Abstract
Background
The benefit of adrenalectomy (ADX) for adrenal metastasis is not established. We evaluated outcomes after ADX for patients with adrenal metastasis.
Methods
We retrospectively analyzed the records of 90 patients who underwent ADX for metastatic disease. Overall survival (OS) after ADX was calculated using the Kaplan–Meier method. Clinical factors were evaluated for associations with OS using a Cox regression model, and with operative factors using the Wilcoxon two-sample or Fisher’s exact test.
Results
The most common primary tumor types were melanoma (35, 39 %) and lung cancer (32, 35 %). A total of 49 (54 %) patients had isolated adrenal metastasis; 55 (61 %) underwent laparoscopic resection (LADX). Median OS was 2.46 years (range < 1 month–15 years), and 5-year survival rate was 38 % (6 % standard error). Most patients experienced disease progression (56, 62 %) despite achieving disease-free status following ADX (78, 86 %). When compared with the open approach, LADX was associated with smaller tumor size, as well as reduced blood loss, operative time, and length of stay (all p < 0.0001), and no difference in OS (p = 0.4122) or complications (p = 1). Isolated adrenal bed recurrence was similar in LADX (N = 3, 5 %) and open ADX (N = 2, 6 %) (p = 1), and did not affect OS (p = 0.2). Larger tumors were associated with shorter median OS (p = 0.0014).
Conclusions
ADX for metastasis can be safely performed in selected patients. Some patients with adrenal metastasis achieve prolonged survival following ADX. Compared with an open approach, LADX has no measurable oncologic disadvantage, minimizes morbidity, and should be considered when tumor characteristics permit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastases to the adrenal glands are reported at autopsy in 27–38 % of patients with a known extra-adrenal cancer [1–3]. Clinically, adrenal metastases are being identified more frequently owing to the increased use of cross-sectional imaging. While surveillance protocols in patients with a history of malignancy have increased the identification of incidental adrenal lesions, and approximately half of these are metastases, up to 50 % are benign lesions warranting standard biochemical evaluation [4–6]. New lesions, those with suspicious imaging characteristics, and those identified in relevant cancers (non-small-cell lung cancer [NSCLC], renal cell carcinoma, and melanoma) are more likely to represent metastasis [6]. The presence of distant metastases, including those to the adrenal gland frequently indicates advanced-stage disease, at which time surgical intervention is not commonly recommended.
However, recently published guidelines from the American Association of Endocrine Surgeons (AAES)/American Association of Clinical Endocrinologists (AACE) indicate that although adrenal metastasectomy is rarely indicated, it may be considered in those patients with an isolated adrenal metastasis [7]. Although the benefits of performing adrenalectomy (ADX) for metastatic disease are still debated, several retrospective series have identified select groups of patients whose survival appears to have been prolonged with surgical intervention [8–11]. These include highly selected patients whose metastatic disease is metachronous, from NSCLC, renal cell carcinoma, melanoma, and lesions <5 cm [8–11]. We evaluated our own experience with patients who underwent ADX for adrenal metastasis.
Materials and methods
Institutional databases were queried to identify patients who underwent ADX for adrenal metastasis from a non-adrenal primary tumor from 1990 to 2012. In all cases, metastatic diagnosis was confirmed by pathologic evaluation after ADX. Patients with a primary adrenal malignancy or whose normal adrenal gland was resected as part of an en bloc resection were excluded from the analysis. There were no exclusion criteria for age, gender, or ethnicity.
Clinical data abstracted from medical records included patient demographics, type of primary cancer, interval from diagnosis of the primary tumor to identification of adrenal metastasis, extent of metastatic disease, operative approach (open ADX or laparoscopic ADX [LADX]), perioperative factors (adrenal procedures, additional procedures performed at ADX, intraoperative and postoperative complications, tumor laterality, tumor area [size], tumor weight, total and ADX operative time, blood loss, length of stay [LOS], and disease status after ADX), recurrence, progression of disease, and date of last follow-up or death. Subgroups of LADX included traditional anterior/anterolateral and posterior retroperitoneoscopic approaches (PRA). Timing of metastasis was measured from the diagnosis of the primary malignancy; metastasis was considered synchronous if it occurred within 6 months of the primary tumor diagnosis and metachronous if diagnosed beyond 6 months. The extent of disease at diagnosis of adrenal metastasis was determined to be isolated (adrenal only) or non-isolated (other metastatic sites in addition to adrenal) based on imaging studies and clinical opinion of the multidisciplinary team treating the patient.
We calculated descriptive statistics for the entire group and key subgroups, such as operative approach and primary tumor type. Differences in clinical and operative factors were assessed using the Wilcoxon two-sample test or Fisher’s exact test, as appropriate. Overall survival (OS) was measured from the date of ADX to the date of death from any cause or last follow-up, and calculated using the Kaplan–Meier method. The progression of disease was defined as the time from ADX to the date of primary tumor detection at any site, and included only the patients who were considered disease-free after ADX. Clinical factors were evaluated for potential associations with OS and disease progression using a Cox regression model. Continuous factors were selected for log transformation to fit the proportional hazard assumption. Multivariate OS analysis was conducted using backward elimination based on the likelihood ratio test and included all the factors with p ≤ 0.20 in the univariate analysis. A p value of 0.05 or less was regarded as statistically significant. All analyses were carried out using SAS software version 9.3 (SAS Institute, Carey, NC, USA). This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Results
A total of 90 patients underwent ADX for adrenal metastasis from 1990 to 2012. Their median age was 59 (range 4–84) years. Table 1 summarizes the characteristics of the patients and their adrenal metastatic disease. The most common primary tumor types were melanoma (N = 35, 39 %) and lung cancer (32, 36 %). More than half of the patients (49, 54 %) had isolated adrenal metastasis, and the majority of metastases were metachronous (74, 84 %). Most patients achieved disease-free status after ADX (78, 87 %). The median time from primary malignancy diagnosis to development of adrenal metastasis was 1.84 (range 0–24.5) years. The median time to ADX after diagnosis of metastasis was 0.26 (range 0–2.5) years. The median follow-up after ADX was 1.21 years (range 0–15.3) years. Multiple treatments were used for the various primary tumor types, including chemotherapy and/or targeted therapy, radiation, or surgery; no attempt was made to evaluate the contributions of these therapies to individual patient outcomes.
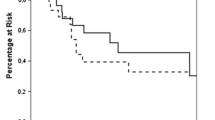
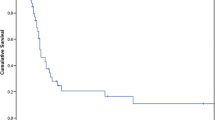
The median OS was 2.46 years (range < 1 month–15.1 years), and the 5-year survival rate was 38 % (standard error [SE] 6 %). The 1-year survival following ADX was 70 %, and 3- and 5-year survival estimates were 44, and 38 %, respectively. Of the 61 patients followed-up for at least 5 years, 16 remained alive 5 years following ADX; 29 patients were followed for <5 years. There was no statistically significant difference in OS in patients who underwent ADX for adrenal metastases from lung cancer compared with those with melanoma or other tumor metastases (1.59 vs. 2.03 vs. 3.99 years, respectively; p = 0.465, Fig. 1). On univariate analysis, failure to achieve disease-free status after ADX was associated with shorter OS (0.52 vs. 2.74 years for disease-free patients; hazard ratio [HR] 4.036, p = 0.0002; Fig. 2). Larger tumors were associated with shorter OS (HR 1.011, p = 0.0014). Log of operative time (HR 2.536, p = 0.0414) and log of blood loss (HR 1.244, p = 0.0068) were also significant on univariate analysis. However, in the multivariate analysis (Table 2), operative time (HR 1.2, p = 0.0329), older age at diagnosis (HR 1.03, p = 0.0341), and failure to achieve disease-free status after ADX (HR 3.6, p = 0.0032) remained statistically significant. All patients who were not disease-free after ADX died within 2.77 years.
Table 3 compares operative factors by ADX approach. The majority of patients (55, 61 %) underwent LADX, which was associated with smaller tumor size (p < 0.0001), reduced blood-loss (p < 0.0001), shorter operative time (p = 0.0325), and shorter LOS (p = 0.0007) when compared with open ADX. Most patients with metastatic lung cancer and melanoma underwent LADX instead of open ADX. A total of 36 (40 %) patients underwent concurrent procedures in addition to ADX; these patients were more likely to undergo open ADX (p = 0.0001). LOS was positively associated with having a concurrent procedure (median 6 days vs. 2 days, p = 0.0002), longer operative time (p < 0.0001), and blood loss (p < 0.0001). No difference in median OS was observed between open and LADX (2.3 vs. 2.46 years, p = 0.4122; Fig. 3).
There was also no significant difference in the rate of adrenal bed recurrence (29 % for LADX vs. 11 % for open ADX, p = 0.07), or the rate of isolated recurrence in the adrenal bed (5 % for LADX vs. 6 % for open ADX, P = 1). Furthermore, adrenal bed recurrence did not affect OS (p = 0.2). An analysis was also performed to determine whether there were differences between LADX subgroups (anterior LADX and PRA) and open ADX subgroups (Table 4). Reduced operative time (p = 0.0084), blood loss (p < 0.0001), tumor size (p < 0.0001), and LOS (p = 0.0055) were observed in the PRA subgroup compared with the others. There were 19 complications in 18 patients (21 %), including one death occurring 5 days following an open bilateral ADX, likely from pulmonary embolism. No difference was found in the rate of complications between LADX (12, 20 %) and open ADX (7, 20 %; p = 1). The complications observed intraoperatively were bleeding (four), pneumothorax (two), splenic tear (one), superior vena cava syndrome (one), and hypotension (one). Postoperative complications were intercostal nerve injury, hematoma, hypotension, adrenal insufficiency, pleural effusion, prolonged ileus, deep venous thrombosis, urinary tract infection, and a drain infection (one patient each). The occurrence of perioperative complications was associated with longer LOS (median 6 vs. 2 days, p = 0.0007); LOS was prolonged when postoperative complications occurred (median 8 vs. 3 days, p = 0.005), but not following an intra-operative complication (median 5 vs. 3 days, p = 0.186).
Eradication of the primary tumor could not be achieved in 12 (13 %) patients. The intent of ADX in most of these patients was palliative (8, 67 %). Palliative ADX was performed to obtain tissue for experimental therapy (e.g. tumor-infiltrating lymphocytes) and symptom relief (mass effect for one patient; failure of medical therapy for ectopic adrenocorticotrophic hormone syndrome for another patient). The disease progression analysis included the 78 patients who achieved disease-free status following ADX. The majority (56, 72 %) experienced disease progression. The median time to progression was 0.87 years (95 % confidence interval CI 0.67–1.12). The 5-year disease-free status rate for this group was 19 % (SE 5 %). Larger tumor size and increased operative blood loss were negatively associated with disease progression and were significant in the multivariate analysis (Table 5).
Discussion
Our series suggests adrenal metastectomy can be safely performed in carefully selected patients. In addition, the observation that one-fourth of patients who underwent adrenal metastectomy were alive at 5 years following ADX suggests that carefully selected patients can benefit from the procedure. While median follow-up was relatively short, our 3- and 5-year actuarial survival estimates of 43.7 and 38.05 %, respectively, are similar to those in other studies that have reported 3-year survival rates ranging from 38 to 45 % after ADX for adrenal metastasis [11, 12] and 5-year survival of 35 % [10] and 31 % [9]. One-fourth of the patients remain without evidence of disease following ADX, and a significant number of patients who experienced disease progression survived 5 years following ADX.
Current AAES/AACE guidelines for the management of adrenal masses suggest that adrenal metastasectomy is rarely indicated but should be considered for palliation and/or in the case of an isolated adrenal metastatic lesion [7]. Our data would support these guidelines. In addition, we would consider ADX in highly selected patients with limited extra-adrenal disease, favorable tumor biology, and an excellent performance status if all disease can be potentially eliminated with resection and other therapies.
Selection criteria for ADX in patients with adrenal metastases remain to be definitively established. We acknowledge that evaluations of patients with adrenal metastasis should always consider the natural history of the specific tumor, the tumor biology, and availability of alternative treatment options. Unlike earlier reports, our series did not demonstrate statistically significant associations of OS with key clinical factors previously reported in the literature. Isolated adrenal metastasis and a longer interval between primary tumor treatment and development of adrenal metastasis were previously reported to be associated with longer survival after adrenal metastectomy [8–10]. Our series included a large proportion of patients with metachronous metastasis and isolated metastasis, thus, it may be difficult to discern a difference in these factors based on the homogeneity of the group. In addition, we did not measure the effects of additional therapies that may have contributed to survival and potentially confounded these measures.
Specific malignancies have been reported to be associated with longer survival after adrenal metastectomy in retrospective series [8, 9]. In fact, a large European retrospective series reported that patients with metastasis from renal cell carcinoma had the longest OS after ADX (median 84 months), while patients with metastasis from other tumors such as colorectal cancer, NSCLC, and others had a median OS of 24–29 months [10]. Our series failed to demonstrate a significant difference in OS following ADX by the type of primary tumor. Our data included only a small number of renal cell cancer patients, which may account for the lack of an association between tumor type and OS. The strongest predictor of OS was disease status following ADX, a finding consistent with guidelines and other studies [8, 10].
We found that LADX minimized morbidity (reduced operative time and blood loss) while maintaining a similar OS and complication rate when compared with open ADX. We did not observe a higher rate of isolated adrenal bed recurrence after LADX than after open ADX; recurrence in the adrenal bed was usually seen in association with systemic progression. This suggests that tumor bed recurrence following ADX was usually a function of tumor biology rather than operative approach, and therefore LADX seems to have no oncologic disadvantage when compared with open ADX for patients with a small-to-medium-sized adrenal metastasis. Our findings are consistent with prior reports that LADX is equivalent to open ADX in terms of local recurrence and OS [13].
The above are important considerations for referring providers, especially when the patient’s prognosis is poor based on the primary tumor type alone. For example, the majority of lung cancer patients in our series underwent LADX, and an increase in patient volume was seen with the introduction of LADX. This may reflect an increased willingness of the medical oncologist to consider a more aggressive approach rendering the patient “no evidence of disease.” Therefore, when permissible, the preferred approach for ADX for metastasis is laparoscopic. The PRA approach has the lowest morbidity as reported above by measure of blood loss and operative time, and a significantly shorter LOS; at our institution, the PRA has recently become preferable to the traditional anterior or anterolateral approaches [14].
One limitation of our study is its retrospective nature, which can introduce selection and ascertainment bias. However, a prospective randomized trial of this clinical scenario would be difficult, and would require patient accrual at multiple institutions given the rarity of diagnosis of adrenal metastasis. Several factors are acknowledged as sources of selection bias, including the lack of an evaluation of patients who underwent ADX compared with a similar group who did not. Referral bias is also evident, as the volume of patients increased following introduction of LADX. The favorable outcome observed in our patient cohort could reflect the benefits of systemic therapies given to most of these patients; owing to the variety of underlying primary malignancies, the disease burden, and treatments provided, we could not evaluate their individual effects.
In conclusion, ADX for metastasis can be safely performed in carefully selected patients. Laparoscopic resection appears to have no measurable oncologic disadvantage compared with an open approach, minimizes morbidity and LOS, and should be considered when tumor characteristics permit. Following ADX for adrenal metastases, a significant number of patients may achieve prolonged survival. Systemic disease progression is common, but isolated adrenal bed recurrence is not. Control of the primary tumor is the strongest predictor of survival following adrenal metastectomy. Further evaluation is necessary to identify selection criteria for ADX and to assess alternative measures of benefit following ADX for metastatic disease to the adrenal gland.
References
Abrams HL, Spiro R, Goldstein N (1950) Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer 3(1):74–85
Lo CY, VanHeerden JA, Soreide JA et al (1996) Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg 83:528–531
Lam KY, Lo CY (2002) Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol 56:95–101
Aron D, Terzolo M, Cawood TJ (2012) Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab 26:69–82
No authors listed (2002) NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“Incidentaloma”) NIH Consens State Sci Statements 19(2):1–23
Lenert JE, Barnett CC, Kudelka AP et al (2001) Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery 130:1060–1067
Zeiger MA, Thompson GB, Duh QY et al (2009) AACE/AAES adrenal incidentaloma guidelines. Endocr Pract 15(5):450–453
Mittendorf EA, Lim SJ, Schacherer CW et al (2008) Melanoma adrenal metastasis: natural history and surgical management. Am J Surg 195:363–369
Vazquez BJ, Richards ML, Lohse CM et al (2012) Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg 36:1400–1405
Moreno P, de la Quintana Basarrate A, Musholt TJ et al (2013) Adrenalectomy for solid tumor metastases: results of a multicenter European study. Abstract presented at American Association of Endocrine Surgeons, Chicago (IL), April 14
Heniford BT, Arca MJ, Walsh RM et al (1999) Laparoscopic adrenalectomy for cancer. Semin Surg Onc 16:293–306
Luketich JD, Burt ME (1996) Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg 63:1614–1616
Strong VE, D’angelica M, Tang L et al (2007) Laparoscopic adrenalectomy for isolated adrenal metastasis. Annals Surg Onc 14:3392–3400
Perrier ND, Kennamer DL, Bao R et al (2008) Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases. Annals Surg 248:666–674
Acknowledgments
The authors would like to thank Mr. Roland Bassett for his assistance on the statistical analysis and Ms. Melissa Burkett for editorial assistance in manuscript preparation.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero Arenas, M.A., Sui, D., Grubbs, E.G. et al. Adrenal Metastectomy is Safe in Selected Patients. World J Surg 38, 1336–1342 (2014). https://doi.org/10.1007/s00268-014-2454-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2454-x