Abstract
Background
The role of adrenalectomy in management of isolated metastatic adrenal tumors is increasingly established. Laparoscopy is becoming the preferred approach for these resections. We evaluated surgical and oncological outcomes of patients who underwent laparoscopic versus open adrenal metastasectomy and assessed the effect of such surgery on postoperative adjuvant therapy and survival.
Methods
We reviewed our institutional experience with adult patients who underwent an adrenal metastasectomy from 1997 to 2013. We assessed preoperative tumor size, operating room (OR) time, status of resection margin, and length of stay (LOS), as well as oncological outcomes including the use of adjuvant chemotherapy and radiotherapy within 1 year of surgery and 5-year survival. The χ 2 test, Mann–Whitney U test, and Kaplan–Meier curve were used for statistical analysis.
Results
Thirty-eight patients were identified. Lung was the primary site of malignancy (52.6 % of cases). Of the metastasectomies, 55.2 % (n = 21) were performed laparoscopically and 44.7 % (n = 17) were open. In the laparoscopic group, median tumor size was 2.6 cm versus 4.8 cm in the open group (p = 0.09). Median OR time and complication rates were similar between the 2 groups. The laparoscopic group, however, trended toward a shorter LOS (3 days laparoscopic vs. 4 days for open; p = 0.07). At 1 year, 37 % of all patients had not required any adjuvant chemotherapy or adjuvant radiotherapy.
Conclusions
This series confirms that adrenal metastasectomy leads to favorable oncological outcomes in select patient groups, with over one-third of patients not requiring adjuvant therapy for at least 1 year after their resection. Laparoscopic approach leads to excellent oncological resection margins without increasing OR time and with a possible reduction in LOS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metastatic adrenal tumors represent the second most common etiology of an adrenal mass after benign adrenal adenomas [1]. The prevalence of adrenal metastasis at postmortem examination is reported to be as high as 3.1 % [2]. The majority of these lesions are asymptomatic, however, and are often a sign of widespread malignant spread. Isolated malignant spread to the adrenal gland was traditionally encountered infrequently. However, increased utilization of routine high resolution imaging during oncological follow-up of patients has lead to increasing identification of isolated adrenal metastasis.
Management of adrenal metastases varies depending on the clinical scenario, and can include close observation, chemotherapy, local ablative therapy, radiotherapy, or surgical resection [3–8]. Several case series have documented improved survival after a surgical adrenalectomy in carefully selected patients who presented with isolated adrenal metastasis [4, 9–13].
We set out to evaluate surgical and oncological outcomes of patients who underwent adrenal metastasectomy, comparing a laparoscopic to an open approach. We also studied the impact of surgical intervention on postoperative need for adjuvant therapy and patient survival.
Patients and methods
A retrospective review of our institutional experience with adult patients who underwent an adrenal metastasectomy from 1997 to 2013 was performed with institutional review board approval. Data were initially gathered using ICD-9 codes for all adrenalectomies performed at Brigham and Women’s Hospital. The data were sorted by individual chart review to identify and exclude adrenalectomies for primary adrenal pathology, those performed concomitantly with an ipsilateral nephrectomy, or those done through a retroperitoneal approach. Only those with pathological confirmation of the metastatic nature of the adrenal tumor were included for final analysis.
Baseline patient characteristics, including site of the primary tumor, were reviewed. We recorded preoperative tumor size (maximal tumor dimension reported on preoperative imaging), tumor volume (on the basis of dimensions provided by the final pathological examination), status of resection margin, operative times [operating room (OR) time], length of stay (LOS), postoperative complications, the use of adjuvant therapy (chemotherapy or radiotherapy) within 1 year of the procedure, and calculated survival rates. We used an intention-to-treat analysis, including converted cases in the laparoscopic group. The time interval between the resection of the primary cancer and the date of detection of adrenal recurrence either by concerning imaging characteristics, increase in size of adrenal lesion, or percutaneous biopsy was recorded and compared between the groups.
Median values are reported unless otherwise stated. Chi square test (and Fisher’s exact test, when appropriate) and nonparametric tests, including the Mann–Whitney U test, were used for statistical analysis. The Kaplan–Meier curve was used to analyze the survival data.
Results
We identified 38 patients who had undergone adrenal metastasectomy for isolated adrenal disease and met our inclusion criteria. Twenty-one cases (55.2 %) were resected laparoscopically (laparoscopic-adrenal group), and 17 (44.7 %) were performed open (open-adrenal group). There were 16 male and 22 female patients, with 66 % of the metastases affecting the left side versus the right (25 vs. 13, respectively, p = 0.07, in comparison to a normal binomial distribution, i.e., 50 of 50). Patient characteristics are summarized in Table 1. There was no statistical difference between the groups.
The time interval between primary tumor resection and the diagnosis of the adrenal metastasis ranged between 0 and 77 months with a median of 19 months for the laparoscopic-adrenal group. The open-adrenal group had a range of 0–127 months with a median interval of 18 months (Table 1).
The primary sources of the metastatic lesions are shown in Fig. 1 and expanded on in Table 2. The most common primary tumor site was lung (20 patients, 52.6 %), followed by kidney (9 patients, 23.7 %), then melanoma (2 patients, 5.2 %).
The median OR time in the laparoscopic-adrenal group was 159 min (range 77–464 min) versus 165 min (range 39–305 min) for the open-adrenal group (p = 0.58). There were three conversions in the laparoscopic group (14 % conversion rate).
The size of the adrenal tumors removed laparoscopically tended to be smaller than the metastasis approached via an open technique (median diameter 2.6 cm vs. 4.8 cm, respectively, p = 0.09). The median volume of laparoscopic adrenal mass (16.5 mm3) was significantly smaller compared to open adrenal mass (97.4 mm3, p = 0.04), suggesting that smaller tumors were removed using the laparoscopic approach. The largest adrenal tumor in the laparoscopic group was 12.5 × 9.2 × 5 cm and the largest in the open adrenalectomy group was 14 × 11 × 7.2 cm. A negative resection margin was achieved in 36 total patients, with complete resections in 95.2 % (20 of 21) of the laparoscopic cases and 94 % (16 of 17) of open cases (Table 3). Three cases required a wedge resection of adjacent structures as a result of adherent tumor, one in the open-adrenal group and two in the laparoscopic-adrenal group. Periaortic or peri-inferior vena cava lymph nodes were resected and submitted for pathology in some cases.
There were no postoperative mortalities. The complication rate for the laparoscopic-adrenal group was 19 % (4 of 21), including hypoxia requiring an unexpected stay in the intensive care unit but no reintubation, acute renal failure that resolved, blood transfusion for an inferior vena cava injury, and a patient who developed left lower lobe pneumonia and Clostridium difficile colitis, both of which were treated on an outpatient basis. In the open-adrenal group, the complication rate was 23.5 % (4 of 17). The complications were hypotension requiring an unexpected stay in the intensive care unit, two patients who developed transient acute renal failure, and splenic injury requiring splenectomy (Table 3). The median hospital stay showed a trend toward shorter LOS in the laparoscopic-adrenal group versus the open-adrenal group (3 vs. 4 days, respectively; p = 0.07).
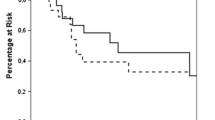
Of the 38 patients, the most recent resection was performed in 2013 and the most remote operation was performed in 1997. Documented follow-up period ranged from 1 to 168 months. The median follow-up in the laparoscopic-adrenal group was 31 months compared to 28 months in the open-adrenal group (p = 0.9) (Table 4). Postoperatively, 36.8 % (14 of 38) required no adjuvant chemotherapy, and 76 % (29 of 38) required no adjuvant radiotherapy within 1 year of surgery (Table 4). Mean estimated survival was 108.4 months in the laparoscopic group and 95.7 months in the open group (p = 0.1). The estimated 5-year survival was 85.1 % in the laparoscopic group and 69.2 % in the open group (p = 0.1) (Fig. 2).
Discussion
Increasing intensity of follow-up and imaging of patients with malignancies has led to increased diagnosis of metastatic lesions to the adrenal gland. Often these lesions are associated with other abnormalities and are an indication of general spread of the disease. There are, however, scenarios when metastasis can be limited to one adrenal gland. In these situations, there are data to support the role of surgical adrenalectomy to help improve patient survival. In 1982, Twomey et al. [14] were the first to report long-term survival of 6 and 14 years in two patients who had undergone an isolated adrenalectomy for large cell lung cancer. Luketich and Burt [3] found a median survival of 31 months for isolated non-small cell lung cancer patients undergoing adrenal metastasectomy versus 8.5 months’ survival in patients treated with only chemotherapy in a total of 14 patients. The improved survival documented in this study led to increased utilization of adrenalectomy in the rare group of patients with isolated adrenal metastasis. Several other studies have since confirmed survival benefits after adrenalectomy for isolated adrenal disease. In a series of 52 patients, Lo et al. [9] reported a 1-year survival of 73 % and a 2-year survival of 40 % with a median survival of 14 months. Another study of 77 patients, by Paul et al. [15], also showed a median survival of 23 months.
In addition to the increased utilization of adrenalectomy for adrenal metastasis, the last 20 years have also seen a shift in the surgical approach to these lesions, with increased popularity of the laparoscopic approach. Heniford et al. [13] published a small series in 1999 of laparoscopic adrenal metastasectomy for lesions of median size of 5.9 cm (1.8–12 cm), and reported successful negative resection margins in 100 % of cases with a conversion rate of approximately 10 %. Valeri et al. [16] similarly reported a series of laparoscopic adrenalectomy for malignancy with a conversion rate of 17 % and complication rate of 11 %. Our laparoscopic group had a conversion rate of 14 % and complication rate of 19 %, which is consistent with these studies.
We also found a trend toward decreased LOS, with the laparoscopic-adrenal group having a median LOS of 3 days compared to 4 days for the open-adrenal group (p = 0.07). In their study comparing laparoscopy versus open adrenalectomy, Strong et al. [17] demonstrated that laparoscopy allowed for shorter operative time, less estimated blood loss, shorter LOS, and fewer complications. We were unable to demonstrate many of these advantages in our review, which may be due to the smaller number of patients in our study, underpowering our analysis. We had an equivalent conversion rate to the above series and similarly used an intention-to-treat analysis that included converted case in the laparoscopic-adrenal group.
Despite potential improved surgical outcomes, the oncological outcomes of laparoscopic approach have remained in question with concerns about inadequate oncological resection. In the largest series to date of patients undergoing adrenal metastasectomy, Strong et al. [17] reported a 5-year survival of 31 % in 92 patients. When they compared the survival rate between the open and laparoscopic groups, they found no differences, suggesting equal oncological outcomes between the two approaches. In our study, the 5-year survival rates were 85 and 69 % for laparoscopic and open groups, respectively. This showed a trend toward better outcomes in the laparoscopic-adrenal group, although it should be noted that this group had a small average tumor size. Our survival rates are higher than in the above series or other published reports. There are several potential explanations for this observation. First, the adrenal tumors in our series were generally smaller (2.6 and 4.8 cm for laparoscopic-adrenal and open-adrenal, respectively) than those reported above; for example, in the study by Strong et al., the tumor size was 3.8 cm in the laparoscopic group and 6.4 cm in the open group. This could have significant effect, as tumors <4.5 cm are thought to have significantly better outcomes than larger tumors [17]. Nearly all tumors in our series were resected with intention for cure and no palliative resections were noted, further biasing our data toward improved survival data. For example, in some studies, over 50 % of adrenalectomies were performed for palliation rather than with a curative intent [18]. Another reason for the improved survival could be the mix of primary tumors. There are studies that reveal certain primary tumors to result in better survival after an adrenalectomy. Vazquez et al. [18] found that surgical resection improved overall survival in patients with metastatic disease from primary disease in soft tissue, kidney, lung, and pancreas. Metastatic malignancies that did not have improve overall survival were observed in colon, thyroid, ovary, liver, and other sites [18]. Another explanation could be the increased frequency of positron emission tomography–computed tomography (PET–CT) scans in surveillance and preoperative assessment of these patients. PET imaging leads to improved staging of several malignancies, including lung cancer, and is more sensitive at detecting distant metastasis [19]. In our series, 71 % of the laparoscopic-adrenal and 41 % of the open-adrenal patients had a PET scan preoperatively, which likely led to better staging and ensured that there was no evidence of subclinical metastatic disease elsewhere.
A unique insight from this study is the impact of surgery on subsequent adjuvant therapy for these patients. Although other studies have studied the survival of patients after metastasectomy, to our knowledge, none has evaluated the impact of surgery on subsequent chemotherapy or radiotherapy. Over 37 % (14 of 38) of patients after resection did not require adjuvant chemotherapy, and 76 % (29 of 38) of patients did not require adjuvant radiotherapy within at least a year of their metastasectomy. Such data provide further evidence that in carefully selected patients, adrenal metastasectomy can lead to prolonged survival.
This series, with resection of clinically solitary adrenal metastasis in 38 patients, confirms that adrenal metastasectomy, laparoscopic or open, can lead to good oncological outcomes in select patient groups. The data in this study confirm that laparoscopic adrenalectomy can be performed without increasing OR time and with a trend toward significant reduction in LOS. In addition, laparoscopic resection has similar oncologic outcomes and leads to our conclusion that laparoscopic adrenalectomy for selected patients is appropriate, safe, and feasible.
References
Uberoi J, Munver R (2009) Surgical management of metastases to the adrenal gland: open, laparoscopic, and ablative approaches. Curr Urol Rep 10:67–72
Lam KY, Lo CY (2002) Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol 56:95–101
Luketich JD, Burt ME (1996) Does resection of adrenal metastasis from non-small cell lung cancer improve survival? Ann Thorac Surg 62:1614–1616
Higashiyama M, Doi O, Kodama K, Yokouchi H, Imaoka S, Koymam H (1994) Surgical treatment of adrenal metastasis following pulmonary resection for lung cancer: comparison of adrenalectomy with palliative therapy. Int Surg 79(2):124–129
Siemer S, Lehmann J, Kamradt J, Loch T, Remberger K, Humke U, Ziegler M, Stockle M (2004) Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol 171:2155–2159
Sancho JJ, Triponez F, Montet X, Sitges-Serra A (2012) Surgical management of adrenal metastases. Arch Surg 397:179–194
Duh QY (2007) Laparoscopic adrenalectomy for isolated adrenal metastasis: the right thing to do and right way to do it. Ann Surg Oncol 14(12):3288–3289
Duh QY (2005) Resecting isolate adrenal metastasis: Why and how? Ann Surg Oncol 10(10):1138–1139
Lo CY, van Heerden JA, Soreide JA et al (1996) Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg 83:528–531
Piga A, Bracci R, Porfiri E, Cellerino R (1996) Metastatic tumors of the adrenals. Minerva Endocrinol 20:79–83
Zheng QY, Zhang GH, Zhang Y, Guo YL (2012) Adrenalectomy may increase survival of patients with adrenal metastases. Oncol Lett 3:917–920
Kebebew E, Siperstein AE, Clark OH, Duh QY (2002) Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg 137:948–951
Heniford BT, Arca MJ, Walsh RM, Gill IS (1999) Laparoscopic adrenalectomy for cancer. Semin Surg Oncol 16:293–306
Twomey P, Montgomery C, Clark O (1982) Successful treatment of adrenal metastases from large-cell carcinoma of the lung. JAMA 248(5):581
Paul CA, Virgo KS, Wade TP, Audisio RA, Johnson FE (2000) Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol 17(1):181–187
Valeri A, Borrelli A, Presenti L, Lucchese M, Venneri F, Mannelli M, Regio S, Borrelli D (2001) Adrenal masses in neoplastic patients: the role of laparoscopic proceure. Surg Endosc 15:90–93
Strong VE, D’Angelica M, Tang L, Prete F, Gonen M, Coit D, Touijer KA, Fong Y, Brennan MF (2007) Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 14(12):3392–3400
Vazquez BJ, Richards ML, Lohse CM, Thompson GB, Farley DR, Grant CS, Huebner M, Moreno J (2012) Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg 36:1400–1405
Pieterman RM, van Putten JW, Meuzelarr JJ, Mooyaart EL, Vaalburg W, Koeter GH, Fidler V, Pruim J, Groen HJ (2000) Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 343(4):254–261
Disclosures
Judy Y. R. Chen, Ali Ardestani, and Ali Tavakkoli have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J.Y.R., Ardestani, A. & Tavakkoli, A. Laparoscopic adrenal metastasectomy: appropriate, safe, and feasible. Surg Endosc 28, 816–820 (2014). https://doi.org/10.1007/s00464-013-3274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3274-z