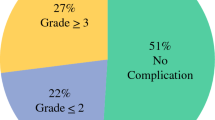
Abstract
Background
The PERISCOPE I study was designed to assess the safety and feasibility of (sub)total gastrectomy, cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy (HIPEC) with oxaliplatin and docetaxel for gastric cancer patients who have limited peritoneal dissemination. The current analysis investigated changes in perioperative management together with their impact on postoperative outcomes.
Methods
Patients with resectable gastric cancer and limited peritoneal dissemination were administered (sub)total gastrectomy, CRS, and HIPEC with oxaliplatin (460 mg/m2) and docetaxel (escalating scheme: 0, 50, 75 mg/m2). Of the 25 patients who completed the study protocol, 14 were treated in the dose-escalation cohort and 11 were treated in the expansion cohort (to optimize perioperative management).
Results
A significant proportion of the patients in the dose-escalation cohort (n = 7, 50%) had ileus-related complications. In this cohort, enteral nutrition was started immediately after surgery at 20 ml/h, which was increased on day 1 to meet nutritional needs. In the expansion cohort, enteral nutrition was administered at 10 ml/h until day 3, then restricted to 20 ml/h until day 6, supplemented with total parenteral nutrition to meet nutritional needs. Ileus-related complications occurred for two patients (18%) of the expansion cohort. The intensive care unit (ICU) readmission rate decreased from 50 (n = 7) to 9% (n = 1; p = 0.04).
Conclusion
The implementation of a strict nutritional protocol during the PERISCOPE I study was associated with a decrease in postoperative complications. Based on these results, a perioperative care path was described for the gastric cancer HIPEC patients in the PERISCOPE II study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A combination of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is increasingly used for the treatment of peritoneal dissemination of various cancer types.1,2,–3 The origin of peritoneal gastric cancer dissemination is the subject of HIPEC surgery investigation.4,5
Recently, several nationwide database studies reported a survival benefit of HIPEC treatment for selected gastric cancer patients.6,7,–8 To date, results from a randomized controlled trial to assess the role of CRS and HIPEC in the treatment of gastric cancer patients with peritoneal dissemination are lacking.
Studies have associated HIPEC surgery with considerable morbidity and mortality rates.9,10 Various published reports have addressed the perioperative management of patients undergoing CRS and HIPEC, primarily for peritoneal dissemination of colorectal cancer.11,12,13,–14 The chemotherapeutic agents most commonly used in these HIPEC procedures are oxaliplatin, cisplatin, and mitomycine C.11 A careful postoperative start of enteral nutrition is recommended in most published papers.15,16
The dose-finding PERISCOPE I study (treatment of PERItoneal dissemination in Stomach Cancer patients with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy) was designed to assess the safety and feasibility of a CRS-HIPEC procedure with 460 mg/m2 of hyperthermic (41–42 °C) oxaliplatin followed by normothermic docetaxel in escalating dosages (0, 50, and 75 mg/m2) for gastric cancer patients with limited peritoneal dissemination.17 A diverse spectrum of postoperative complications was encountered, with fairly high rates of intestinal complications.18 During the PERISCOPE I study, adaptations were made to the postoperative care path.
The current analysis aimed to investigate the changes in the perioperative management of the PERISCOPE I patients over time, together with the impact of those changes on postoperative outcomes. Based on this, the goal was to describe the postoperative care path to be used in the PERISCOPE II study.5,19
Methods
The PERISCOPE I Study
All the patients were treated in the PERISCOPE I study, a dose-finding phases 1 and 2 study, with treatment-related toxicity as the primary outcome measure.17 The trial was conducted at two Dutch centers experienced in HIPEC and gastric cancer surgery: the Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital in Amsterdam and the Sint Antonius Hospital in Nieuwegein.
The study protocol has been published previously.17 In short, gastric cancer patients with a resectable primary tumor and limited synchronous peritoneal metastasis and/or tumor-positive peritoneal cytology were eligible for inclusion in the study provided they had no disease progression during systemic chemotherapy. The PERISCOPE I study was approved by the Medical Ethics Committee of the Netherlands Cancer Institute, and written informed consent was obtained from all the patients.
For the current analysis, only patients who completed the entire study protocol were selected (i.e., all the patients described in this paper underwent systemic chemotherapy followed by an operative procedure consisting of a [sub]total gastrectomy with D2 lymph node dissection, CRS, and HIPEC). An open HIPEC technique was used, with a fixed dose (460 mg/m2) of hyperthermic (41–42 °C) oxaliplatin followed by normothermic (37 °C) docetaxel in a dose-escalation scheme (0, 50, 75 mg/m2) to establish the maximum tolerated dose of intraperitoneal docetaxel. At dose level 3 (75 mg/m2 docetaxel), treatment-related toxicity was unacceptable. At that time, 14 patients were included in the study as the dose-escalation cohort (Table 1). Dose level 2 (50 mg/m2) was defined as the maximum tolerated dose of intraperitoneal docetaxel for this procedure. To optimize perioperative care protocols, 11 extra patients were treated at this dose-level (460 mg/m2 oxaliplatin followed by 50 mg/m2 docetaxel). These patients were included in the expansion cohort. In all the patients, after HIPEC and BII or Roux-en-Y reconstruction, a feeding jejunostomy was inserted routinely.
Anesthesiologic Management
All the patients received combined epidural anesthesia and general anesthesia. Standard anesthesiologic monitoring plus hemodynamic monitoring using stroke volume variation and cardiac output measurements was used to assess the fluid status (EV1000; Edwards Life Science, Ivrine, CA, USA).
In an effort to achieve normovolemia and optimal oxygen delivery to the tissues, fluid support and vasopressors (noradrenaline) were given during the operation. For the majority of the patients (92%) dexamethasone was administered just before the docetaxel chemoperfusion to prevent a possible allergic reaction. Body temperature was measured continuously during the procedure. Peroperative blood gas analysis was performed at regular intervals during the operation for 18 patients who underwent surgery in the Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital. After the operation, all the patients were extubated in the operating room and then transferred to the intensive care unit (ICU).
Data Collection and Statistics
Clinical data were derived from the prospective database of the PERISCOPE I study. Postoperative complications were recorded based on the National Cancer Institute Common Terminology Criteria for Adverse Events 4.03.20 Ileus, abdominal infection, intestinal perforation, anastomotic leakage, duodenal leakage, wound infection, and gastrointestinal fistula were grouped as abdominal complications, whereas pneumonia, aspiration pneumonia, pneumothorax, respiratory failure, and pleural effusion were grouped as respiratory complications. A subset of both categories (ileus, intestinal perforation, gastrointestinal fistula, and aspiration pneumonia) was seen as ileus-related complications. Additional data regarding preoperative nutritional status, peroperative fluid management, postoperative ICU stay, and nutritional management were retrospectively derived from anesthesia protocols, ICU medical files (MetaVision, Essen-Kettwig, Germany), and electronic patient records.
Differences between the groups were analyzed with Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. The results are shown as medians and ranges. A p value lower than 0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 25 patients in this study, 19 underwent surgery in the Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, and 6 underwent surgery in the Sint Antonius Hospital. The median age of the patients was 61 years (range, 33–75 years), and 16 patients (64%) were men.
Preoperative Nutritional Details
The majority of the patients (n = 20, 80%) had experienced weight loss at the time of gastric cancer diagnosis. Before the operation, 22 of the patients (88%) were seen by a dietician, and nutritional support was given (via an enteral tube in 4 patients) to 17 of these patients (68%),.
Peroperative Details
The median duration of the operation (including HIPEC) was 7 h (range, 3–10 h). A total gastrectomy was performed for 19 of the patients (76%), and 6 of the patients (24%) had a subtotal gastrectomy. During the operation, intravenous fluids were administered at a median volume of 6.5 L (range, 3.6–10.5 L). The median blood loss was 610 ml (range, 100–1810 ml). To four of the patients, blood products (e.g., packed cells or fresh frozen plasma) were given. Peroperative glucose and lactate levels were known for 18 of the patients.
In all the patients, glucose and lactate levels rose during the HIPEC phase of the procedure (Fig. 1). Plasma lactate peaked at the end of the intraperitoneal chemoperfusion, at a median value of 4.2 mmol/L (range, 3.0–7.8 mmol/L). For 11 of the patients, the intraoperative peak concentration had been 2.3 mmol/L or higher. The body temperature of all the patients increased during the hyperthermic part of the procedure. It peaked at the end of the oxaliplatin chemoperfusion at a median value of 38.1 °C (range, 36.7–39.1 °C).
Postoperative Nutritional Details
The median ICU stay (including readmissions) was 1 day (range, 1–33 days). Enteral nutrition via the surgical jejunostomy was started immediately after the patient’s arrival in the ICU, at 20 ml/h for the dose-escalation cohort and at 10 ml/h for the expansion cohort. In the dose-escalation cohort, enteral nutritional intake via the jejunostomy was increased every hour on postoperative day 1 until the calculated nutritional needs were reached.21 In the expansion cohort, enteral nutrition was administered at 10 ml/h until day 3, then restricted to 20 ml/h until day 6, with total parenteral nutrition (TPN) started routinely about day 3 to meet nutritional needs. After day 6, the enteral nutrition was increased provided the patient had no ileus-related symptoms. In the dose-escalation cohort, TPN was given to five patients (33%), starting on median day 5 (range, day 2 to day 8). In the dose-expansion cohort, 10 patients (91%) received TPN, starting on median day 3 (range, day 2 to day 8). Based on the differences in postoperative nutritional management, the amounts of enteral nutrition per day differed significantly between the two groups in the early postoperative period (Table 2).
Postoperative Complications
Overall, 17 patients (68%) experienced one or more serious adverse events (SAEs). The patients in the dose-escalation cohort had a more complicated postoperative course than the patients in the expansion cohort, although the difference did not reach statistical significance (86% vs 45%; p = 0.081; Table 3). The number of SAEs was significantly higher in the dose-escalation cohort than in the expansion cohort (p = 0.021).
The complications in this study included 25 abdominal complications (6 abdominal infections, 6 cases of ileus, 5 anastomotic leakages, 3 intestinal perforations, 3 wound infections, 1 duodenal leakage, and 1 gastrointestinal fistula) and 16 respiratory complications (9 cases of pneumonia; 3 cases of aspiration pneumonia, 2 cases of pneumothorax, 1 pleural effusion, and 1 respiratory failure). Ileus-related complications, defined as ileus, intestinal perforation, gastrointestinal fistula, and aspiration pneumonia, occurred for seven patients (50%) in the dose-escalation cohort compared with two patients (18%) in the expansion cohort (p = 0.208).
The proportion of patients readmitted to the ICU was significantly higher in the dose-escalation cohort (50%) than in the expansion cohort (9%) (p = 0.04). Three patients, all in the dose-escalation cohort, died within 60 days after surgery (1 patient due to early disease progression and 2 patients due to postoperative complications).
The intraoperative peak concertation of plasma lactate was associated with the re-intervention rate. That is, 6 (55%) of the 11 patients with a peak level of 4 mmol/L or higher needed a re-intervention versus no patients in the group with a peak level below 4 mmol/L (p = 0.038)
Discussion
The PERISCOPE I study was the first dose-finding feasibility study of gastric cancer patients undergoing HIPEC surgery with oxaliplatin and docetaxel. The two participating centers had extensive experience in both HIPEC treatment and gastric cancer surgery before the start of the study. Nevertheless, serious postoperative complications occurred more frequently than anticipated.
The current analysis aimed to describe the changes in perioperative management of the PERISCOPE I patients over time and the impact of these changes on postoperative outcomes. The study led to the development of a perioperative care path for the gastric cancer HIPEC patients in the PERISCOPE II study (Table 4).5
A significant proportion of the patients in the dose-escalation cohort (50%) had ileus-related complications. Although ileus-related complications are common after HIPEC surgery, its sequelae in the PERISCOPE I cohort required a change in postoperative management.22 It is hypothesized that these sequelae are caused by the loss of the stomach’s reservoir function that normally helps to prevent ileus-related complications such as an aspiration pneumonia and intestinal perforations.
In our study, the gastrectomy patients with a paralytic ileus due to CRS and HIPEC who received enteral nutrition via the jejunostomy in an amount that met their nutritional needs were at an increased risk for the development of one or more SAEs (86%). Alternatively, for the patients whose enteral nutrition was restricted during the first postoperative days, the risk for the development of one or more SAEs was lower (45%). To meet the nutritional needs and prevent a catabolic state, TPN was started.
Previously, Shannon et al. 23 suggested starting TPN after gastrectomy and HIPEC as early as postoperative day 1 or 2. In our opinion, TPN should be started after day 3 (i.e., after the initial systemic inflammatory response to the operation has faded away) to prevent metabolic complications.24,25 In our study, to prevent small bowel atrophy and improve gut motility, a small amount of enteral nutrition was given via the jejustomy during the first week to a maximum of 20 ml/h. This strategy is contradictory to current recommendations in HIPEC literature, but in the PERISCOPE I study, the implementation of this strict nutritional protocol was associated with a decrease in the rate of postoperative complications and ICU readmissions (50% vs. 9%).11,12,26
The results of the peroperative blood gas analyses showed that the glucose and lactate levels rose during the HIPEC phase of the surgical procedure in the PERISCOPE I study. A rise in plasma lactate levels during HIPEC with oxaliplatin has been related to the use of dextrose 5% as carrier solution for oxaliplatin, causing hyperglycemia and the metabolic relation between glucose and lactate.15 However, in the PERISCOPE I study, Dianeal PD04 (1.36% glucose) was used as the carrier solution for oxaliplatin. Most likely, the rise in glucose and lactate levels was due to a combination of the 1.36% glucose in the Dianeal, inadequate tissue perfusion after blood and fluid loss, and the use of hyperthermic chemotherapeutics. The latter also explains the increase in body temperature and heart rate during the HIPEC phase.27,28
A high peak lactate level has been associated with a worse surgical outcome.29 Similarly, in the PERISCOPE I cohort, the patients with an intraoperative peak lactate level of 4 mmol/L or higher had a higher re-intervention rate (50%) than those with lactate levels below 4 mmol/L (0%).
The small study population and the three different doses of intraperitoneal docetaxel limited the conclusions that can be drawn from the comparison between the dose-escalation cohort and the expansion cohort. Another limitation of the current analysis was its retrospective design (i.e., the two cohorts were formed after completion of the study). However, notwithstanding the relatively small sample, this study did show that HIPEC procedures in combination with gastric cancer surgery are complex and require a different postoperative management protocol than HIPEC procedures for other cancer patients.
In the PERISCOPE I study, it appeared feasible to treat gastric cancer patients after systemic chemotherapy with a combination of a (sub)total gastrectomy, cytoreductive surgery, and HIPEC using 460 mg/m2 of hyperthermic oxaliplatin followed by 50 mg/m2 of normothermic docetaxel. Over time, a strict perioperative management protocol was adopted to counteract the predominantly ileus-related complications. This protocol has become part of the experimental arm in the randomized PERISCOPE II study.
References
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32.
Van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. (2018) Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378: 230–40.
Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–9.
Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183.
Koemans WJ, van der Kaaij RT, Boot H, Buffart T, Veenhof A, Hartemink KJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination: the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19:420.
Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtroder C, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23:11–22.
Rihuete Caro C, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano A, Perez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44:1805–10.
Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol. 2019;37:2028–40.
ill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692–8.
Wu Z, Li Z, Ji J. Morbidity and mortality of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in advanced gastric cancer. Transl Gastroenterol Hepatol. 2016;1:63.
Raspe C, Flother L, Schneider R, Bucher M, Piso P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43:1013–27.
Webb CA, Weyker PD, Moitra VK, Raker RK. An overview of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for the anesthesiologist. Anesth Analg. 2013;116:924–31.
Cooksley TJ, Haji-Michael P. Postoperative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J Surg Oncol. 2011;9:169.
Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol. 2012;19:4244–51.
De Somer F, Ceelen W, Delanghe J, De Smet D, Vanackere M, Pattyn P, Mortier E. Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit Dial Int. 2008;28:61–6.
Padmakumar AV. Intensive care management of patient after cytoreductive surgery and HIPEC: a concise review. Indian J Surg Oncol. 2016;7:244–8.
van der Kaaij RT, Braam HJ, Boot H, Los M, Cats A, Grootscholten C, et al. Treatment of peritoneal dissemination in stomach cancer patients with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): rationale and design of the PERISCOPE study. JMIR Res Protoc. 2017;6:136.
van der Kaaij RT, Wassenaar ECE, Koemans WJ, Sikorska K, Grootscholten C, Los M, et al. Treatment of PERItoneal disease in Stomach Cancer patients with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy (HIPEC): initial results of the PERISCOPE I. Br J Surg. 10.1002/bjs.11588 2020.
ClinicalTrials.gov. Identifier: NCT03348150. Gastrectomy + cytoreductive surgery + HIPEC for gastric cancer with peritoneal dissemination. (PERISCOPE II). Retrieved 1 January 2020 at https://ClinicalTrials.gov/show/NCT03348150
U.S. Department of Health and Human Services NCI. Common terminology criteria for adverse events (CTCAE). 2009; https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–82.
Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg. 2009;249:900–7.
Shannon NB, Tan GHC, Chia CS, Soo KC, Teo MC. Does having a gastrectomy delay time to feeding and prolong hospital stay in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Int J Hyperthermia. 2018;34:518–23.
Leijte GP, Custers H, Gerretsen J, Heijne A, Roth J, Vogl T, et al. Increased plasma levels of danger-associated molecular patterns are associated with immune suppression and postoperative infections in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Front Immunol. 2018;9:663.
Klein CJ, Stanek GS, Wiles CE III. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc. 1998;98:795–806.
Zhao XF, Wu N, Zhao GQ, Liu JF, Dai YF. Enteral nutrition versus parenteral nutrition after major abdominal surgery in patients with gastrointestinal cancer: a systematic review and meta-analysis. J Investig Med 2016;64:1061–74.
Shime N, Lee M, Hatanaka T. Cardiovascular changes during continuous hyperthermic peritoneal perfusion. Anesth Analg. 1994;78:938–42.
Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Perioperative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia. 2008;63:389–95.
Creagh-Brown BC, De Silva AP, Ferrando-Vivas P, Harrison DA. Relationship between peak lactate and patient outcome following high-risk gastrointestinal surgery: influence of the nature of their surgery: elective versus emergency. Crit Care Med. 2016;44:918–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koemans, W.J., Houwink, A., van der Kaaij, R.T. et al. Perioperative Management of Gastric Cancer Patients Treated With (Sub)Total Gastrectomy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Lessons Learned. Ann Surg Oncol 28, 4647–4654 (2021). https://doi.org/10.1245/s10434-020-09465-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09465-8