomes of adrenal metastasectomy in broader clinical contexts including synchronous and metachronous disease and extra-adrenal metastases in a multi-institutional study of all lung cancer pathologies.
Methods
Study Population and Variables
We performed a retrospective multi-institutional cohort study of patients (n = 122) undergoing adrenal metastasectomy for metastatic lung cancer at six institutions (Brigham and Women’s Hospital, Indiana University, Massachusetts General Hospital, Medical College of Wisconsin, University of Pennsylvania, Vanderbilt University) between 2001 and 2015. Patients were excluded if they underwent concurrent ipsilateral nephrectomy. Three patients included in the prior publication were excluded due to discrepancy in histopathologic subtyping.6 The electronic medical records were queried for patient demographics, tumor characteristics, biochemical data, imaging, and histopathology. Institutional Review Board (IRB) approval was obtained independently at all study sites to meet the guidelines of their responsible governmental agencies.
Definitions and Outcomes
Time to adrenal metastasis was defined as the time between the diagnosis of the primary tumor and the diagnosis of the adrenal metastasis. Adrenal lesions were identified as ipsilateral or contralateral based on the relationship to the primary lung tumor. Lung cancer subtypes were categorized as small cell, non-small cell (squamous cell carcinoma, adenocarcinoma, large cell carcinoma, sarcomatoid carcinoma, and not otherwise specified), and unknown based on previously reported histopathology.13 The interval between the diagnosis of the primary tumor and the adrenal metastasis was characterized as synchronous (0–6 months) or metachronous (> 6 months)11 and the extent of disease was defined as oligometastatic if limited to 1–4 sites.14 Adrenalectomy resection status was assessed as R0, R1, or R2 based on published criteria.15 Operations were evaluated based on whether the approach was laparoscopic, retroperitoneoscopic, open, or laparoscopic converted to open. Therapies were defined with respect to adrenalectomy. Chemotherapy, immunotherapy, and radiation administered before adrenalectomy were defined as therapies of the primary tumor. Therapies administered after adrenalectomy were defined as adjuvant, which may have include treatment for other sites of disease. The primary outcomes were disease-free survival (DFS) and overall survival (OS). DFS was defined as time from adrenalectomy to disease recurrence, as determined by clinical assessment. OS was defined as time from adrenalectomy to death.
Statistical Analysis
Descriptive statistics are provided as percentages for categorical variables, median and interquartile range (IQR) for nonparametric variables, and mean and standard deviation (SD) for parametric variables. Univariable Cox regression analysis was utilized to identify relationships between clinical characteristics and survival outcomes; covariates demonstrating modest significance (p < 0.20) on univariate analyses and those with clinical relevance were tested in the multivariable Cox regression models and removed by manual backward elimination until variables demonstrating p < 0.10 remained. The number of covariates was limited to maintain model stability. For evaluation of DFS and OS, Kaplan–Meier survival analysis and log rank testing were performed. Alpha was set to 0.05 for all statistical analysis. Statistical analysis was performed using STATA version 15.1 (StataCorp LLC, College Station, TX).
Results
Study Cohort Characteristics
122 patients were included in the study (Table 1). The mean age was 60.5 years and 49.2% were female. Most patients had undergone resection (61.5%) and/or radiation (37.7%) of the primary lung tumor. Median time to adrenal metastasis was 11 months (IQR: 0–19 months), with lesions more often being metachronous (59.0%) than synchronous (37.7%). Median time to adrenal metastasis was 17 months (IQR: 12–24 months) when excluding patients with synchronous disease. Less than half (41.8%) of metastases were ipsilateral to the primary tumor. 109 patients (89.3%) had non-small cell carcinoma, four (3.3%) had small cell carcinoma, and nine (7.4%) had unknown pathology. The most common subtype of NSCLC was adenocarcinoma (56.6%). In this retrospective multi-institutional study, race data were not uniformly collected.
Treatment of Adrenal Metastasis
Adrenal metastasectomy was most often performed laparoscopically (60.7%) (Table 2), while 28.7% of patients underwent an intended open operation, and 7.4% of procedures were converted to open. Notably, patients who underwent open or laparoscopic converted to open transabdominal adrenalectomy had a median tumor size of 4.9 cm (IQR: 3.2–7.3 cm), which was significantly larger than the median tumor size of patients who underwent a laparoscopic or retroperitoneoscopic approach (3.5 cm; IQR: 2.6–4.9 cm, p = 0.02). There was no difference in the adequacy of oncologic resection between patients who underwent an open or laparoscopic converted to open procedure versus a laparoscopic or retroperitoneoscopic procedure (p = 0.20). Most operations achieved an R0 (69.7%) or R1 (22.1%) resection. The use of adjuvant therapy was common, with 45.1% of patients undergoing chemotherapy and 21.3% undergoing radiation therapy.
Survival Outcomes
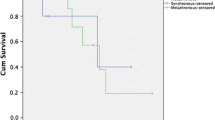
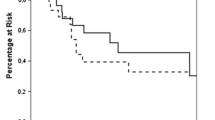
Recurrent disease was identified in 71 (58.2%) patients. Median DFS was 40 months (1 year: 64.8%; 5 year: 42.9%, Fig. 1a). On univariate analysis, factors associated with shorter DFS included R1 or R2 resection and radiation therapy of the primary tumor (Table 3). On the final multivariable logistic regression model, factors associated with longer DFS included primary tumor resection (HR: 0.001; p = 0.005), longer time to adrenal metastasis (HR: 0.94; p = 0.005), and ipsilateral metastases (HR: 0.13; p = 0.004). Shorter DFS correlated with older age (HR: 1.11; p = 0.01), R1 resection (HR: 8.94; p = 0.01), adjuvant radiation (HR: 9.45; p = 0.02), and open adrenalectomy (HR: 10.0, p = 0.03). There was no statistically significant difference in DFS between patients with small cell carcinoma and non-small cell carcinoma (p = 0.75) nor between histologic subtypes of non-small cell carcinoma (p = 0.94) (Fig. 2a). Tumor size was not an independent predictor of worse DFS.
Subgroup analyses were performed to further assess the association between the timing of metastasis development and DFS. When limiting the analysis only to patients with metachronous disease, time from adrenal metastasis was no longer significant in the model for DFS. Similarly, utilizing synchronicity rather than time to adrenal metastasis in the model of all patients demonstrated a significant association between synchronicity and DFS (HR: 5.0; p = 0.05). When the analysis was limited only to patients without extra-adrenal metastatic disease at the time of adrenalectomy in order to represent the true disease-free interval, there was a protective effect on DFS (HR: 0.96; p = 0.028), however, this was not sustained when excluding synchronous patients.
55 patients (45.1%) died during the study period. Median OS was 47 months (1 year: 80.2%; 5 year: 35.2%, Fig. 1). On univariate analysis, longer OS was associated with a history of resection of the primary tumor and adjuvant chemotherapy (Table 4). Factors associated with shorter OS included radiation of the primary tumor, small cell histology, extra-adrenal metastases at adrenalectomy, and R1 or R2 resection. On the final multivariable Cox regression model, longer OS was associated with ipsilateral metastatic disease (HR: 0.55; p = 0.02) and adjuvant (HR: 0.35; p = 0.02) or primary (HR: 0.29; p = 0.05) chemotherapy. Shorter OS was associated with extra-adrenal metastases at adrenalectomy (HR: 3.52; p = 0.007), small cell histology (HR: 15.0; p = 0.04), and a history of lung radiation therapy (HR: 3.37, p = 0.002). Patients with small cell carcinoma were more likely to have a shorter OS compared to those with NSCLC (p = 0.0001), but there was no difference among NSCLC subtypes (p = 0.61) (Fig. 2b).
Discussion
Adrenal metastasectomy for lung cancer is being performed with increasing frequency, yet the indications for surgery are incompletely defined.12 It is particularly relevant to develop guidelines for metastasectomy while the treatment paradigm for advanced lung cancers is being transformed by the development of targeted agents and immunotherapy.16,17,18,19,20 As lung cancer therapies evolve, criteria for adrenalectomy in the setting of metastatic lung cancer may continue to expand. Prior studies have focused on NSCLC,11 although there have been some case reports of durable survival using adrenal metastasectomy for small cell lung cancer.21,22 This is the first multi-institutional study to investigate the factors associated with survival outcomes in patients undergoing adrenal metastasectomy for all lung cancer pathologic subtypes, characterizing the utility of surgery in a broad clinical context. A range of lung cancer pathologies were incorporated, including squamous cell carcinoma, adenocarcinoma, large cell carcinoma, sarcomatoid carcinoma, and small cell carcinoma.
In this study, we observed durable survival despite advanced disease. The median time to adrenal metastasis in our cohort was 11 months, similar to the 15.7 months identified in another series of 43 patients undergoing adrenal metastasectomy for the more favorable pathology of NSCLC.8The median OS was 47 months, higher than the median OS of 6.5 months reported for unresected patients with a single NSCLC lesion in the adrenal gland3 and 11.5 months for a group of patients with unresected metastatic NSCLC limited to the thoracic cavity or in a single focus outside the thoracic cavity.23
There were several factors related to tumor biology that impacted survival in this study. We found that the laterality of the metastasis impacted both DFS and OS, with ipsilateral metastases portending improved survival. This finding contrasts with the results by Porte et al., who found no difference in survival based on tumor laterality in a smaller study of 43 patients.8 The impact of laterality on survival may be explained by the hypothesis that lung metastases to the adrenal are initially spread via lymphatic channels to the ipsilateral side before metastasizing hematogenously.24 In our cohort, we found that the presence of extra-adrenal metastases at the time of adrenalectomy was also associated with shorter OS. This finding contrasts that of Russo et al., who found no difference in OS between patients with and without extra-adrenal metastatic disease in 68 patients with NSCLC, with a median survival of up to 3.3 years.25 The difference in our findings may be secondary to increased sample size allowing for increased statistical power, along with our inclusion of patients with small cell histology. The association between extra-adrenal metastases at the time of adrenalectomy and survivorship was strong, with an HR twice as high as that for contralateral disease. With regard to the time to adrenal metastasis, in our cohort, a longer interval between the diagnosis of the primary cancer and the adrenal metastasis only correlated with improved DFS after adrenal metastasectomy when patients with synchronous and metachronous disease were included, but not when synchronous patients were excluded. Similarly, although the time to adrenal metastasis had an association with reduced DFS for those patients without extra-adrenal metastatic disease at the time of adrenalectomy [thereby representing the true disease-free interval (DFI)], this was not sustained when excluding synchronous patients. Thus, synchronicity is likely the more relevant feature. The literature regarding the similar variable DFI is conflicting. Some studies suggest that a shorter DFI may be associated with more aggressive tumor biology and, therefore, a more limited benefit from adrenalectomy.11,26 For OS, we did not identify a difference based on either the time to adrenal metastasis or presence of synchronous disease. These findings may be explained by a recent quantitative framework that suggests that metastatic seeding begins as early as 3.5 years prior to the diagnosis of lung cancer.27 With respect to the diameter of the adrenal lesions, large adrenal metastases (greater than 4.5 cm) have previously been associated with decreased survival.28 Indeed, we found that adrenal metastases smaller than 4.5 cm in diameter were associated with improved DFS, although OS was not impacted; the impact of size on disease control may be related to resectability, which is discussed below. Lastly, we considered the effect of tumor histology on survival. We identified that the subtype of NSCLC had no differential effect on survival, but small cell histology was associated with shorter OS. Small cell histology had the highest HR in the multivariable model for OS at 15.0. Taken together, these findings suggest that tumor biology significantly impacts disease progression and survivability.
Factors associated with both primary and metastatic tumor control also correlated with survival outcomes. A history of primary tumor resection was associated with improved DFS, whereas a history of lung radiation was associated with shorter OS. These findings suggest that complete resection of the primary tumor may slow cancer progression. With regard to control of the adrenal metastasis, prior studies have shown no DFS or OS difference based on operative approach.28,29,30 In contrast, in the present study, open adrenalectomy was associated with decreased DFS but not OS. As all surgical decisions were made a priori, tumor size potentially confounds surgical approach. Although tumor size was not an independent predictor of worse survival in our study, patients who underwent open or laparoscopic converted to open resection had larger tumors than those who underwent a laparoscopic or retroperitoneoscopic approach. Oncologic resection adequacy was not different between groups. Thus, the open approach may be a marker for more advanced tumor biology necessitating a more aggressive resection to obtain adequate margins. Positive margins and the need for adjuvant radiation were associated with worse DFS. As immunotherapy becomes increasingly utilized and produces a robust antitumor response in select patients, we suspect that the improved disease control and survival will increase the number of patients who are candidates for adrenal metastasectomy, particularly given early studies that demonstrate a smaller response of adrenal metastases to pembrolizumab compared with other sites of NSCLC metastases.31 In contrast, one study of NSCLC patients demonstrated a more robust response of adrenal metastases to nivolumab, which could diminish the role of surgery for strong responders to immunotherapy.32 Further research into the role of adrenal metastasectomy after immunotherapy is required.
This study has several limitations. First, the analysis in a retrospective study is inherently limited by the data available and lack of randomization. However, a randomized approach between surgical and nonsurgical approaches would not be feasible in this setting. We also lack a nonsurgical comparator group based on the design of this study, which may have allowed for further insight into the association of surgery on survival but would have introduced selection bias. Even so, selection bias remains a factor, as management decisions are inherently made based on presenting clinical features that may not be presented in this study, such as patient fitness for surgery. Next, due to the small sample size, our study may be under-powered to evaluate all subgroups, including patients with small cell histology and those treated with immunotherapy. To improve the statistical power of our study, multi-institutional data across a long study period were utilized. However, the heterogeneity and the long period of time encompassed by the study period precluded a standardized postoperative imaging protocol. In addition, all of the participating study institutions were large academic referral centers, which may reduce generalizability of our results. We encompassed six institutions to provide some heterogeneity of practice patterns. Finally, any therapy provided prior to adrenalectomy was considered treatment of the primary lesion, but it is conceivable that some patients underwent adrenal-directed therapy prior to undergoing adrenalectomy. Similarly, 5 of the 26 patients who underwent adjuvant radiation exhibited extra-adrenal metastatic disease at the time of adrenalectomy, so some adjuvant therapy may have been directed toward other organs. These distinctions can be addressed in future prospective work to better understand the impact of neoadjuvant and adjuvant therapy on survivability after adrenal metastasectomy.
In summary, durable survival is observed in patients undergoing adrenal metastasectomy. SCLC and extra-adrenal metastases are relative contraindications to adrenal metastasectomy due to decreased survival. However, synchronicity, adrenal size, and surgical approach do not appear to significantly impact overall survival. We therefore recommend adrenal metastasectomy for patients with isolated, ipsilateral adrenal metastases of NSCLC, with a priority placed on obtaining adequate oncologic margins.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27.
Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84.
Eberhardt WEE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, et al. The IASLC lung cancer staging project. J Thorac Oncol. 2015;10(11):1515–22.
Soffen EM, Solin LJ, Rubenstein JH, Hanks GE. Palliative radiotherapy for symptomatic adrenal metastases. Cancer. 1990;65(6):1318–20.
Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996;62(6):1614–6.
Wachtel H, Roses RE, Kuo LE, Lindeman BM, Nehs MA, Tavakkoli A, et al. Adrenalectomy for secondary malignancy: patients, outcomes, and indications. Ann Surg [Internet]. 2020. https://doi.org/10.1097/SLA.0000000000003876.
Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82(2):389–94.
Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001;71(3):981–5.
Pfannschmidt J, Schlolaut B, Muley T, Hoffmann H, Dienemann H. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer Amst Neth. 2005;49(2):203–7.
Chen JYR, Ardestani A, Tavakkoli A. Laparoscopic adrenal metastasectomy: appropriate, safe, and feasible. Surg Endosc. 2014;28(3):816–20.
Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non–small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26(7):1142–7.
Bartlett EK, Simmons KD, Wachtel H, Roses RE, Fraker DL, Kelz RR, et al. The rise in metastasectomy across cancer types over the past decade: metastasectomy trends. Cancer. 2015;121(5):747–57.
Clark SB, Alsubait S. Non small cell lung cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited 2021 Apr 13]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK562307/.
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10.
Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988;61(11):2310–4.
Antonia SJ, Lopez-Martin JA, Bendell JC, Ott PA, Taylor MH, Eder JP, et al. Checkmate 032: nivolumab (N) alone or in combination with ipilimumab (I) for the treatment of recurrent small cell lung cancer (SCLC). J Clin Oncol. 2016;34(15_suppl):100.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu Y-L, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311.
Pant-Purohit M, Cheng L, Einhorn L. Apparent surgical cure for metastatic small cell lung cancer. J Thorac Oncol. 2008;3(6):682–3.
Pham DC, Awad Z, Hoppe BS, Hew J, Ning K. Metastasectomy of solitary adrenal metastasis from small cell lung cancer. J Investig Med High Impact Case Rep. 2017;5(4):232470961774090.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
Bazhenova L, Newton P, Mason J, Bethel K, Nieva J, Kuhn P. Adrenal metastases in lung cancer: clinical implications of a mathematical model. J Thorac Oncol. 2014;9(4):442–6.
Russo AE, Untch BR, Kris MG, Chou JF, Capanu M, Coit DG, et al. Adrenal metastasectomy in the presence and absence of extraadrenal metastatic disease. Ann Surg. 2019;270(2):373–7.
Howell GM, Carty SE, Armstrong MJ, Stang MT, McCoy KL, Bartlett DL, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol. 2013;20(11):3491–6.
Hu Z, Li Z, Ma Z, Curtis C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. 2020;52(7):701–8.
Strong VE, D’Angelica M, Tang L, Prete F, Gönen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14(12):3392–400.
Adler JT, Mack E, Chen H. Equal oncologic results for laparoscopic and open resection of adrenal metastases. J Surg Res. 2007;140(2):159–64.
Romero Arenas MA, Sui D, Grubbs EG, Lee JE, Perrier ND. Adrenal metastectomy is safe in selected patients. World J Surg. 2014;38(6):1336–42.
Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S, et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother. 2018;67(12):1825–32.
Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4(1):84.
Acknowledgment
Research reported in this publication was partially supported by the National Institutes of Health, National Center for Advancing Translational Sciences grant KL2-TR001879 (H.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krumeich, L.N., Roses, R.E., Kuo, L.E. et al. Survival After Adrenalectomy for Metastatic Lung Cancer. Ann Surg Oncol 29, 2571–2579 (2022). https://doi.org/10.1245/s10434-021-11192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11192-7