Abstract
Background
Multiple trials have demonstrated a survival benefit for adjuvant chemotherapy after resection of pancreatic adenocarcinoma. This study aimed to identify the rate for completion of adjuvant chemotherapy, factors associated with completion, and its impact on survival after surgical resection.
Methods
The Surveillance Epidemiology and End Results Medicare-linked data was used to identify patients who underwent upfront resection for pancreatic adenocarcinoma from 2004 to 2013. Billing codes were used to quantify receipt and completion of chemotherapy. Factors associated with completion of chemotherapy were identified using multivariable regression. Kaplan–Meier and Cox proportional-hazards modeling were used to examine survival.
Results
The inclusion criteria were met by 2440 patients. Of these patients, 65% received no adjuvant chemotherapy, 28% received incomplete therapy, and 7% completed chemotherapy. The factors associated with chemotherapy completion were nodal metastases and treatment at a National Cancer Institute-designated cancer center (p ≤ 0.05). Comorbidities decreased the odds of completion (p ≤ 0.05). The median overall survival (OS) was 14 months for the patients who received no adjuvant chemotherapy, 17 months for those who received incomplete adjuvant chemotherapy, and 22 months for those who completed adjuvant chemotherapy (p ≤ 0.05). More recent diagnosis, comorbidities, T stage, nodal metastases, and no adjuvant chemotherapy were associated with an increased hazard ratio for death (p ≤ 0.05). Evaluation of 15 or more nodes and completion of chemotherapy decreased the hazard ratio for death (p ≤ 0.05).
Conclusions
Only 7% of the Medicare patients who underwent upfront resection for pancreatic cancer completed adjuvant chemotherapy, yet completion of adjuvant chemotherapy was associated with improved OS. Completion of adjuvant chemotherapy should be the goal after upfront resection, but neoadjuvant chemotherapy may ensure that patients receive systemic chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite advances in multidisciplinary management, pancreatic adenocarcinoma remains the fourth leading cause of cancer-related death in the United States, accounting for an estimated 44,330 deaths in 2018.1 Surgical resection is the mainstay of therapy with curative intent, but the 5-year overall survival (OS) rate after pancreatectomy alone is merely 10–20%.2,3,–4
Several randomized controlled trials (RCTs) have demonstrated improved OS and disease-free survival (DFS) with completion of adjuvant chemotherapy.2,3,5,6,–7 As such, the National Comprehensive Cancer Network (NCCN) guidelines recommend resection followed by chemotherapy for resectable pancreatic cancer.8 Despite this recommendation, receipt of chemotherapy is not universal. In RCTs with highly selected patients who have completely recovered from surgery, the rates for completion of chemotherapy range from 54 to 79%.5,9,10,11,–12 Single-institution series and cohort studies report even lower rates of 40–60%).13,14
A variety of factors may contribute to why a significant proportion of patients do not receive or complete chemotherapy after resection. Patients often present with poor performance statuses and disease-related comorbidities at the time of diagnosis.15 Surgical resection itself carries high morbidity. Up to 50% of patients experience a major postoperative complication.13,16,17,–18 Postoperative complications can result in delayed administration of chemotherapy and decreased likelihood of its administration.16,17 Furthermore, early disease progression after resection occurs in up to 34% of patients, precluding them from receiving adjuvant chemotherapy.13,14 The combination of these factors likely results in low rates of initiation and completion of adjuvant chemotherapy.
Although RCTs have demonstrated that adjuvant chemotherapy improves survival after resection, patients may not initiate or complete adjuvant chemotherapy for a myriad of reasons. The rates of initiation and completion of adjuvant chemotherapy have not been well established outside RCTs or single-institution series. Therefore, this study sought to determine the rates of receipt and completion of adjuvant chemotherapy on a population level, factors associated with completion of chemotherapy, and its potential impact on survival of patients with pancreatic adenocarcinoma in the United States.
Methods
Using the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) linkage with Medicare, patients who received a diagnosis of pancreatic adenocarcinoma from 2004 to 2013 were identified. The SEER registries provide cancer surveillance for 18 geographic areas, representing 34.6% of the U.S. population.19,20 The registry collects patient, tumor, and treatment characteristics, as well as vital status. The SEER-Medicare database links patients in the SEER program to corresponding Medicare claims.19
Patients 66 years old or older with a diagnosis of pancreatic adenocarcinoma were included in the study. Patients who had metastatic disease at diagnosis, a history of another primary malignancy, reception of any preoperative therapy, or treatment with postoperative chemoradiation were excluded from the study.
Tumor stage was determined by the SEER-derived American Joint Committee on Cancer (AJCC) stage group variable, which is a combination of AJCC 6th- and 7th-edition staging. The study was approved by the University of Minnesota’s Institutional Review Board.
Receipt of adjuvant chemotherapy was identified using Medicare claims. Initiation of adjuvant chemotherapy was defined as the receipt of a unique billing code for intravenous chemotherapy or a specific J code corresponding to fluorouracil, gemcitabine, or folinic acid within 12 weeks from the date of surgery. The 12-week cutoff for initiation of chemotherapy was based on the European Study Group for Pancreatic Cancer (ESPAC)-3 data showing no difference in OS with initiation of adjuvant chemotherapy up to 12 weeks postoperatively.11
During the period of this study, the two most common adjuvant chemotherapeutic agents used for pancreatic cancer were gemcitabine and fluorouracil. In a fluorouracil-based regimen, folinic acid typically is administered intravenously followed by fluorouracil for 5 consecutive days, and repeated every 28 days for six cycles.21,22 In a gemcitabine-based approach, gemcitabine is administered by intravenous infusion once a week for 3 of 4 weeks, and repeated six times.10,21 We defined one cycle of chemotherapy using a typical gemcitabine cycle that involves receipt of a unique Medicare claim for chemotherapy on 3 separate days during a month. Not only are gemcitabine regimens more common, but this approach also allows for the most inclusive definition of a chemotherapy cycle.
Completion of adjuvant chemotherapy was defined as completion of six cycles within 10 months after the date of surgery. This extended time frame allowed for prolonged recovery from surgery and possible delays or adjustments in treatment schedules, including missed doses or missed claims. Chemotherapy administered after 10 months likely does not reflect adjuvant chemotherapy and was not included in the analysis.
The patients who met the inclusion criteria were classified into three cohorts (no chemotherapy, incomplete chemotherapy, and complete chemotherapy) based on the number of adjuvant chemotherapy cycles received. Multivariable logistic regression analyses were performed to evaluate factors associated with completion of adjuvant chemotherapy. All models included date of diagnosis, patient age, gender, race, Charlson Comorbidity Index (CCI), T stage, nodal status, number of lymph nodes evaluated, hospital designation (NCI-designated cancer center), type of surgical resection, and receipt of adjuvant chemotherapy.
Trends over time were analyzed using the Cochran-Armitage test for trend. Survival was analyzed using Kaplan–Meier and Cox proportional-hazard modeling. For all models, sensitivity analyses were performed to ensure that the observed effects were not a product of coding classifications. Results were considered statistically significant for a two-tailed p value of 0.05 or lower. For all statistical analyses, SAS statistical software, version 9.3 (SAS Institute, Cary, NC, USA) was used.
Results
Patient Population
The inclusion criteria were met by 2440 patients. The patient characteristics for the entire cohort that underwent upfront surgical resection are presented in Table S1. The median age of all the patients was 74 years. The majority of the patients (81%) were non-Hispanic white, and 46% were male. Half of the cohort had a CCI of 0. Most of the tumors were T stage 3, larger than 2 cm, and node-positive. A Whipple procedure was performed for 70% of the patients.
After upfront surgical resection, 65% of the patients received no adjuvant chemotherapy, 28% received incomplete adjuvant chemotherapy and 7% completed six cycles of adjuvant chemotherapy. An audit of the Medicare claims data was performed to determine the specific chemotherapy regimens used. Of the patients who completed chemotherapy, 97% had at least one specific J code for gemcitabine, and 8% had at least one specific J code for fluorouracil. The patient characteristics based on receipt of adjuvant chemotherapy (no chemotherapy, incomplete chemotherapy, or complete chemotherapy) are presented in Table 1.
Factors Associated With Completion of Adjuvant Chemotherapy
Multivariable logistic regression was used to evaluate factors associated with completion of adjuvant chemotherapy (Table 2). A greater severity of comorbidities (CCI ≥ 2) was associated with significantly decreased odds for completion of chemotherapy (odds ratio [OR], 0.57; 95% confidence interval [CI], 0.36–0.91). Positive nodal status (OR, 1.67; 95% CI, 1.14–2.46) and treatment at an NCI-designated cancer center (OR, 4.25; 95% CI, 2.68–6.76) were associated with significantly increased odds for completion of chemotherapy. Date of diagnosis was not a significant factor for completion of chemotherapy.
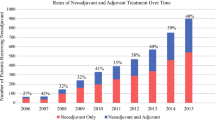
Further evaluation of receipt and completion of chemotherapy over time was performed. Annual rates of initiation and completion of chemotherapy are shown in Fig. 1. No significant trend by year was observed (p = 0.49). However, evaluation by period showed a significant decrease in initiation of chemotherapy in the more recent period (2009–2014) compared with the earlier period (2004–2008). During 2009–2014, 25% of the patients initiated chemotherapy, and 7% completed it compared with 31% and 7%, respectively, in the 2004–2008 period (Table 1; p = 0.002).
Rates for initiation and completion of chemotherapy by year of diagnosis. Numbers represent the percentage of patients with a diagnosis of pancreatic adenocarcinoma who underwent upfront surgical resection and subsequently received complete, incomplete, or no chemotherapy in the adjuvant setting (p = 0.49)
Adjuvant Chemotherapy and OS
The median OS was 14 months for the patients who received no adjuvant chemotherapy, 17 months for those who received incomplete adjuvant chemotherapy, and 22 months for those who completed adjuvant chemotherapy (Fig. 2, p ≤ 0.05). A Cox proportional-hazards model evaluated factors associated with OS (Table 3). More recent year of diagnosis (2009–2013 vs 2004–2008), higher level of comorbidities (CCI ≥ 2 vs 0), higher T stage (T stage 3 and 4 vs T stage 1 and 2), nodal positivity (vs negativity), and receipt of no adjuvant chemotherapy (vs incomplete chemotherapy) all were associated with a significant increase in the hazard ratio for death (p < 0.05). Extent of lymphadenectomy (≥ 15 nodes examined) and completion of adjuvant chemotherapy (vs incomplete chemotherapy) were associated with a significantly decreased hazard ratio for death (p < 0.05).
Kaplan–Meier survival curve for all the patients in the SEER-Medicare-linked data who underwent upfront surgical resection for pancreatic adenocarcinoma from 2004 to 2013 stratified by receipt of adjuvant chemotherapy. The median overall survival was 14 months for the patients who received no adjuvant chemotherapy, 17 months for those who received incomplete adjuvant chemotherapy, and 22 months for those who completed adjuvant chemotherapy (p < 0.05)
Discussion
This study used a large national database to evaluate rates for initiation and completion of adjuvant chemotherapy after upfront resection for pancreatic adenocarcinoma in the Medicare population. Approximately one-third of the patients initiated adjuvant chemotherapy, but only 7% of the population completed adjuvant chemotherapy after upfront resection.
Receipt of adjuvant chemotherapy was associated with improved survival. Furthermore, this study uniquely demonstrated a significant difference in survival between the patients who received no adjuvant chemotherapy (median OS, 14 months), those who received incomplete adjuvant chemotherapy (median OS, 17 months), and those who completed chemotherapy (median OS, 22 months). (p < 0.05). Even after control was used for other relevant variables, completion of adjuvant chemotherapy was associated with improved survival compared with no adjuvant chemotherapy and incomplete adjuvant chemotherapy.
Administration of adjuvant chemotherapy after resection with curative intent has been demonstrated to improve DFS and OS.2,3,5,7,22,23 In 2001, the initial results of the ESPAC-1 trial were published, demonstrating that adjuvant fluorouracil-based chemotherapy improved survival over surgery.3,22 In 2007, Charite Onkologie (CONKO)-001 further solidified adjuvant chemotherapy as the standard of care by demonstrating a 10% improvement in 5-year OS with gemcitabine compared with observation.10 The subsequent ESPAC-3v2 and ESPAC-4 trials more clearly defined optimal adjuvant chemotherapy regimens.3,5,11,21 Recently, a modified FOLFIRINOX regimen was evaluated in the adjuvant setting, demonstrating improved DFS and OS compared with adjuvant gemcitabine, but with an increased rate of serious adverse events.12
Despite the survival benefits attributed to adjuvant chemotherapy, only 35% of Medicare patients initiated chemotherapy, and only 7% completed the courses. Prior RCTs have demonstrated that at best, 54–79% of patients receive adjuvant chemotherapy per protocol.5,9,10,12,22 In CONKO-001, 87% of the patients received at least one cycle of treatment, whereas only 62% completed treatment per protocol, and 9.7% received no adjuvant chemotherapy.10 In retrospective reviews and large institutional series, the rates of chemotherapy administration are significantly less.13,14,16,24,25,–26 In a population-based study of 203 patients, 41.9% of the patients completed adjuvant chemotherapy, whereas 20.2% received incomplete chemotherapy, and 37.9% received no adjuvant chemotherapy.13 Another study evaluated NCCN compliance and found that only 35% of the patients received the recommended multi-modality care.25
The current study used a population-based data set and further demonstrated low rates of initiation and completion of adjuvant chemotherapy in the Medicare population. The dramatic difference between this study and prior studies in the rates for initiation and completion of adjuvant chemotherapy likely is related to patient selection. An RCT includes only patients who have completely recovered from resection, have a good performance status, and show no evidence of disease. Although this is necessary for conducting a well-designed trial, it does not reflect the reality of patient recovery and treatment after surgery.
The current study represented the full gamut of patients who underwent upfront surgical resection for pancreatic cancer. Furthermore, the SEER-Medicare database also included patients in a variety of health care systems, not just large tertiary referral centers, thus providing a near-complete cross-section of patients and outcomes.
The poor receipt of guideline-compliant care with respect to administration of adjuvant chemotherapy certainly is multifactorial, including both patient and provider variables. Prior studies have shown that up to 50% of patients may experience postoperative complications,17 with almost one-fourth of the patients experiencing at least one serious complication.16 Findings have shown that the presence of a serious complication significantly increases the odds of not receiving adjuvant chemotherapy.16
In addition to postoperative complications, other factors reported as barriers to the receipt of optimal therapy include advanced age, poor performance status, early disease progression, and treatment center characteristics.13,14,15,16,–17,25,27 In a large review of the California Cancer Registry, the receipt of guideline-compliant care significantly decreased with increasing age.25 In the current review of SEER-Medicare data, advanced age and comorbidities were similarly associated with decreased odds of chemotherapy completion. The low rates of chemotherapy administration after upfront resection in the Medicare population, particularly those of advanced age, is notable because a previous review of SEER-Medicare showed only a very small benefit of surgery over chemotherapy alone for patients 80 years old or older.28
The two positive predictors of adjuvant chemotherapy completion were treatment at an NCI-designated cancer center and nodal metastases. Treatment at an NCI-designated cancer center was associated with more than a fourfold increase in the odds of a patient completing chemotherapy, in line with prior studies showing that patients who received care at high-volume centers have improved outcomes after pancreatectomy,29,30 and are more likely to receive guideline-compliant care.25,27
In this analysis of SEER-Medicare data, the presence of nodal disease was independently associated with an increased likelihood of completion of adjuvant chemotherapy. This finding may be in response to the fact that nodal metastases are a negative prognostic factor.31,32,33,34,–35 Providers may be more attuned to the importance of adjuvant chemotherapy in the setting of an NCI-designated cancer center and for patients with nodal metastases.
Importantly, receipt and completion of adjuvant chemotherapy did not increase over time. The two major trials that established adjuvant chemotherapy guidelines (ESPAC-1 and CONKO-001) were published in 2001 and 2007, respectively.3,10 The current study was designed to compare the early period (2004–2008) with the later period (2009–2013) to account for any change in practice caused by the publication of CONKO-001.10 However, no significant increase was observed. In the early period (2004–2008), 31% of the patients initiated chemotherapy and 7% completed chemotherapy, whereas the corresponding rates in the late period (2009–2014) were 25% and 7% (p = 0.002). The fact that use and completion of adjuvant chemotherapy did not increase after the publication of CONKO-001 suggests that obstacles other than a perceived paucity of high-level data to support adjuvant chemotherapy existed at that time.
Given the low rates of receipt and completion of adjuvant chemotherapy, a neoadjuvant approach to chemotherapy may enable more patients to receive systemic treatment. Some of the theoretical advantages of neoadjuvant chemotherapy are early initiation of systemic therapy to treat micro-metastatic disease, ability to select out favorable tumor biology before potentially morbid operations, and potential to downstage the primary tumor.36,37,38,–39 Over time, some centers have begun to adopt a neoadjuvant approach for pancreatic cancer, which was accepted at first only for borderline resectable/locally advanced tumors but has spread to use for resectable cancer. However, a recent National Cancer Database (NCDB) study of 18,332 patients with a diagnosis of stages 1 to 3 pancreatic cancer from 2003 to 2011 who underwent surgical resection found that only 1736 patients (9.5%) received neoadjuvant therapy.40
The inherent limitations of this study stemmed from its retrospective nature and its data source. All retrospective studies are subject to reporting and selection bias. In the SEER-Medicare database, information on chemotherapy is claims-based and subject to missing or erroneous claims. However, previous studies have validated this practice.41,42,43,44,–45 Furthermore, augmentation of SEER data with Medicare claims significantly improves the reliability of chemotherapy reporting.45 Although we were unable to obtain granular information on the regimens for each patient and his or her unique clinical course, the data set did allow evaluation, quantification, and characterization of adjuvant chemotherapy.
Completion of adjuvant chemotherapy in this study was defined by a count of chemotherapy codes/claims for a typical gemcitabine regimen. Therefore, the current study may have overreported the actual completion rate for adjuvant chemotherapy because patients who received 5-fluorouracil (5-FU) require more doses of chemotherapy to complete a cycle. This approach was adopted to avoid introducing bias into the study by underreporting completion of adjuvant chemotherapy and because gemcitabine was the most common chemotherapy used. It was impossible to determine whether adjuvant chemotherapy was recommended to patients and the specific reasons why this may or may not have occurred. Finally, the SEER data set does not contain margin status data from the surgical pathology specimen. Nonetheless, adjuvant chemotherapy is recommended after upfront surgical resection regardless of the margin status.46
Despite these limitations, this was the largest and most comprehensive analysis to evaluate adjuvant chemotherapy completion for patients with pancreatic cancer in the United States.
In conclusion, this analysis of the Medicare population demonstrated that initiation and completion of adjuvant chemotherapy after upfront surgical resection of pancreas cancer occur infrequently, only for 35% and 7% of the patients, respectively. Also, this study demonstrated that completion of adjuvant chemotherapy was associated with better survival than experienced by patients who received incomplete or no chemotherapy. The main obstacles to completion of adjuvant chemotherapy appear to be advanced age, comorbidities, recovery after surgical resection, and the health care delivery system. Completion of systemic adjuvant chemotherapy should be the goal after surgical resection for patients with pancreatic cancer because it is associated with improved survival, but this goal remains elusive. A neoadjuvant approach to chemotherapy administration may obviate some of these obstacles and help to ensure that more patients receive and complete systemic chemotherapy for pancreatic cancer.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85.
Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–75.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–15.
Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Clinical Practice Guidelines: Hepatobiliary Cancers. 2018. Version 1 Retrieved 30 May 2018 at https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
Sinn M, Bahra M, Liersch T, et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35:3330–7.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–12.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma: a population-based cohort study. Acta Oncol. 2016;55:265–77.
Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–24; discussion 24–5.
Aloia TA, Aloia TE, Lee JE, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–55.
Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–7.
Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873–81.
Schwarz L, Bruno M, Parker NH, et al. Active surveillance for adverse events within 90 days: the standard for reporting surgical outcomes after pancreatectomy. Ann Surg Oncol. 2015;22:3522–9.
SEER-Medicare Linked Database. 2018. Retrieved May 2, 2019 at https://healthcaredelivery.cancer.gov/seermedicare/.
Health NIo. About the SEER Program–SEER. National Institute of Health. 2019. Retrieved February 26, 2019 at https://seer.cancer.gov/about/overview.html.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Mirkin KA, Greenleaf EK, Hollenbeak CS, Wong J. Time to the initiation of adjuvant chemotherapy does not impact survival in patients with resected pancreatic cancer. Cancer. 2016;122:2979–87.
Weinrich M, Bochow J, Kutsch AL, et al. High compliance with guideline recommendations but low completion rates of adjuvant chemotherapy in resected pancreatic cancer: a cohort study. Ann Med Surg London. 2018;32:32–7.
Visser BC, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB Oxford. 2012;14:539–47.
Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol. 2011;34:567–72.
Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–34.
Marmor S, Burke EE, Virnig BA, Jensen EH, Tuttle TM. A comparative analysis of survival outcomes between pancreatectomy and chemotherapy for elderly patients with adenocarcinoma of the pancreas. Cancer. 2016;122:3378–85.
Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–45.
McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–53.
Li HB, Zhou J, Zhao FQ. A prognostic nomogram for disease-specific survival in patients with pancreatic ductal adenocarcinoma of the head of the pancreas following pancreaticoduodenectomy. Med Sci Monit. 2018;24:6313–21.
Valsangkar NP, Bush DM, Michaelson JS, et al. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17:257–66.
Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–44.
Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery.2007;141:610–18.
Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–40; discussion 240.
Lim KH, Chung E, Khan A, et al. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192–200.
Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–96.
Heinrich S, Schäfer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–22.
Reni M, Balzano G, Zanon S, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2–3 trial. Lancet Gastroenterol Hepatol. 2018;3:691–7.
Mirkin KA, Hollenbeak CS, Wong J. Survival impact of neoadjuvant therapy in resected pancreatic cancer: a prospective cohort study involving 18,332 patients from the National Cancer Data Base. Int J Surg. 2016;34:96–102.
Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):Iv-55–61.
Lamont EB, Lauderdale DS, Schilsky RL, Christakis NA. Construct validity of Medicare chemotherapy claims: the case of 5FU. Med Care. 2002;40:201–11.
Lamont EB, Herndon JE II, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. J Natl Cancer Inst. 2005;97:1080–3.
Du XL, Key CR, Dickie L, Darling R, Geraci JM, Zhang D. External validation of medicare claims for breast cancer chemotherapy compared with medical chart reviews. Med Care. 2006;44:124–31.
Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54:e55–64.
Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, version 1.2019. J Natl Compr Canc Netw. 2019;17:202–10.
Acknowledgment
The authors acknowledge Stephanie Lundgren and the entire Division of Surgical Oncology at the University of Minnesota for their assistance with this project. Ariella Altman is supported by the Institute of Basic and Applied Research in Surgery and the VFW fund of the University of Minnesota Department of Surgery. Keith Wirth is supported by NIH/NIDDK T32K108733 (MPI: Yamamoto and Beilman).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Altman, A.M., Wirth, K., Marmor, S. et al. Completion of Adjuvant Chemotherapy After Upfront Surgical Resection for Pancreatic Cancer Is Uncommon Yet Associated With Improved Survival. Ann Surg Oncol 26, 4108–4116 (2019). https://doi.org/10.1245/s10434-019-07602-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07602-6