Abstract
In this study, we investigated the clinical relevance of CD274 (PD-L1) protein expression by tumor cells and tumor-infiltrating immune cells in colorectal cancer (CRC). To this end, 186 microsatellite instability-high (MSI-H) and 153 microsatellite stable (MSS) CRCs were subjected to immunohistochemistry (IHC) analysis for the expression of CD274 and mismatch repair proteins. CD274 expression was evaluated in tumor cells at the center (TC) and periphery (TP), and immune cells at the center (IC) and periphery (IP) of CRC. IHC slides stained for CD3 and CD8 were scanned using an Aperio ScanScope for precise calculation of tumor-infiltrating T cell density. Additionally, samples were screened for the B-Raf (BRAF)-V600E mutation using a Cobas 4800 System and IHC. In total, CD274TC, CD274TP, CD274IC, and CD274IP were observed in 43 (23.1%), 47 (25.3%), 107 (57.5%), and 102 (54.8%) of the MSI-H CRCs examined, and in three (2.0%), four (2.6%), 47 (30.7%), and 56 (36.6%) of the 153 MSS CRCs tested. Meanwhile, intratumoral heterogeneity of CD274 expression in tumor cells and immune cells was detected in 24 (12.9%) and 47 (25.3%) MSI-H CRCs, respectively. Notably, in both MSI-H and MSS CRC, CD274IC and CD274IP were independently associated with improved prognosis (P < 0.05), while BRAF mutation was associated with CD274TP, poor differentiation, sporadic type, and hMLH1(−)/hMSH2(+)/hMSH6(+)/PMS2(−) in MSI-H CRC (P < 0.006). In conclusion, CD274 expression in tumor-infiltrating immune cells was an independent factor for improved prognosis in CRC patients. A deeper understanding of CD274 status may yield improved responses to future CRC immunotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remarkable development of immunotherapeutics for several cancers has changed the anti-cancer therapeutic paradigm. Particularly, targeting of PDCD1 (PD-1) and its ligand, CD274 (PD-L1), has demonstrated excellent anti-tumor effects in various tumors [1, 2]. The PDCD1/CD274 checkpoint is thought to comprise a key mechanism of host immune system evasion in malignancy [3], and anti-PDCD1 antibodies have been approved by the US Food and Drug Administration (FDA) for metastatic non-small-cell lung cancer patients [4]. Interestingly, CD274 expression has primarily been observed in MSI-H CRCs, and is only rarely seen in microsatellite stable (MSS) CRCs [5, 6]. Moreover, a recent study indicated that PDCD1 blockade using drugs such as pembrolizumab comprises a specific and highly effective method for treating microsatellite instability-high (MSI-H) CRC [7]. As such, the FDA has granted a priority review to evaluate the efficacy of pembrolizumab for treating MSI-H advanced CRC.
Many studies have examined the prognostic impact of CD274 status and its predictive value in various malignancies. Recent studies showed that CD274 was expressed in several malignant tumors, where it tended to be correlated with decreased survival [1, 8]. However, the prognostic value of CD274 expression remains to be determined, as other studies have detected debatable correlations [9–11], or even no correlation [5, 6, 12], between prognosis and CD274 expression in CRC patients.
Interestingly, a previous report observed an improved response to PDCD1 blockade therapy in patients exhibiting CD274 expression on tumor-infiltrating immune cells [13]. In particular, abundant tumor-infiltrating immune cells are frequently observed in MSI-H CRC [14]. Thus, further evaluation of CD274 expression by immune cells, as well as tumor cells, is needed to facilitate CRC immunotherapy.
Frequently, certain subsets of cancer patients will show decreased responses to targeted therapies due to regional heterogeneity of target molecules, and immunotherapy seems to be no exception. CD274 expression likely has more regional heterogeneity than other mutational alterations, because the PDCD1/CD274 axis is part of a dynamic immune reaction. Indeed, certain recent studies suggest that the value of CD274 immunohistochemistry (IHC) as a predictive and prognostic marker is debatable due to frequent regional heterogeneity [2, 15]. Therefore, regional heterogeneity of CD274 expression should be evaluated in detail in CRC.
This study was conducted to investigate the clinical relevance of CD274 expression by tumor cells and immune cells in CRC, focusing primarily on the MSI-H subgroup. We also analyzed intratumoral heterogeneity and precisely determined the tumor-infiltrating lymphocyte density in CRC using an Aperio image analysis system.
Materials and methods
Patients and samples
In total, 339 CRC patients who underwent surgical resection at Seoul National University Bundang Hospital were included in our study. The patient cohort was composed of two groups: 186 MSI-H CRC patients who were surgically treated between 2003 and 2012, and for comparison, 153 consecutive MSS CRC patients who underwent surgical treatment during 2005. Patients who had received pre-operative chemotherapy or radiotherapy were excluded from the cohort. Among the MSI-H CRC patients, 104 received post-operative chemotherapy, while 74 were only treated surgically; the post-operative history for the remaining eight patients was unobtainable. Meanwhile, 107 and 46 of the MSS CRC patients received post-operative chemotherapy or surgery alone, respectively. Two pathologists (Kyu Sang Lee and Hye Seung Lee) histologically reviewed each CRC case. Clinicopathological data were obtained from hospital medical and pathologic reports. Cancer stage was determined according to the American Joint Committee on Cancer (AJCC), 7th edition. Suspected Lynch syndrome (LS) patients were selected from the MSI-H CRC cohort according to the Bethesda guideline (2004) [16]. These patients did not fulfill the diagnostic criteria for LS, as the MMR mutation test was not performed. Follow-up information collected included patient outcome and the interval between the date of surgical resection and the date of death by any cause or censoring (overall survival).
The use of medical record data and patient tissue samples in this study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (reference: B-1511/322-306).
Tissue array method
A tissue microarray (TMA) was constructed using representative lesions of formalin-fixed paraffin-embedded (FFPE) CRC tissues (SuperBioChips Laboratories, Seoul, South Korea) [17]. Two TMA (2 mm in diameter) single cores were placed at the tumor center and periphery.
Microsatellite instability
Microsatellite instability (MSI) was examined by fragmentation assay analysis using an automated DNA sequencer (ABI 3731 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA) with the following five microsatellite markers, according to previously described methods: BAT-26, BAT-25, D5S346, D17S250, and D2S123 [18].
Immunohistochemistry
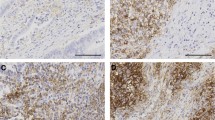
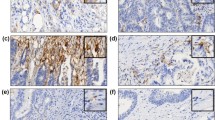
IHC analysis was performed using antibodies specific to CD274 (PD-L1, E1L3 N, 1:50; Cell Signaling Technology, Danvers, MA, USA), CD3 (1:100; Dako, Glostrup, Denmark), CD8 (1:100; Dako), B-Raf (BRAF) (Ventana, Tucson, AZ, USA), MLH1 (Ventana), MSH2 (Cell Marque, Rocklin, CA, USA), MSH6 (Cell Marque), and PMS2 (Ventana). Immunostaining was conducted using the Ventana Bench mark XT autostainer (Ventana) and the ultraView Universal DAB kit (Ventana), according to the manufacturer’s recommendations. Normal colonic epithelial cells were utilized as internal negative controls. Tissues were considered CD274-positive when more than 5% of neoplastic cells showed membrane staining of any intensity (Fig. 1) [5]. Meanwhile, tissues were considered BRAF positive when at least 80% of the tumor cells exhibited moderate to strong diffuse cytoplasmic staining (Fig. 1) [19]. Mild cytoplasmic staining in neoplastic cells was considered equivocal. Lastly, tissues containing neoplastic cells that exhibited nuclear staining for the mismatch repair (MMR) proteins MLH1, MSH2, MSH6, and PMS2, respectively, were considered positive.
Immunohistochemical staining of colorectal cancer patient samples. Images of tumor cells exhibiting a a lack of CD274 expression and b CD274 expression at the cell surface (×40). c CD274 expression on tumor-infiltrating immune cells (×40). d CD3 expression on tumor-infiltrating lymphocytes (×40). e Aperio image analysis using the Nuclear v9 algorithm: blue score 0; yellow score 1; orange score 2; red score 3 (×40). f B-Raf (BRAF) expression (×40). All immunohistochemistry images depict representative results
CD3 and CD8 IHC slides were scanned on an Aperio ScanScope (Aperio Technologies, Inc., Vista, CA, USA) at 20× magnification. For precise calculation, CD3+ and CD8+ tumor-infiltrating lymphocytes (TILs) were quantified with an ImageScope computerized image analysis system (Aperio Technologies) using the Nuclear v9 algorithm. A score of 2–3 indicated CD3+ and CD8+ T cells (Fig. 1). TIL density was calculated by dividing the percentage (%) of positive nuclei by the core area (mm2). We arbitrarily defined the cutoff value for TIL density as the median, and thereby divided TIL density into two groups: high and low.
BRAF mutation analyses
To identify BRAF mutations, DNA was harvested from 40 MSI-H CRC patient tissues including 26 BRAF IHC-positive and 14 equivocal tissues, using a Cobas DNA Sample Preparation Kit (Roche, Basel, Switzerland), according to the manufacturer’s instructions and as described previously [20]. Samples were then screened for the BRAF (V600E) mutation using a Cobas 4800 System (Roche).
Statistical analyses
Categorical variables were compared using the Chi square or Fisher’s exact test, as appropriate. The correlation between TIL density and CD274 expression was analyzed by determining Pearson’s correlation coefficients. The McNemar test was used to determine intratumoral CD274 expression heterogeneity. The association between survival and CD274 expression was evaluated using Kaplan–Meier curves with the log-rank test and Cox’s proportional hazards model. A threshold of P < 0.05 was considered statistically significant. To prevent inflation of type I error, data were subjected to multiple testing correction by Bonferroni adjustment [21]. The adjusting P values in Tables 1, 2, and 3 were 0.003, 0.004, and 0.006, respectively. IBM SPSS statistics version 21 (IBM, Armonk, NY, USA) was utilized for all statistical analyses.
Results
Frequency and clinicopathological features of CD274 expression in MSI-H and MSS CRC patients
We investigated CD274 expression in CRC in four different tissue lesions and cell types: tumor cells at the center (TC) and at the periphery (TP), and immune cells at the center (IC) and at the periphery (IP). CD274-expressing immune cells consisted primarily of macrophages, plasma cells, and lymphocytes. In total, CD274TC was observed in 43 (23.1%) of the 186 MSI-H samples, while CD274TP was observed in 47 (25.3%) samples, CD274IC in 107 (57.5%) samples, and CD274IP in 102 (54.8%) samples. Table 1 summarizes the correlations detected between clinicopathological features and CD274 expression in MSI-H CRCs. CD274 expression in tumor cells at both the center and periphery tended to be associated with old age, high grade of histologic differentiation, non-mucinous type, lymphatic invasion, and CD3+ and CD8+ T cell infiltration, in accordance with the previous studies (P < 0.003) [5, 6]. Meanwhile, CD274 expression on tumor-infiltrating immune cells at the center and periphery tended to be associated with CD3+ and CD8+ TIL infiltration (P < 0.003).
In contrast to MSI-H tissues, CD274 expression on tumor cells was rarely observed in the 153 MSS CRC tissues examined; CD274TC and CD274TP were observed in only three (2.0%) and four (2.6%) cases, respectively. In contrast, CD274 expression was frequently observed on immune cells, with CD274IC and CD274IP being observed in 47 (30.7%) and 56 (36.6%) cases, respectively. The correlations detected between clinicopathological features and CD274 expression on tumor-infiltrating immune cells of MSS CRCs are summarized in Table 2. Notably, CD274IP was associated with the absence of metastasis of MSS CRC (P < 0.004).
Correlation between peripheral CD274 expression and TIL density in MSI-H CRC patients
We investigated the correlation between CD274 expression and TIL density at the tumor periphery in the 186 MSI-H CRC patients. The density (%/mm2) of CD3+ TILs was higher [median, interquartile range (IQR): 422, 196–685] than that of CD8+ TILs [median, IQR: 125, 65.6–242]. CD274TP expression exhibited a moderate positive correlated with CD3+ (ρ, 0.538; P < 0.001) and CD8+ (ρ, 0.546; P < 0.001) TILs, according to Dancey and Reidy’s categorization method (2004) [22]. Meanwhile, CD274IP expression was moderately correlated with CD3+ (ρ, 0.438; P < 0.001), but weakly correlated with CD8+ TILs (0.365; P < 0.001).
Intratumoral heterogeneity of CD274 expression in MSI-H and MSS CRC patients
All 339 CRC cases were screened for CD274 expression at the tumor center and periphery to evaluate intratumoral heterogeneity (Supplementary Table 1). Intratumoral heterogeneity of CD274 expression was not uncommon in CRC. Among the 186 MSI-H CRC cases, discordance between CD274TC and CD274TP was observed in 24 (12.9%) cases, and discordance between CD274IC and CD274IP was observed in 47 (25.3%) cases. In the MSS CRC cohort, evaluation of intratumoral heterogeneity of CD274 expression of tumor cells was meaningless due to the low incidence of positivity. However, discordance between CD274IC and CD274IP was found in 37 (24.1%) out of 153 MSS CRC cases. The difference in intratumoral heterogeneity of CD274 expression between MSI-H and MSS CRC was not significant.
Prognostic impact of CD274 expression in MSI-H and MSS CRC patients
In MSI-H CRC, CD274TC and CD274TP were not associated with survival (Supplementary fig. 1; P > 0.05). Conversely, CD274IC and CD274IP were significantly associated with improved survival (Fig. 2; P = 0.003 and P = 0.005, respectively). However, the density of CD3+ and CD8+ TILs did not correlate with patient survival (Supplementary fig. 1; P > 0.05). As mentioned above, survival analysis of CD274 expression of tumor cells in the MSS cohort was meaningless due to a low incidence of positivity. However, CD274IC and CD274IP were significantly associated with improved prognosis in the MSS cohort (Fig. 2; P = 0.014 and P < 0.001, respectively).
Kaplan–Meier survival curves illustrating the prognostic effect of CD274 expression on tumor-infiltrating immune cells in colorectal cancer (CRC). CD274 expression on tumor-infiltrating immune cells at a the tumor center and b the tumor periphery in microsatellite instability-high CRC tissues. CD274 expression on tumor-infiltrating immune cells at c the tumor center and d the tumor periphery of microsatellite stable CRC tissues
Notably, multivariate Cox proportional hazards analysis indicated that CD274 expression on tumor-infiltrating immune cells independently predicted improved prognosis in both MSI-H and MSS CRC cohorts (Table 3).
Detection of the BRAF-V600E mutation in MSI-H CRC patients
We investigated BRAF IHC status in 186 MSI-H CRC cases. To validate BRAF-V600E IHC, 26 IHC-positive and 14 IHC-equivocal cases were subjected to BRAF (V600E) mutation analysis using the Cobas 4800 System. Each of the 26 BRAF IHC-positive cases, but none of the IHC-equivocal cases, contained the V600E mutation. Notably, BRAF mutation was associated with CD274TP, distinguishing expression pattern of MMR proteins [hMLH1(−)/hMSH2(+)/hMSH6(+)/PMS2(−)], sporadic type, and high grade of histologic differentiation (P < 0.006), in agreement with previous studies (Table 4) [23, 24]. While BRAF mutation did not predict overall survival in MSI-H CRC patients (P = 0.987; Supplementary Figure 1), this mutation was significantly associated with worse prognosis in a subgroup of patients who had received post-operative chemotherapy (P = 0.041; Supplementary Figure 1). As such, BRAF mutation seems to be associated with worse prognoses only at the later stages of MSI-H CRC, which is an indication for post-operative chemotherapy.
Discussion
The high density of TILs that is commonly observed in MSI-H CRCs [14] suggests that these cancers vigorously induce a host immune reaction, which could be due to the higher mutational burden of these tumors; next-generation sequencing (NGS) studies have reported that MSI-H CRCs contain 10–50 times more mutations than MSS CRCs [25]. These molecular alterations produce abnormal neoantigens, which have the potential to result in increased numbers of TILs. Giannakis et al. showed that increased neoantigen load was positively correlated with TILs density and improved prognosis in CRC [26]. Actually, these data indicate that neoantigen load is more significant than MSI in clinical implication. Moreover, a recent study demonstrated that MSI-H CRCs show stronger expression of immune-regulation gene clusters than MSS CRCs [27, 28]. These clusters predominantly consist of gene-related T-helper 1 (Th1) and immune checkpoint receptors including PDCD1, CD274, CTLA-4, and LAG-3 [28, 29]. Notably, elevated expression of immune checkpoint molecules can create an immune-suppressive microenvironment [30], which could yield improved responses to immune checkpoint blockade.
The relationship between CD274 expression on tumor cells and prognosis in CRC is highly variable and controversial. While Droeser et al. demonstrated that CD274 expression was associated with better prognosis in MMR-proficient CRC [9], another study reported that the occurrence of CD274 expression on tumor cells with PDCD1 expression on TILs resulted in a worse CRC prognosis [11]. In the current study, CD274 expression on tumor cells was associated with a tendency toward a favorable prognostic value; however, this result had limited significance (Supplementary fig. 1) [6].
Interestingly, CD274 positivity was detected in >50% of tumor-infiltrating immune cells in MSI-H CRCs and >30% of those in MSS CRCs. CD274 expression on tumor and immune cells is thought to have distinct implications. However, a few other studies have also examined the clinicopathological implications of CD274 expression on immune cells. CD274 expression on immune cells shows a tendency towards decreased survival in gastric and uterine cervix adenocarcinomas [31, 32]. On the contrary, CD274 expression on immune cells was associated with improved survival in advanced urothelial carcinoma and spinal chordoma [33, 34]. Notably, our data indicated that CD274 expression on tumor-infiltrating immune cells was an independent factor for improved prognosis in both MSI-H and MSS CRC. Interestingly, in contrast to our data, Wang et al. showed that CD274 positivity on immune cells signifies worse prognoses in consecutive CRC [10]. However, a recent study supported our results that CD274 expression on immune cells results in improved survival of patients with stage IIIb CRC [35]. Similarly, another study also demonstrated that CD274 expression on immune cells showed a tendency toward improved survival in MSI-H CRCs, but this result was not statistically significant [6]. Moreover, this latter study suggested that favorable prognoses resulting from CD274 expression on immune cells was considered an effect of a high density of tumor-infiltrating T cells [6]. To rule out the causes of the favorable prognosis suggested by other groups, we precisely counted and analyzed tumor-infiltrating CD3+ and CD8+ T cells using an Aperio image analysis system, instead of the traditional method of counting by eye. Despite these elaborative analyses, the presence of CD3+ and CD8+ TILs was not significantly associated with prognosis for any of the cutoff values tested. These results support the conclusion that the prognostic value of the CD274 expression on immune cells was not a reflection of high TIL density.
CD274 has been shown to be expressed on macrophages, dendritic cells, T and B lymphocytes, and to protect tissues from excessive immune reaction [36]. While the mechanism by which expression of CD274 on TILs leads to improved patient prognoses remains unclear, there are several possible hypotheses. First, it is conceivable that active anti-tumor immune reactions could enable the positive selection of tumor cells exhibiting mutations in genes encoding human leukocyte antigen (HLA) and/or antigen-processing machinery (APM) [26]. In this case, expression of CD274 on TILs could relieve the anti-tumor immune reaction, leading to reduced numbers of such mutant tumor cells and thereby to better patient outcomes. Alternatively, Di Caro et al. suggested that chemotherapeutic effects on tumor cells seem to be enhanced under immune-escape conditions [37]. CD274 expression on TILs could, therefore, have enhanced the chemotherapeutic responsiveness of tumor cells by promoting an immune-escape condition in CRC. Another contributing factor for favorable prognosis is interferon (IFN)-r-producing tumor-infiltrating T cells. Droeser et al. demonstrated that CD274 mRNA expression was significantly correlated with IFN-γ gene expression in CRC specimens [9], and that IFN-γ might play a role in the tumor surveillance and cytotoxic anti-tumor function. However, further studies are necessary to clarify this possibility.
Recently, several studies detected regional heterogeneity of CD274 expression in various cancers, including lung cancer, melanoma, and renal cell carcinoma [15, 38, 39]. These data suggest that we should be careful in evaluating CD274 expression in routine small biopsies due to the potential for false-negative results. To the best of our knowledge, our study is the first to evaluate intratumoral heterogeneity (central and peripheral portions of the primary tumor) in CD274 expression in CRCs; our data indicate that such heterogeneity is common in resected CRC specimens (Supplementary Table 1), which are more reliable than biopsy specimens. A possible reason for this regional discordance is that various immune reactions might further affect the peripheral portion of tumors than the central portion. Additionally, hypoxic conditions in the central portion might induce intratumoral heterogeneity of CD274 expression. Several studies suggested that hypoxia can promote the expression of CD274 on tumor cells and TILs via hypoxia-inducible factor-1α (HIF-1α) up-regulation [40, 41].
Recent molecular studies indicated that CRC is a heterogeneous disease, arising from several genetic pathways. These pathways are crucial in determining patient prognosis and treatment [42, 43]. In non-small cell lung cancer, recent studies indicated that the genomic landscape determined patient response to PDCD1 blockade therapy [44], and that CD274 expression was associated with EGFR mutations [45]. Our study and others demonstrated that mutation of BRAF is significantly associated with CD274 expression in CRC tumor cells, but not in immune cells [5, 6]. Another recent study suggested that CD274 positivity is associated with the serrated pathway and stem cell features in CRC [24]. Thus, the diverse genetic alterations that correlate with CD274 expression in CRC should be further investigated.
To conclude, our study comprehensively evaluated CD274 expression in MSI-H CRCs, as well as in MSS CRCs. Notably, we found that CD274 expression on tumor-infiltrating immune cells was an independent predictive factor for improved prognosis in both MSI-H and MSS CRCs. Our findings indicate that the CD274 status may be helpful in predicting CRC patient outcomes. Moreover, our results indicate that discordance of CD274 expression between the central and peripheral portions of CRC tumors is not uncommon. Thus, evaluation of various tumor portions is recommended to enhance the validity of CD274 expression results. Further investigation of the mechanism underlying CD274 expression in immune cells as well as the predictive and prognostic role of this protein in CRC is needed.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- C:
-
Center
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- FFPE:
-
Formalin-fixed and paraffin-embedded
- HR:
-
Hazard ratio
- IC:
-
Immune cells at the center
- IHC:
-
Immunohistochemistry
- IP:
-
Immune cells at the periphery
- LS:
-
Lynch syndrome
- MSI-H:
-
Microsatellite instability-high
- MSS:
-
Microsatellite stable
- P:
-
Periphery
- PCR:
-
Polymerase chain reaction
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death ligand 1
- TC:
-
Tumor cells at the center
- TP:
-
Tumor cells at the periphery
References
Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19(5):1021–1034. doi:10.1158/1078-0432.CCR-12-2063
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14(4):847–856. doi:10.1158/1535-7163.MCT-14-0983
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L (2008) B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111(7):3635–3643. doi:10.1182/blood-2007-11-123141
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M (2016) PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol 29(9):1104–1112. doi:10.1038/modpathol.2016.95
Kim JH, Park HE, Cho NY, Lee HS, Kang GH (2016) Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer 115(4):490–496. doi:10.1038/bjc.2016.211
Asaoka Y, Ijichi H, Koike K (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 373(20):1979. doi:10.1056/NEJMc1510353#SA1
Wu P, Wu D, Li L, Chai Y, Huang J (2015) PD-L1 and survival in solid tumors: a meta-analysis. PLoS One 10(6):e0131403. doi:10.1371/journal.pone.0131403
Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49(9):2233–2242. doi:10.1016/j.ejca.2013.02.015
Wang L, Ren F, Wang Q, Baldridge LA, Monn MF, Fisher KW, Sheng W, Zhou X, Du X, Cheng L (2016) Significance of programmed death ligand 1 (PD-L1) immunohistochemical expression in colorectal cancer. Mol Diagn Ther 20(2):175–181. doi:10.1007/s40291-016-0188-1
Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ, Shike M, Stadler ZK, Vakiani E, Klimstra DS, Shia J (2016) Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol 29(11):1433–1442. doi:10.1038/modpathol.2016.139
Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, Inamura K, Cao Y, Song M, Nowak JA, Liao X, Nosho K, Chan AT, Giannakis M, Bass AJ, Hodi FS, Freeman GJ, Rodig S, Fuchs CS, Qian ZR, Ogino S (2016) Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. doi:10.1136/gutjnl-2016-311421
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. doi:10.1038/nature14011
Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR (2001) Histopathological identification of colon cancer with microsatellite instability. Am J Pathol 158(2):527–535. doi:10.1016/S0002-9440(10)63994-6
Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvee S, Washetine K, Mouroux J, Venissac N, Poudenx M, Otto J, Sabourin JC, Marquette CH, Hofman V, Hofman P (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27(1):147–153. doi:10.1093/annonc/mdv489
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, Park do J, Kim JH, Yang HK, Lee BL, Kim WH (2007) Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res 13(14):4154–4163. doi:10.1158/1078-0432.ccr-07-0173
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58(22):5248–5257
Toon CW, Walsh MD, Chou A, Capper D, Clarkson A, Sioson L, Clarke S, Mead S, Walters RJ, Clendenning M, Rosty C, Young JP, Win AK, Hopper JL, Crook A, von Deimling A, Jenkins MA, Buchanan DD, Gill AJ (2013) BRAFV600E immunohistochemistry facilitates universal screening of colorectal cancers for Lynch syndrome. Am J Surg Pathol 37(10):1592–1602. doi:10.1097/PAS.0b013e31828f233d
Nam SK, Yun S, Koh J, Kwak Y, Seo AN, Park KU, Kim DW, Kang SB, Kim WH, Lee HS (2016) BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS One 11(3):e0151865. doi:10.1371/journal.pone.0151865
Greenhalgh T (1997) How to read a paper. Statistics for the non-statistician. I: Different types of data need different statistical tests. BMJ 315(7104):364–366
Christine D, John R (2004) Statistics without maths for psychology: using SPSS for windows. Prentice Hall, London
Capper D, Voigt A, Bozukova G, Ahadova A, Kickingereder P, von Deimling A, von Knebel Doeberitz M, Kloor M (2013) BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer 133(7):1624–1630. doi:10.1002/ijc.28183
Inaguma S, Lasota J, Wang Z, Felisiak-Golabek A, Ikeda H, Miettinen M (2017) Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol 30(2):278–285. doi:10.1038/modpathol.2016.185
Timmermann B, Kerick M, Roehr C, Fischer A, Isau M, Boerno ST, Wunderlich A, Barmeyer C, Seemann P, Koenig J, Lappe M, Kuss AW, Garshasbi M, Bertram L, Trappe K, Werber M, Herrmann BG, Zatloukal K, Lehrach H, Schweiger MR (2010) Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 5(12):e15661. doi:10.1371/journal.pone.0015661
Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA (2016) Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. doi:10.1016/j.celrep.2016.03.075
Lal N, Beggs AD, Willcox BE, Middleton GW (2015) An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. Oncoimmunology 4(3):e976052. doi:10.4161/2162402X.2014.976052
Boissiere-Michot F, Lazennec G, Frugier H, Jarlier M, Roca L, Duffour J, Du Paty E, Laune D, Blanchard F, Le Pessot F, Sabourin JC, Bibeau F (2014) Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology 3:e29256. doi:10.4161/onci.29256
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5(1):43–51. doi:10.1158/2159-8290.CD-14-0863
Kim R, Emi M, Tanabe K (2007) Cancer immunoediting from immune surveillance to immune escape. Immunology 121(1):1–14. doi:10.1111/j.1365-2567.2007.02587.x
Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ (2016) Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. doi:10.1136/gutjnl-2015-310839
Heeren AM, Punt S, Bleeker MC, Gaarenstroom KN, van der Velden J, Kenter GG, de Gruijl TD, Jordanova ES (2016) Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 29(7):753–763. doi:10.1038/modpathol.2016.64
Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, Signoretti S (2015) Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 26(4):812–817. doi:10.1093/annonc/mdv009
Zou MX, Peng AB, Lv GH, Wang XB, Li J, She XL, Jiang Y (2016) Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am J Transl Res 8(7):3274–3287
Shigehiro Koganemaru NI, Miura Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, Tanabe Y, Moriyama J, Fujii T, Matoba S, Kuroyanagi H, Takano T (2016) Prognostic value of programmed death-ligand 1 (PD-L1) expression in patients with stage III colorectal cancer. J Clin Oncol 34(suppl; abstr 3568) [Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting 2016]
He J, Hu Y, Hu M, Li B (2015) Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep 5:13110. doi:10.1038/srep13110
Di Caro G, Marchesi F, Laghi L, Grizzi F (2013) Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med 17(9):1088–1095. doi:10.1111/jcmm.12117
Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, Hersey P, Scolyer RA (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 28(3):245–253. doi:10.1111/pcmr.12340
Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, Hodi FS, Choueiri TK, McDermott DF, Freeman GJ, Signoretti S (2015) Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 3(10):1158–1164. doi:10.1158/2326-6066.CIR-15-0043
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74(3):665–674. doi:10.1158/0008-5472.CAN-13-0992
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211(5):781–790. doi:10.1084/jem.20131916
Colussi D, Brandi G, Bazzoli F, Ricciardiello L (2013) Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci 14(8):16365–16385. doi:10.3390/ijms140816365
Kocarnik JM, Shiovitz S, Phipps AI (2015) Molecular phenotypes of colorectal cancer and potential clinical applications. Gastroenterol Rep (Oxf) 3(4):269–276. doi:10.1093/gastro/gov046
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128. doi:10.1126/science.aaa1348
Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25(10):1935–1940. doi:10.1093/annonc/mdu242
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by a Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Republic of Korea (NRF-2016R1D1A1B03931744).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, K.S., Kwak, Y., Ahn, S. et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother 66, 927–939 (2017). https://doi.org/10.1007/s00262-017-1999-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1999-6