Abstract
Background
In colorectal cancer (CRC), the indication for immune checkpoint inhibitors is determined by the microsatellite instability status of the tumors. However, an optimal biomarker for their indication has not been fully identified to date. This study aimed to establish the clinicopathologic importance of the Immunoscore (IS) in CRC and to clarify the relationships between the IS, programmed death-ligand 1 (PD-L1) expression, and tumor-associated macrophages.
Methods
In 132 cases, CRC was diagnosed and surgically treated in our department from 2009 to 2010. Immunohistochemical staining using primary antibodies PD-L1, CD3, CD8, CD68, and CD163 was performed. The IS was determined according to the proposal of an international task force. Statistical analyses were performed to investigate the correlation between the IS, clinicopathologic variables, and expression of immune checkpoint molecules.
Results
The overall survival (OS) and relapse-free survival (RFS) in the high-IS group (I3–4) were significantly better than in the low-IS group (I0–2) (OS: P = 0.0420; RFS: P = 0.0226). The positivity rate for PD-L1 on tumor cells (tPD-L1) was only 0.8%, whereas that for PD-L1 on interstitial tumor-infiltrating mononuclear cells (iPD-L1) was 18.2%. The iPD-L1-positive group showed significantly better survival in terms of both OS and RFS than the iPD-L1-negative group (OS: P = 0.0278; RFS: P = 0.0253). The findings showed significant correlation between the IS and iPD-L1 expression (P < 0.0001).
Conclusions
The study found that a high IS was a good indicator of a better prognosis and significantly correlated with iPD-L1 expression in CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC), one of the most common malignant diseases worldwide, is the second leading cause of cancer-related death in Japan.1 Although a variety of anticancer drugs have been developed, the number of CRC-related deaths has not been significantly reduced.2,3,4 The host immune response, including tumor-infiltrating lymphocytes (TILs), plays an important role in CRC prognosis5,6 and in other malignant diseases,7,8,9 and interest in the use of immune checkpoint inhibitors as part of a new therapeutic strategy has increased.
The mechanism behind immune checkpoint inhibition is blockade of the programmed death-1 (PD-1)/PD-ligand 1 (PD-L1) pathway.10 Whereas PD-1 is strongly expressed on activated lymphocytes, particularly on TILs, PD-L1 is expressed not only on antigen-presenting cells but also on tumor cell surfaces. The binding of both molecules causes the immune response of T cells to be suppressed and results in immune tolerance.11 The immune checkpoint inhibitors nivolumab and pembrolizumab are both reported to be effective in malignant melanoma, non-small cell lung cancer, renal cell carcinoma, and malignant lymphoma.12,13,14,15,16
Galon et al.6 first proposed the use of the Immunoscore (IS) system, defined by evaluation of TILs, and reported that the IS was a significantly better prognostic indicator in CRC.17,18 However, the use of the IS alone to determine the use of immune checkpoint inhibitors needs to be verified.
Expression of PD-L1 is observed not only in tumor cells (TCs) but also in tumor-infiltrating mononuclear cells (TIMCs). The expression of PD-L1 on TCs correlates with a poor prognosis in various malignancies.19,20 However, few studies have investigated the association between PD-L1 expression in TIMCs and the prognosis for CRC patients.
The presence of macrophages in the interstitium of PD-L1-expressing tumors has been reported previously.21,22 Studies also have reported that tumor-associated macrophages (TAMs), particularly M2-type macrophages, form an environment favorable for tumor growth.23,24
Therefore, this study aimed primarily to establish the clinicopathologic importance of the IS in CRC and secondarily to clarify the relationships between the IS, PD-L1 expression, and TAMs.
Materials and Methods
Patients and Samples
From 2009 to 2010, CRC was diagnosed in 192 patients and surgically treated in our department. After written informed consent was obtained, formalin-fixed paraffin-embedded tissue specimens were obtained from each patient. Cases with neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy and multiple cancer cases were excluded. The study enrolled 132 patients.
The clinicopathologic features of the patients are shown in Table S1. Postoperative pathologic staging was determined according to the seventh edition of the UICC-TNM classification of malignant tumors. Clinical outcome records and pathologic reports were reviewed retrospectively.
This study was conducted in accordance with the provisions of the Declaration of Helsinki and approved by the Institutional Review Board of Kurume University Hospital (no. 300).
Immunohistochemistry
Paraffin-embedded tissue samples were cut at a thickness of 4 μm and spread on coated slide glasses. The slide glasses were labeled with the following antibodies using BenchMark ULTRA (Ventana Automated Systems, Inc., Tucson, AZ, USA) and Bond-Max autostainer (Leica Microsystems, Newcastle, UK). The primary antibodies (with dilutions) used were: CD3 (× 300, clone LN10; Leica Microsystems), CD8 (× 200, clone 4B11; Leica Microsystems), CD68 (× 1200, clone KP1; DakoCytomation, Glostrup, Denmark), CD163 (× 100, clone 10D6; Leica Microsystems), and PD-L1 (× 100, clone E1L3N; Cell Signaling Technology, Inc., Danvers, MA, USA).
Immunostaining with CD3, CD8, CD68, and PD-L1 was performed by the fully automated Bond-III system (Leica Microsystems). Antigen retrieval was performed using onboard heat-induced retrieval with epitope retrieval solution 2 (ER2, EDTA-based buffer, pH 9.0; Leica Microsystems) for 10 min at 99 °C. Slide glasses were incubated with each antibody for 30 min at room temperature. A Bond Polymer Refine Detection System (Leica Microsystems) was used, and slide glasses were incubated with secondary antibody for 30 min at room temperature. All slides were visualized using diaminobenzidine (DAB).
BenchMark ULTRA was used to stain CD163. Each slide was heat-treated using Ventana’s ULTRA cell conditioning 1 (CC1; Ventana Automated Systems, Inc.) retrieval solution for 30 min at 95 °C and incubated with the CD163 antibody for 30 min at 37 °C. This automated system used the streptavidin biotin complex method with 3,3′ DAB as the chromogen (Ventana UltraVIEW DAB detection kit).
Image Analysis and Evaluation of PD-L1 Expression
All stained slides were scanned and digitized using NanoZoomer2.0-HT: C9600-13 (Hamamatsu Photonics KK, Shizuoka, Japan). The scanned images were analyzed using NDP.view2: U12388-01 software (Hamamatsu Photonics KK), and five points of the center of the tumor (CT) and the invasive margin (IM) each were captured and stored as JPEG images with a × 200 field of view. The captured images were processed and quantified using image-processing software, Image J 1.50i (U.S. National Institutes of Health, Bethesda, MY, USA).25
For evaluation, the primary deconvolution of the image was performed, followed by the selection of the red image and creation of the binary image. The color density threshold was set to be constant, and the auto counting of positive cells was performed using particle count.
The median value was calculated from the measured values of the five points measured at the CT and IM. The cutoff value of PD-L1 expression was determined using receiver operating characteristic curves. Expression of PD-L1 was evaluated by distinguishing between TC expression (tPD-L1) and TIMC expression (iPD-L1).
Evaluation of the IS and TAMs
The IS was quantified according to the protocol proposed by the international task force and classified into five stages according to the density of CD3- and CD8-positive lymphocytes in the CT and IM. Specifically, we classified the IS from I0 to I4.26,27 Similar to the evaluation procedure for PD-L1 expression, the expression of CD3 and CD8 was measured by the CT and IM at five points respectively, and the median of each was taken as the cutoff value. Evaluation of CD68 and CD163 was performed according to the procedure used for PD-L1, CD3, and CD8 evaluations.
Statistical Analysis
The correlations between PD-L1 expression, the IS, and the clinicopathologic characteristics of the patients were analyzed using a Chi square test. Survival curves were estimated by the Kaplan–Meier method, and statistical significance was evaluated using a log-rank test. Overall survival (OS) was defined as the time from surgery to death, and relapse-free survival (RFS) was defined as the time from surgery to disease recurrence. Uni- and multivariate analyses were performed using the Cox hazards model. All statistical analyses were conducted using JMP software version 12.0 (SAS Institute Inc., Cary, NC, USA), and a P value lower than 0.05 was considered statistically significant.
Results
Staining Results of Each Marker
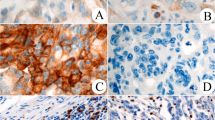
Representative PD-L1-stained images are shown in Fig. 1a–c. PD-L1 was expressed on both TCs and TIMCs. Figure 1a shows a PD-L1-negative case, and Fig. 1b shows a (PD-L1 on tumor cells) tPD-L1-positive case. Figure 1c shows an (PD-L1 on intestinal tumor infiltrating mononuclear cells) iPD-L1-positive case. Only 1 case (0.8%) was positive for tPD-L1, whereas 24 cases (18.2%) were positive for iPD-L1. No PD-L1-positive cases were observed in either tPD-L1 or iPD-L1. Representative examples of CD3 and CD8 positivity in the CT and IM are shown in Fig. 1d–g.
a–c Immunohistochemical staining of representative programmed death-ligand 1 (PD-L1) expression (× 50 and × 200). a PD-L1 expression negativity. b PD-L1 expression positivity on tumor cells. c PD-L1 expression positivity on tumor-infiltrating mononuclear cells. d–g Immunohistochemical staining of representative CD3 and CD8 (× 50 and × 200). d CD3-positivity at the center of the tumor. e CD3-positivity at the invasive margin. f CD8-positivity at the center of the tumor. g CD8-positivity at the invasive margin
IS and Clinicopathologic Variables
We first performed the IS scoring for the lesion. The IS was classified into five stages, I0–I4, according to evaluation of CD3 and CD8 in the CT and IM as follows: I0 (35 cases, 26.5%), I1 (21 cases, 15.9%), I2 (21 cases, 15.9%), I3 (20 cases, 15.2%), and I4 (35 cases, 26.5%). The IS was divided into two groups, a high-score group (I3–4) and a low-score group (I0–2), and each of these was analyzed. The IS was not significantly associated with any of the clinicopathologic features (data not shown).
The IS and Survival Analysis
In the I3–4 group, the OS and RFS ratio was significantly higher than in the I0–2 group (OS: P = 0.0420; RFS: P = 0.0226) (Fig. 2a, b). Further examination by tumor-node-metastasis (TNM) stage showed that the OS and RFS did not differ significantly in the stages I and IV cases. In the stage III cases, those in the I3–4 group had significantly better prognoses than those in the I0–2 group (OS: P = 0.0390; RFS: P = 0.0125). Even in the stage II cases, the I3–4 group tended to show a good prognosis (OS: P = 0.2138; RFS: P = 0.0792) (Fig. 2c–f; Fig. S1).
Kaplan–Meier curves of a overall survival (OS) and b relapse-free survival (RFS) according to the Immunoscore (IS) in patients with colorectal cancer. c–f Kaplan–Meier curves of RFS according to the IS by each tumor-node-metastasis (TNM) stage. The solid line represents the group with a high score (I3–4), and the dashed line represents the group with a low score (I0–2)
To analyze the effects of clinicopathologic variables and the IS on OS and RFS in stages II and III cases, uni- and multivariate analyses were performed (Table 1). The univariate analysis of OS showed a significant difference only in the IS (P = 0.0116). In the multivariate analysis, only the IS was an independent prognostic factor (hazard ratio [HR], 2.71; 95% confidence interval [CI], 2.72–2.87; P = 0.0026). In the univariate analysis of RFS, a significant difference was observed between the IS (P = 0.0019) and N stage (P = 0.0208), and in the multivariate analysis, the IS and N stage were extracted as independent prognostic factors (HR, 11.7; 95% CI, 2.38–210; P = 0.0006, and HR, 3.32; 95% CI, 1.17–11.8; P = 0.0229, respectively).
Correlation Between iPD-L1 Expression, the IS, and Clinicopathologic Characteristics
The relationships between iPD-L1 expression, the IS, and clinicopathologic features are shown in Table 2. The iPD-L1-positive group exhibited a significantly higher proportion of right-sided (P = 0.0178), early TNM stage (P = 0.0026), T1–2 (P = 0.0035), and N0 (P = 0.0145) cases. The iPD-L1-positive and I3–4 groups were significantly correlated (P < 0.0001).
Localization of PD-L1 Expression and Survival Analysis
We used CD68 as a marker to assess M1 macrophage distribution and CD163 as a marker of M2 macrophage. The results from the immunohistochemical staining of CD68 and CD163 performed to clarify the localization of PD-L1 expression are shown in Fig. 3a–f. The PD-L1-positive TIMCs were positive for both CD68 and CD163, suggesting the possibility of the presence of macrophages, especially M2-type macrophages. The OS and RFS were significantly better in the iPD-L1-positive cases (P = 0.0278) than in the iPD-L1-negative cases (P = 0.0253) (Fig. 3g, h).
Immunohistochemical staining of CD68 and CD163 in tumor-infiltrating mononuclear cells with positive programmed death-ligand 1 (PD-L1) expression (iPD-L1). iPD-L1-positivity a × 25 and b × 200. CD68 expression at the same position c × 25 and d × 200. CD163 expression at the same position e × 25 and f × 200. Kaplan–Meier curves of g overall survival and h relapse-free survival according to the expression of PD-L1 on the tumor-infiltrating mononuclear cells (iPD-L1) in patients with colorectal cancer
Discussion
Our study showed that the IS is an independent prognostic factor for both OS and RFS in stages II and III CRCs. We observed an almost complete lack of tPD-L1 expression. The iPD-L1-positive cases and the IS were significantly correlated. Furthermore, iPD-L1 expression was associated with a good prognosis, suggesting that PD-L1 may be expressed in M2-type macrophages.
Determined by the density of CD3 and CD8 in the CT and IM, the IS is an excellent prognostic factor for CRC.28,29,30 Typically, postoperative adjuvant chemotherapy is recommended for stage II high-risk cases and stage III cases.4 As per regular clinicopathologic analysis, stage II low-risk cases are excluded from adjuvant therapy. However, our results suggested that the IS can identify truly high-risk cases that could not have been identified by traditional risk analysis.
We also compared the significance of the IS by stage I and stage IV cases but could not identify the influence of the IS on the prognosis in each stage. This could be attributed to the fact that in stage I cases, radical resection is possible and the involvement of the immune response should be relatively low. In stage IV cases, although the effect of pre-surgical factors would be excluded, most cases receive a variety of postoperative combination chemotherapy, possibly causing the true benefit of immune status for OS and RFS to be canceled.
Studies show that PD-L1 is expressed not only on TCs but also on TIMCs and that tumor PD-L1 expression is correlated with a poor prognosis in various carcinomas.19,20 However, analysis by microsatellite instability (MSI) status is advanced in CRCs, and the clinicopathologic evaluation of the PD-L1 expression site has not been determined. Lee et al.31 showed that PD-L1 expression in TCs correlated with a poor prognosis in mismatch-repair-deficient CRCs.
In contrast, Li et al.32 reported that PD-L1 expression in TCs is associated with a better prognosis in CRCs. However, few studies have examined the relationship between PD-L1 expression in TIMCs and prognosis in CRC cases.
The relationships between PD-L1 in TIMC expression and clinicopathologic features have been reported in other carcinomas. Some reports state that PD-L1 in TIMC expression correlates with a poor prognosis in esophageal squamous cell carcinoma, gastric cancer, and uterine cervix adenocarcinoma33,34,35 and a favorable prognosis in urothelial carcinoma and head and neck cancer.36,37 Koganemaru et al.38 reported that PD-L1 tumor expression is associated with a poor prognosis, whereas high PD-L1 in TIMC expression is related to a better prognosis in stage III CRCs. In addition, Lee et al.39 reported that PD-L1 in tumor-infiltrating immune cell expression correlated with a good prognosis in CRCs.
In our study, high iPD-L1 expression correlated significantly with OS improvement, supporting the results of the aforementioned studies. As for the analysis of PD-L1, in addition to the implication of its expression on tumors or their marginal interstitium, the results may vary depending on the diagnostic reagents used. Therefore, to interpret all analysis results in a similar manner, it is necessary to unify these reagents and make a common diagnosis.
Tumor-associated macrophages are important to the formation of a tumor microenvironment. When macrophages are activated in the tumor microenvironment, they are polarized into M1 and M2 types. The M2 macrophages produce angiogenic factors and cell growth factors, forming an environment favorable for cancer growth.23,24
In this study, the immunohistochemical staining of CD68, which is characteristic of common macrophages, and that of CD163, which is characteristic of M2 macrophages, suggested that PD-L1-positive TIMCs may have M2-type macrophages. The presence of M2 macrophages is correlated with a poor prognosis in various carcinomas.40,41,42 However, Edin et al.43 showed that the high infiltration of M1 and M2 macrophages correlates with a good prognosis in CRCs, and that the local role of TAMs is controversial. Although it has been established that blockade of the PD-1/PD-L1 pathway activates T cells, little is known about the role of this pathway in TAMs, and further investigation is warranted.
Based on these results, we hypothesized that M2 macrophages may be exhausted and may not be able to fulfill the tumor growth function, just as the lymphocytes may be exhausted and unable to attack the tumor cells. As a result of the exhaustion, the possibility exists that PD-L1 is expressed on macrophages. However, to prove this hypothesis, it will be necessary to investigate the functional mechanism by examining IFNγ, a regulator of PD-L1 expression, and M2 macrophage secretion factors.
Our study had several limitations. First, because it was performed at a single center, the generalizability of the results may be low. Second, patient selection bias was a possibility due to the nature of the retrospective study. Third, the PD-L1 expression on TCs was weak. However, it has been suggested that the antibody we used (E1L3N) in this study, is comparable with other antibodies used in other studies.44,45 In CRCs, PD-L1 expression on TCs is observed in approximately 12–30% of MSI-high cases. The corresponding value is very low in microsatellite-stable (MSS) cases.21,39,46 Although the prevalence of MSI-high is estimated to be 10–15% worldwide, it is as low as 3–7% in Japan.47,48 Thus, it is possible that there was almost no PD-L1 expression on TCs. Fourth, due to the low proportion of MSI-high cases in Japan, we did not investigate the MSI status as a routine exam. In future studies, we plan to study the role of MSI status in IS.
Conclusions
Our findings suggest that the IS is a good indicator of a better prognosis and significantly correlated with iPD-L1 expression in CRC. Therefore, evaluation of PD-L1 expression should distinguish between TIMCs and TCs. Further research is necessary to clarify the significance of PD-L1 expression on TIMCs, especially on M2-type macrophages.
Change history
29 January 2019
In the original article, Akihiko Kawahara’s first name is incorrect. It is correct as reflected here.
References
Cancer Statistics in Japan 2016. http://ganjoho.jp/data/reg_stat/statistics/brochure/2016/cancer_statistics_2016.pdf.
Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463–76.
Elez E, Argilés G, Tabernero J. First-line treatment of metastatic colorectal cancer: interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16:52.
Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2017;23:1–34.
Reissfelder C, Stamova S, Gossmann C, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Investig. 2015;125:739–51.
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Bremnes RM, Busund L-T, Kilvær TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11:789–800.
Kollmann D, Ignatova D, Jedamzik J, et al. Expression of programmed cell death protein 1 by tumor-infiltrating lymphocytes and tumor cells is associated with advanced tumor stage in patients with esophageal adenocarcinoma. Ann Surg Oncol. 2017;24:2698–706.
Matkowski R, Gisterek I, Halon A, et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–51.
Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:1–14.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:451–61.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–62.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9.
Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8.
Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–7.
Mino-Kenudson M. Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med. 2016;13:157–70.
Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24.
Korehisa S, Oki E, Iimori M, et al. Clinical significance of programmed cell death-ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer. 2018;142:822–32.
Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers Basel. 2014;6:1670–90.
Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–39.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis HISTORICAL commentary NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J Pathol. 2014;232:199–209.
Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pagès F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–82.
Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711.
Anitei M-G, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the Immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–9.
Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51.
Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433–42.
Li Y, Liang L, Dai W, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55.
Jiang Y, Lo AWI, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017;8:30175–89.
Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2016;66:794–801.
Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. 2016;29:753–63.
Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–7.
Kim HR, Ha S-J, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956.
Koganemaru S, Inoshita N, Miura Y, et al. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108:853–8.
Lee KS, Kwak Y, Ahn S, et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother. 2017;66:927–39.
Tiainen S, Tumelius R, Rilla K, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66:873–83.
Mei J, Xiao Z, Guo C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget. 2016;7:34217–28.
Waniczek D, Lorenc Z, Śnietura M, Wesecki M, Kopec A, Muc-Wierzgoń M. Tumor-associated macrophages and regulatory T cells infiltration and the clinical outcome in colorectal cancer. Arch Immunol Ther Exp Warsz. 2017;65:445–54.
Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a m1 or m2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045.
Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–8.
Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 2017;3:256–9.
Kim JH, Park HE, Cho NY, Lee HS, Kang GH. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115:490–6.
Umar A, Richard Boland C, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8.
Asaka SI, Arai Y, Nishimura Y, et al. Microsatellite instability-low colorectal cancer acquires a KRAS mutation during the progression from Dukes’ A to Dukes’ B. Carcinogenesis. 2009;30:494–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yomoda, T., Sudo, T., Kawahara, A. et al. The Immunoscore is a Superior Prognostic Tool in Stages II and III Colorectal Cancer and is Significantly Correlated with Programmed Death-Ligand 1 (PD-L1) Expression on Tumor-Infiltrating Mononuclear Cells. Ann Surg Oncol 26, 415–424 (2019). https://doi.org/10.1245/s10434-018-07110-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07110-z