Abstract
Introduction
Chemotherapy is increasingly administered prior to resection in patients with early-stage pancreatic adenocarcinoma, but the national prevalence of this practice is poorly understood. Our objectives were to (1) describe the utilization of upfront chemotherapy management of stage I pancreatic cancer; (2) define factors associated with the use of upfront chemotherapy and subsequent resection; and (3) assess hospital-level variability in upfront chemotherapy and subsequent resection.
Methods
The National Cancer Database was used to identify patients treated for clinical stage I pancreatic adenocarcinoma. Outcomes were receipt of upfront chemotherapy and surgical resection after upfront chemotherapy. Associations between patient/hospital factors and both initial management and subsequent resection were assessed by multivariable logistic regression.
Results
A total of 17,495 patients were included, with 26.6% receiving upfront chemotherapy. Upfront chemotherapy was more likely in patients who were ≥ 80 years of age (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.39–1.93), had T2 tumors (OR 2.56, 95% CI 2.36–2.78), or were treated at a low-volume center (OR 2.10, 95% CI 1.63–2.71). Among patients receiving upfront chemotherapy, only 33.5% underwent subsequent resection. Resection was more likely in patients with T1 tumors (OR 1.22, 95% CI 1.04–1.43) and in those treated at high-volume centers (OR 4.03, 95% CI 2.90–5.60). Only 20.4% of hospitals performed resection in > 50% of patients after upfront chemotherapy.
Conclusion
Rates of surgical resection after upfront chemotherapy are relatively low, and the proportion of patients who eventually undergo resection varies considerably between hospitals. The use of surgery after upfront chemotherapy in resectable pancreatic cancer should be considered as an internal quality-of-cancer-care measure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic cancer continues to be a leading cause of cancer death in the US, with an estimated 56,770 new cases and 45,750 deaths in 2019.1 Despite an improved understanding of the molecular drivers of pancreatic cancer, 5-year survival has remained low.2,3 In the treatment of locoregional tumors, surgical resection offers the only opportunity for a cure. Studies have suggested improving postoperative survival rates, with 5-year survival of 10–30%.4,5,6
While surgical resection and adjuvant chemotherapy has remained the standard of care for resectable pancreatic adenocarcinoma, there has been increasing interest in the study and utilization of neoadjuvant therapy for resectable tumors.7,8 Both the National Comprehensive Cancer Network and American Society of Clinical Oncology practice guidelines have integrated neoadjuvant therapy as a consideration in resectable tumors.8,9 These guidelines are designed to allow for early testing of tumor biology, potentially avoiding unnecessary surgery in patients who may ultimately develop rapidly progressive disease.10,11
Despite the growing body of evidence surrounding neoadjuvant therapy and evolving guidelines for resectable disease, national utilization of chemotherapy as the initial treatment modality in resectable pancreatic cancer is poorly described. This is especially important given previous descriptions of underutilization of surgery in resectable disease,12 which may be further exacerbated by shifting treatment paradigms. The objectives of this study were to (1) describe the utilization of upfront chemotherapy in the management of clinical stage I pancreatic cancer; (2) define factors associated with the use of upfront chemotherapy and subsequent resection; and (3) assess hospital-level variability in upfront chemotherapy and subsequent resection.
Methods
Data Source and Study Population
The 2005–2015 National Cancer Database (NCDB) participant user file was the dataset for this retrospective cohort study. This time period was chosen to encompass the majority of the contemporary prospective reports on neoadjuvant chemotherapy in pancreatic cancer that have led to broad acceptance and utilization of neoadjuvant therapy for pancreatic adenocarcinoma,13 while allowing for adequate follow-up. The NCDB is sponsored by the Commission on Cancer of the American College of Surgeons and the American Cancer Society, and uses trained registrars to capture approximately 30% of hospitals and 70% of newly diagnosed cancers nationally.3,14 Data are abstracted by trained and periodically audited registrars.
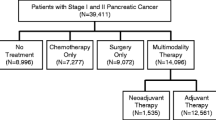
The population for this study was patients with clinical stage 1 pancreatic ductal adenocarcinoma (T1N0M0 or T2N0M0), as defined by the American Joint Committee on Cancer (AJCC) 7th Edition, who underwent some treatment for their disease.13,15,16 Patients were excluded if they did not receive care at the reporting facility or if they were identified in the NCDB as not being surgical candidates (due to age, comorbidities, or early mortality). These selection criteria were intended to minimize the inclusion of patients who may be too frail to undergo surgical procedures. Records with International Classification of Disease for Oncology, 3rd Edition, pancreatic adenocarcinoma histology codes, including cyst-associated adenocarcinoma, were selected. Cases were excluded due to a lack of documentation regarding chemotherapy or radiation (n = 1718), surgery (n = 66), or hospital/socioeconomic characteristics (n = 359; approximately 10% of eligible records).
Outcomes
The primary outcomes of interest were receipt of upfront chemotherapy and surgical resection following upfront chemotherapy. A patient was considered to have undergone upfront chemotherapy if either chemotherapy or chemoradiotherapy was listed before surgery on the NCDB treatment sequence variable, or if the number of days from diagnosis to receipt of chemotherapy was smaller than the number of days to surgery. Among those patients receiving upfront chemotherapy, subsequent resection was defined by a documented definitive surgical procedure after receipt of chemotherapy.
Covariates: Patient Sociodemographic and Clinical Characteristics
Patient-level variables of interest included patient sex, age, race/ethnicity, year of treatment, Charlson–Deyo Score (a composite measure of pre-existing medical comorbidities), and socioeconomic variables.17 Disease-related factors included clinical T stage and tumor location (head, body, tail, and other/overlapping). Hospital-level variables included hospital type (academic vs. non-academic), yearly pancreatectomy volume over the study period (< 5 per year, 5–9 per year, 10–19 per year, and ≥ 20 per year), and census region. Hospital-level rates of upfront chemotherapy and subsequent resection were calculated. Hospital-level rates of both outcomes are presented for the entire cohort and in a subset of hospitals with at least 10 cases.
Statistical Analysis
Associations between patient/hospital characteristics and both outcomes were assessed by multivariable logistic regression models with robust standard errors adjusted for patient clustering within hospitals. Serial hierarchical logistic regression models were then constructed to assess the relative contribution of patient and hospital factors to the variation in hospital-level use of upfront chemotherapy and subsequent resection. These analyses allow for assessment of how much hospital variation in an outcome (e.g. use of upfront chemotherapy) is explained by differences in a group of predictors (e.g. patient/case mix). Empty models were used to quantify the variance due to hospital-level random effects. Two subsequent models were constructed, first adding patient characteristics and, subsequently, hospital characteristics. The decrease in hospital variance attributed to hospital-level random effects was then calculated as previously described, yielding the contribution of patient and hospital factors to the overall hospital variation.18,19 Tests of significance were two-sided, with p-values considered significant at the 0.05 level. Statistical analyses were performed using Stata version 15.1 (StataCorp LP, College Station, TX, USA). This study was considered non-human subjects research by the Northwestern Institutional Review Board and was therefore exempt from approval.
Results
Patient Cohort Characteristics
A total of 17,495 patients with pancreatic adenocarcinoma at 1147 hospitals met the inclusion criteria, with 35.4% of the cohort coming from the first half of the study period (2006–2010) and 64.6% coming from the second half of the study period (2011–2015). Most patients had T2 disease (68.7%) and 53.4% were treated at an academic facility. Additional cohort characteristics can be found in Table 1.
Factors Associated with Receipt of Chemotherapy or Radiation as Initial Therapy
Overall, 26.6% of patients received upfront chemotherapy, 48.7% of whom received multi-agent regimens. Patients were more likely to receive upfront chemotherapy if male (odds ratio [OR] 1.11, 95% confidence interval [CI] 1.04–1.20), ≥ 80 years of age (OR 1.64 vs. age 50–59 years, 95% CI 1.39–1.93), non-Hispanic Black (OR 1.25 vs. non-Hispanic White, 95% CI 1.10–1.42), treated in more recent years (OR 1.14, 95% CI 1.03–1.26), had T2 disease (OR 2.56, 95% CI 2.36–2.78), or were treated at hospitals performing less than five pancreatectomies per year (OR 2.10 vs. hospitals performing > 20 pancreatectomies, 95% CI 1.63–2.71). Patients were less likely to undergo upfront chemotherapy if they were from high-income areas (OR 0.78 vs. low-income areas, 95% CI 0.65–0.94), had more comorbidities (OR 0.82 vs. no comorbidites, 95% CI 0.75–0.90), or had pancreatic tail lesions (OR 0.33 vs. head lesions, 95% CI 0.28–0.39). Sensitivity analyses excluding patients > 80 years and patients with T2 tumors yielded no qualitative changes in other results (Table 2).
Factors Associated with Surgical Resection After Upfront Chemotherapy
The overall rate of surgical resection after upfront chemotherapy was 33.5%. Patients were more likely to undergo subsequent resection if female (OR 1.17, 95% CI 1.03–1.33) or were treated in more recent years (OR 1.90, 95% CI 1.59–2.27). Increasing odds of subsequent resection were observed based on pancreatectomy volume, from 15.8% in hospitals performing less than five pancreatectomies per year, to 31.1% if 5–9 pancreatectomies were performed (OR 2.17, 95% CI 1.65–2.86), to 40.9% if 10–19 pancreatectomies were performed (OR 3.31, 95% CI 2.44–4.49), and to 49.1% if ≥ 20 pancreatectomies were performed (OR 4.03, 95% CI 2.90–5.60). Patients were less likely to undergo resection after upfront chemotherapy if ≥ 80 years of age (OR 0.17 vs. age 50–59 years, 95% CI 0.13–0.24), non-Hispanic Black (OR 0.77 vs. non-Hispanic White, 95% CI 0.60–0.99), Hispanic (24.1%; OR 0.70, 95% CI 0.52–0.94), were from areas with the least education (OR 0.61 vs. most educated, 95% CI 0.45–0.82), or uninsured (OR 0.59 vs. privately insured, 95% CI 0.43–0.80). Sensitivity analyses excluding patients > 80 years of age and patients with T2 tumors yielded no qualitative changes in other results (Table 3).
Hospital Variation in Use of Upfront Chemotherapy and Subsequent Resection
Including all 1147 hospitals, the median hospital-level use of upfront chemotherapy was 28.6% (interquartile range [IQR] 11.8–50.0%, range 0–100%). The median hospital-level rate of subsequent surgical resection was 0% (IQR 0–36.8%, range 0–100%) (Fig. 1a). Overall, 535 hospitals (46.6%) had no documented resections after upfront chemotherapy, while 337 hospitals (29.4%) performed a resection on more than half of such patients. When limiting analyses to hospitals with at least 10 cases, similar variation was observed in the use of upfront chemotherapy (n = 417, median 22.2%, IQR 13.8–35.3%, range 0–96.2%) (Fig. 1b) and subsequent resection (n = 114, median 40.0%, IQR 26.7–54.5%, range 0–93.8%) (Fig. 1c).
Hospital variation in upfront chemotherapy and subsequent surgical resection in patients with clinical stage pancreatic adenocarcinoma. a Hospital variation in the use of upfront chemotherapy (top) and subsequent resection (bottom) including all 1147 hospitals. Boxes encompass the 25–75th percentile. Whiskers are ± 2.5 IQR, and outliers are identified by additional points. The caterpillar demonstrates hospital variation in b upfront chemotherapy (n = 417) and c subsequent resection (n = 114) when restricted to hospitals with at least 10 patients. IQR interquartile range
Variation Attributable to Patient and Hospital Factors
Overall, 2.5% of variation between hospitals in the use of upfront chemotherapy was attributable to patient factors (e.g. patient age, comorbidities, and T stage), 19.4% was attributable to measured hospital factors (e.g. hospital type and volume), and the remaining 78.1% was due to unmeasured factors (Fig. 2a). For subsequent surgical resection, 22.2% of hospital-level variance was attributed to patient factors, 42.8% was attributed to hospital factors, and 35.0% was due to unmeasured factors (Fig. 2b).
Attributable variance in the use of upfront chemotherapy and subsequent surgical resection in patients with clinical stage I pancreatic adenocarcinoma. Figure demonstrates the amount of variation between hospitals that is attributable to individual factors (e.g. patient age, T stage), hospital factors (e.g. volume, academic status), and unmeasured factors (e.g. surgeon preference) for a upfront chemotherapy and b subsequent resection
Discussion
In this study, 26.6% of patients with stage I pancreatic adenocarcinoma and no documented surgical contraindication received upfront chemotherapy. Only 33.5% of patients managed with upfront chemotherapy underwent subsequent resection. Both the use of upfront chemotherapy and subsequent resection rates increased in more recent years and were associated with advanced patient age, higher clinical T stage, and sociodemographic factors. There was considerable hospital variation in both the use of upfront chemotherapy and subsequent resection, with only a small fraction of hospital-level variation being explained by differences in patient characteristics. To our knowledge, this study represents the most comprehensive modern assessment of variation in national management patterns in stage I pancreatic cancer.
It is unsurprising that there has been a significant increase in the use of upfront chemotherapy in early-stage cancers in recent years. Studies have highlighted the benefits of early incorporation of systemic therapy into the treatment pathway of even resectable pancreatic cancer, including avoiding delays in systemic therapy that will almost certainly be necessary, and avoidance of the morbidity of surgery in patients with extremely aggressive tumor biology.10,11 These benefits are especially important when considering evidence that full courses of adjuvant chemotherapy are often not completed, especially in patients who experience surgical complications.20
A striking result of this study is the low rate of subsequent surgical resection after upfront chemotherapy, even when limited to a population that should have high rates of resectability. Even when accounting for patients who may have progressed to unresectable disease while receiving upfront chemotherapy, the observed resection rate in this study is lower than would be anticipated based on the literature, where the resection rate after neoadjuvant therapy is generally more than 50% in borderline resectable cases and approaches 75% in patients who were initially resectable.13 Unfortunately, the NCDB dataset does not possess the granularity of data required for detailed reviews of local progression of disease while receiving upfront chemotherapy. Previous studies have highlighted the national underuse of surgery in the management of stage I pancreatic cancer,12 and these results may reflect persistent erroneous biases against surgical therapy in pancreatic adenocarcinoma.
This study also indicates persistent disparities in the management of early-stage pancreatic cancer, with both racial minorities and socioeconomically disadvantaged patients being less likely to undergo surgical resection after upfront chemotherapy. Previous work has demonstrated disparities in access to surgical evaluation, higher rates of refusal of surgical management, and worse overall outcomes in these populations.21,22,23,24 Further qualitative and quantitative studies are required to better understand these persistent disparities in cancer care.
Finally, hospital-level factors associated with both the use of upfront chemotherapy and subsequent resection were revealing, with low-volume centers being more likely to use upfront chemotherapy and less likely to subsequently perform surgery. This pattern implies that a large fraction of patients at these facilities may be receiving chemotherapy as definitive management despite having potentially resectable primary tumors, although this is difficult to ascertain directly through the NCDB. Moreover, analysis of hospital variation showed that hospital-level differences were driven primarily by the hospital itself (e.g. volume) or unmeasured factors (e.g. surgeon/oncologist preferences) rather than the patient population. Similar to our overall findings, it is unlikely that individual-level local progression of disease while receiving upfront chemotherapy would explain this hospital-level relationship. This indicates that national treatment patterns are driven more by hospital and physician practice patterns than individual treatment plans.
In aggregate, the results of this study highlight the potential need to develop quality measures around surgical resection after upfront chemotherapy in patients who are clinically classified to have early-stage, resectable cancer. Such measures likely should not assess the use of upfront chemotherapy as guidelines are continually evolving and it is impossible to capture the nuance of individual patient treatment decisions in even a robust dataset. Rather, such measures may define goals for the overall rate of resection in patients managed with upfront chemotherapy. While overall resection rates are low after upfront chemotherapy, the fact that many other hospitals achieved relatively high rates of surgical resection implies the potential for improvement at hospitals with lower resection rates.
This study has limitations. The dataset does not allow for reliable delineation between neoadjuvant intent and definitive chemotherapy or radiation therapy, likely causing underestimation of subsequent resection rates. However, we believe our conservative approach is warranted in this cohort (stage I patients without documented surgical refusal or contraindication), most of whom are likely to have resectable primary tumors, as it could be argued that definitive chemotherapy is inappropriate in this patient population. However, the inclusion criteria of the study should make that fraction relatively small. Second, the NCDB dataset is limited in that there is no information on patient preferences (e.g. patient requests on treatment sequence), exclusion from surgery due to treatment adverse effects, or if there was local progression of disease while receiving chemotherapy. We do not believe this would systematically bias the results as such factors would be unlikely to cluster within hospitals. Additionally, the NCDB does not capture the specific chemotherapeutic regimens, which should be explored in future studies. Finally, there were many hospitals with relatively few cases, making estimations of hospital-level rates somewhat unstable. This was mitigated by sensitivity analyses including only hospitals with at least 10 cases, which yielded qualitatively similar results.
Conclusion
Upfront chemotherapy is used in more than one-quarter of patients with stage I pancreatic adenocarcinoma, and the proportion of patients who eventually undergo resection in this group is very low overall. Hospital variation in both the use of upfront chemotherapy and subsequent resection is largely independent of differences in patient populations, implying that variation is mostly due to hospital and physician practice patterns. New quality metrics assessing the rate of surgical resection after upfront chemotherapy in patients with potentially resectable pancreatic adenocarcinoma may be necessary.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34.
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90.
Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg. 2006;10:1212–23; discussion 23–4.
Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–62.
Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–72.
Piatek M, Kusnierz K, Bienkowski M, Peksa R, Kowalczyk M, Nawrocki S. Primarily resectable pancreatic adenocarcinoma—to operate or to refer the patient to an oncologist? Crit Rev Oncol/Hematol 2019;135:95–102.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:1028–61.
Khorana AA, Mangu PB, Katz MHG. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline update summary. J Oncol Pract 2017;13:388–91.
Belli C, Cereda S, Anand S, Reni M. Neoadjuvant therapy in resectable pancreatic cancer: a critical review. Cancer Treat Rev 2013;39:518–24.
Dhir M, Malhotra GK, Sohal DPS, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol 2017;15:183.
Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–80.
Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201–19.
About the Commission on Cancer. 2018. Available at: https://www.facs.org/quality-programs/cancer/coc/about. Accessed 21 Dec 2018.
Cancer Staging Manual. 2018. Available at: https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx. Accessed 21 Dec 2018.
Participant User Files. 2018. Available at: https://www.facs.org/quality-programs/cancer/ncdb/puf. Accessed 21 Dec 2018.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9.
Merlo J, Yang M, Chaix B, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Commun Health 2005;59:729–36.
Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Commun Health 2006;60:290–7.
Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372–7.
Swords DS, Mulvihill SJ, Brooke BS, Skarda DE, Firpo MA, Scaife CL. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery 2019;165(4):751–759.
Khawja SN, Mohammed S, Silberfein EJ, Musher BL, Fisher WE, Van Buren G. Pancreatic cancer disparities in African Americans. Pancreas 2015;44:522–7.
Moaven O, Richman JS, Reddy S, Wang T, Heslin MJ, Contreras CM. Healthcare disparities in outcomes of patients with resectable pancreatic cancer. Am J Surg 2019;217:725–31.
Tohme S, Kaltenmeier C, Bou-Samra P, Varley PR, Tsung A. Race and health disparities in patient refusal of surgery for early-stage pancreatic cancer: an NCDB cohort study. Ann Surg Oncol 2018;25:3427–35.
Funding
As an organization, the American College of Surgeons had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this work represent those of the authors only. RJE (Agency for Healthcare Research and Quality [AHRQ] 5T32HS000078) was supported by a postdoctoral research fellowship and the American College of Surgeons Clinical Scholars in Residence Program. RPM is supported by the Agency for Healthcare Quality (K12HS023011) and an Institutional Research Grant from the American Cancer Society (IRG-18-163-24). ADY is supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (K08HL145139).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors report no conflicts of interest, financial or otherwise, related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ellis, R.J., Ho, J.W., Schlick, C.J.R. et al. National Use of Chemotherapy in Initial Management of Stage I Pancreatic Cancer and Failure to Perform Subsequent Resection. Ann Surg Oncol 27, 909–918 (2020). https://doi.org/10.1245/s10434-019-08023-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08023-1