Abstract
Background
Lobular neoplasia (LN) represents a spectrum of atypical proliferative lesions, including atypical lobular hyperplasia and lobular carcinoma-in-situ. The need for excision for LN found on core biopsy (CB) is controversial. We conducted a prospective multi-institutional trial (TBCRC 20) to determine the rate of upgrade to cancer after excision for pure LN on CB.
Methods
Patients with a CB diagnosis of pure LN were prospectively identified and consented to excision. Cases with discordant imaging and those with additional lesions requiring excision were excluded. Upgrade rates to cancer were quantified on the basis of local and central pathology review. Confidence intervals and sample size were based on exact binomial calculations.
Results
A total of 77 of 79 registered patients underwent excision (median age 51 years, range 27–82 years). Two cases (3 %; 95 % confidence interval 0.3–9) were upgraded to cancer (one tubular carcinoma, one ductal carcinoma-in-situ) at excision per local pathology. Central pathology review of 76 cases confirmed pure LN in the CB in all but two cases. In one case, the tubular carcinoma identified at excision was also found in the CB specimen, and in the other, LN was not identified, yielding an upgrade rate of one case (1 %; 95 % CI 0.01–7) by central pathology review.
Conclusions
In this prospective study of 77 patients with pure LN on CB, the upgrade rate was 3 % by local pathology and 1 % by central pathology review, demonstrating that routine excision is not indicated for patients with pure LN on CB and concordant imaging findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lobular neoplasia (LN) is a term used to encompass a spectrum of atypical proliferative lesions that includes atypical lobular hyperplasia (ALH) and lobular carcinoma-in-situ (LCIS). LCIS was initially considered to be a form of mammary carcinoma and as such was treated with mastectomy.1 However, long-term results of clinical follow-up studies suggested that LCIS and ALH represented markers of generalized increased breast cancer risk, not true precursor lesions, and surgery fell out of favor.2 – 4 Patients with ALH have a relative risk of breast cancer that is 4- to 5-fold higher than the general population, and patients with LCIS have a relative risk as high as 8- to 10-fold.
LN lacks a distinct radiographic correlate, and thus it is frequently diagnosed as an incidental finding in otherwise benign breast biopsy specimens, with a reported incidence ranging from 0.3 to 4 %.3,5 – 9 In the era of image-guided core biopsy (CB), a diagnosis of LN led to concern that the imaging abnormality in question was potentially undersampled, and numerous studies have evaluated the upgrade rate to ductal carcinoma-in-situ (DCIS) or invasive breast cancer after surgical excision of LN.9,10 These rates vary widely, with some studies reporting no upgrades and others reporting upgrades in up to 50 % of patients.11 – 13 Unfortunately, most early studies were retrospective and described small numbers of patients, not all of whom underwent excision.6,8,11 – 18 In some studies, LN in the CB was accompanied by another lesion, which by itself would warrant excision (such as atypical ductal hyperplasia), and in some reports, cases with pleomorphic LCIS or radiographic-pathologic discordance (e.g., ALH or LCIS on CB but a highly suspicious mass on imaging studies) were included.8,14,19 With the exception of a study by Irfan and Brem, many of the earlier studies also failed to report the Breast Imaging Reporting and Data System (BI-RADS) classification of the initial imaging lesion.6 – 8,11 – 21
Given the limitations of the available literature, current National Comprehensive Cancer Network guidelines remain conservative and recommend excision after a CB diagnosis of LCIS.22 However, among providers, this issue remains a matter of debate. The purpose of this study was to determine the upgrade rate to DCIS or invasive cancer after a CB diagnosis of pure LN in a single-arm multicenter prospective study.
Materials and Methods
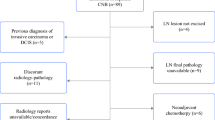
The protocol received institutional review board approval at the Dana Farber/Harvard Cancer Center in November 2004 and was expanded in 2012 to included five additional sites in the Translational Breast Cancer Research Consortium (TBCRC) (Table 1). Eligibility criteria are listed in Fig. 1. In all cases, the breast imaging-detected lesion had a BI-RADS score of 4 or lower, and all pathologic diagnoses of LN were deemed concordant with imaging findings. Image-guided core biopsies were performed as per institutional standards; neither device type nor gauge were standardized for the study. Eligible patients signed informed consent, completed registration, and underwent surgical excision. Hematoxylin and eosin-stained sections (and in some cases immunostains for E-cadherin) were evaluated by the local pathologist to establish the diagnosis and subsequently underwent central pathology review by two breast pathologists (L.G., S.J.S.).
Statistical Considerations
The primary objective was to estimate the frequency with which LN found on core needle biopsy (by local pathology report) was upgraded to invasive breast cancer or DCIS on surgical excision. We aimed to accrue 78 patients based on the desire to use a decision rule with the power to differentiate between a true upgrade rate of 5 versus 15 %. The protocol-specified decision rule stated that if ≤7 of 78 patients were upgraded, we would conclude that the chance of upgrade on excision was too low to continue research on this subject (and that we would not recommend surgical excision for LN). Conversely if ≥8 patients were upgraded, we would continue to investigate which patients required surgical excision. With this design, if the true probability of upgrade was 5 %, there would be a 4 % chance that we would continue research on this issue, and if the true probability of an upgrade was 15 %, there would be a 91 % chance that we would continue to do research on this issue. This decision rule and the calculation of 95 % confidence intervals were based on the exact binomial distribution.
Results
In total, 79 patients were registered on the study. At the time the 78th patient was registered, it was known that one of the previously registered patients was unable to undergo surgical excision due to concomitant illness; the accrual of the 79th patient was thus permitted. An additional patient cancelled surgery and rescheduled to a date long after her CB, prompting her treating physician to remove her from the study. Thus, 77 of 79 patients who underwent surgical excision per protocol guidelines were included in this analysis. Patient characteristics of the 77 eligible participants are listed in Table 2. Median patient age was 51 years (range 27–82 years), and median time between CB and surgical excision was 2 months (range 0.5–15 months). Per local pathology interpretation, the CB diagnosis was ALH in 49 patients (64 %), LCIS in 17 (22 %), and both ALH and LCIS in 11 (14 %) (Table 3).
Primary End Point
On the basis of local pathology review, the diagnosis of 2 of 77 patients was upgraded after surgical excision: one patient was found to have a grade I invasive tubular carcinoma (patient 6), and another patient was found to have intermediate nuclear grade DCIS (patient 59). Therefore, the upgrade rate based on the local pathology diagnosis was 2 (3 %) of 77 (95 % CI 0.3–9). The case upgraded to tubular carcinoma (patient 6) represented an magnetic resonance imaging–guided CB diagnosis of LCIS. The biopsy was performed for an area of abnormal enhancement in a 49-year-old patient undergoing magnetic resonance imaging screening because of a strong family history of breast cancer. In the case upgraded to DCIS, the original mammographic abnormality represented an area of calcifications spanning 8 mm in a 49-year-old woman with distant family history of postmenopausal breast cancer. The CB revealed LCIS, and the subsequent excision demonstrated solid, cribriform, and micropapillary DCIS of intermediate nuclear grade. Of the 18 excision slides, DCIS was present in 3 slides, one of which had CB site changes.
Central Pathology Review
CB slides were available for central pathology review in 76 of 77 cases, and LN was confirmed in 74 cases (96 %). In one case (patient 16), the local pathology report diagnosed LCIS in the CB; however, no LCIS (or ALH) was identified on central pathology review, and patient 6 (whose disease was upgraded to tubular carcinoma at excision per local pathology) was found to harbor a focus of tubular carcinoma in the CB specimen that was similar to that identified in the surgical excision. In a post hoc analysis, based on the 74 cases for which LN and absence of cancer in the CB could be confirmed by central pathology review, there was only 1 upgraded case (1 %, 95 % CI 0.01–7).
Discussion
Although many studies have looked at upgrade rates to carcinoma after excision of LN, various methodologic issues, including their retrospective design with inherent selection bias, failure to describe the BI-RADS classification and/or radiographic-pathologic concordance, and inclusion of pleomorphic LCIS, have made it difficult to interpret the findings. Table 4 summarizes the findings of the more contemporary, larger (comprising more than 20 cases) series on this subject and demonstrates that upgrade rates remain highly variable, ranging from 0 to 27 %.7 , 14,19 – 21,23 – 31 In our prospective study with strict eligibility criteria and central pathology review, we found an upgrade rate for concordant, BI-RADS 4 or lower, pure LN lesions to be 3 % based on the local pathology diagnosis and 1 % based on central pathology review. These findings are in agreement with more recent studies reporting the presence of concordance for the LN lesions in question, and they demonstrate that routine excision for a concordant CB diagnosis of pure LN is not warranted.
Lewis et al. reviewed 201 cases of LN and reported an upgrade rate of 13 %; however, BI-RADS and/or pathologic-radiographic concordance information for these cases was not available.26 Niell et al. reported an upgrade rate of 11 % in 63 concordant LN lesions.28 Shah-Khan et al., however, reported an upgrade rate of 2 % among 101 LN lesions, 90 % of which were concordant with imaging findings.30 Rendi et al. reported three upgrades (4 %) among 68 BI-RADS 4 lesions, without specific concordance information provided.29 Forty of these patients were designated as high risk on the basis of their family history, and all three upgrades were in high-risk patients. Although pathologic-radiographic concordance is likely more important than pure BI-RADS classification, studies reporting both have consistently reported the lowest upgrade rates. Atkins et al. reported no upgrades among 38 concordant lesions.23 Chaudhary et al. reported an upgrade rate of 3 % among 87 concordant lesions.25 Finally, similar to our study, Murray et al. conducted a prospective study of 80 excisions after a CB diagnosis of pure LN and reported two upgrades (3 %) among 72 concordant cases.32 In contrast, in the discordant group, the upgrade rate was 3 (38 %) of 8. Among the three upgraded discordant cases, two were thought to have had insufficient sampling of the target lesion by CB, both of which were upgraded to DCIS, and one represented a spiculated mass, classified as BI-RADS 5, which was upgraded to invasive carcinoma.
As demonstrated here and in other studies of LN, there is a degree of interobserver variability for a diagnosis of ALH versus LCIS, and the distinction is to some degree subjective and dependent on the amount of submitted material. In a series of breast specimens sent for formal consultation at the Universidade Federal de Minas Gerais School of Medicine in Brazil, Gomes et al. reported agreement for a diagnosis of ALH in 8 (47 %) of 17 cases and an agreement for a diagnosis of LCIS in 31 (69 %) of 45 cases.33 In our series of CB specimens, among 49 cases diagnosed as ALH by the local pathologist, there was agreement by central pathology review in 46 cases (94 %), and among 28 cases diagnosed as LCIS, with or without ALH, there was agreement in 14 cases (50 %). Although clinical follow-up studies demonstrate a greater magnitude of subsequent breast cancer risk associated with LCIS than with ALH, clinical management algorithms remain the same; therefore, the clinical significance of the distinction between these two lesions on CB may be less relevant. Patients with ALH and LCIS should be counseled regarding their cumulative increased risk of subsequent breast cancer, which is conferred equally to both breasts and significantly reduced with the use of chemoprevention.34
Our study, with its unique prospective design, predetermined statistical end points, strict eligibility criteria, and central pathology review, represents the most comprehensive evaluation of this subject to date. Although the confidence intervals for the upgrade rates in our study range from 0 to 9 %, the predetermined statistical end points were clear, and our goal was to differentiate between a true upgrade rate of 5 versus 15 %. Considering that a BI-RADS 3 designation implies a less than 2 % risk of upgrade to a cancer diagnosis, our findings suggest that a concordant CB diagnosis of LN is amenable to follow-up imaging at 6 months rather than excision, as the upgrade rates are similar.35 Prospective data to document the yield of 6-month follow-up imaging in patients with concordant diagnosis of pure LN on CB are not available, yet it is our opinion that this practice should be considered in the management algorithms for patients with pure LN who do not undergo excision to allow multidisciplinary groups to collect this information and report their outcomes.
In conclusion, in this prospective multi-institutional study of patients with BI-RADS 4 or lower concordant lesions with LN on CB, we found a very low upgrade rate to invasive cancer or DCIS on excision. These results indicate that routine excision after a diagnosis of LN on CB is not indicated. Patients with a concordant CB diagnosis of pure LN should be counseled regarding their increased lifetime risk of breast cancer and offered participation in high-risk surveillance programs, including a full discussion of lifestyle interventions (diet, exercise) as well as the risks and benefits of chemoprevention.
References
Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17:491
Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100:238–44.
Hoda SA (2001) Lobular carcinoma in situ and atypical lobular hyperplasia. In: Rosen PP, editor Rosen’s breast pathology, 2nd ed. Lippincott Williams & Wilkins, Philiadelphia, PA, pp. 581–618.
Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW Jr. Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2:225–51.
Beute BJ, Kalisher L, Hutter RV. Lobular carcinoma in situ of the breast: clinical, pathologic, and mammographic features. AJR Am J Roentgenol. 1991;157:257–65.
Bauer VP, Ditkoff BA, Schnabel F, Brenin D, El-Tamer M, Smith S. The management of lobular neoplasia identified on percutaneous core breast biopsy. Breast J. 2003;9:4–9.
Foster MC, Helvie MA, Gregory NE, Rebner M, Nees AV, Paramagul C. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004;231:813–9.
Liberman L, Sama M, Susnik B, et al. Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings. AJR Am J Roentgenol. 1999;173:291–9.
O’Driscoll D, Britton P, Bobrow L, Wishart GC, Sinnatamby R, Warren R. Lobular carcinoma in situ on core biopsy—what is the clinical significance? Clin Radiol. 2001;56:216–20.
Londero V, Zuiani C, Linda A, Vianello E, Furlan A, Bazzocchi M. Lobular neoplasia: core needle breast biopsy underestimation of malignancy in relation to radiologic and pathologic features. Breast. 2008;17:623–30.
Renshaw AA, Cartagena N, Derhagopian RP, Gould EW. Lobular neoplasia in breast core needle biopsy specimens is not associated with an increased risk of ductal carcinoma in situ or invasive carcinoma. Am J Clin Pathol. 2002;117:797–9.
Dmytrasz K, Tartter PI, Mizrachy H, Chinitz L, Rosenbaum Smith S, Estabrook A. The significance of atypical lobular hyperplasia at percutaneous breast biopsy. Breast J. 2003;9:10–2.
Middleton LP, Grant S, Stephens T, Stelling CB, Sneige N, Sahin AA. Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol. 2003;16:120–9.
Arpino G, Allred DC, Mohsin SK, Weiss HL, Conrow D, Elledge RM. Lobular neoplasia on core-needle biopsy—clinical significance. Cancer. 2004;101:242–50.
Crisi GM, Mandavilli S, Cronin E, Ricci A Jr. Invasive mammary carcinoma after immediate and short-term follow-up for lobular neoplasia on core biopsy. Am J Surg Pathol. 2003;27:325–33.
Irfan K, Brem RF. Surgical and mammographic follow-up of papillary lesions and atypical lobular hyperplasia diagnosed with stereotactic vacuum-assisted biopsy. Breast J. 2002;8:230–3.
Shin SJ, Rosen PP. Excisional biopsy should be performed if lobular carcinoma in situ is seen on needle core biopsy. Arch Pathol Lab Med 2002;126(6):697−701
Yeh IT, Dimitrov D, Otto P, Miller AR, Kahlenberg MS, Cruz A. Pathologic review of atypical hyperplasia identified by image-guided breast needle core biopsy. Correlation with excision specimen. Arch Pathol Lab Med. 2003;127:49–54.
Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21:1208–16.
Bonnett M, Wallis T, Rossmann M, et al. Histopathologic analysis of atypical lesions in image-guided core breast biopsies. Mod Pathol. 2003;16:154–60.
El-Sheikh TM, Silverman JF. Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol. 2005;29:534–43.
Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:448–75.
Atkins KA, Cohen MA, Nicholson B, Rao S. Atypical lobular hyperplasia and lobular carcinoma in situ at core breast biopsy: use of careful radiologic-pathologic correlation to recommend excision or observation. Radiology. 2013;269:340–7.
Cangiarella J, Guth A, Axelrod D, et al. Is surgical excision necessary for the management of atypical lobular hyperplasia and lobular carcinoma in situ diagnosed on core needle biopsy? A report of 38 cases and review of the literature. Arch Pathol Lab Med. 2008;132:979–83.
Chaudhary S, Lawrence L, McGinty G, Kostroff K, Bhuiya T. Classic lobular neoplasia on core biopsy: a clinical and radio-pathologic correlation study with follow-up excision biopsy. Mod Pathol. 2013;26:762–71.
Lewis JL, Lee DY, Tartter PI. The significance of lobular carcinoma in situ and atypical lobular hyperplasia of the breast. Ann Surg Oncol. 2012;19:4124–8.
Nagi CS, O’Donnell JE, Tismenetsky M, Bleiweiss IJ, Jaffer SM. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112:2152–8.
Niell B, Specht M, Gerade B, Rafferty E. Is excisional biopsy required after a breast core biopsy yields lobular neoplasia? AJR Am J Roentgenol. 2012;199:929–35.
Rendi MH, Dintzis SM, Lehman CD, Calhoun KE, Allison KH. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol. 2012;19:914–21.
Shah-Khan MG, Geiger XJ, Reynolds C, Jakub JW, Deperi ER, Glazebrook KN. Longterm follow-up of lobular neoplasia (atypical lobular hyperplasia/lobular carcinoma in situ) diagnosed on core needle biopsy. Ann Surg Oncol. 2012;19:3131–8.
Zhao C, Desouki MM, Florea A, Mohammed K, Li X, Dabbs D. Pathologic findings of follow-up surgical excision for lobular neoplasia on breast core biopsy performed for calcification. Am J Clin Pathol. 2012;138:72–8.
Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M, Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119:1073–9.
Gomes DS, Porto SS, Balabram D, Gobbi H. Inter-observer variability between general pathologists and a specialist in breast pathology in the diagnosis of lobular neoplasia, columnar cell lesions, atypical ductal hyperplasia and ductal carcinoma in situ of the breast. Diagn Pathol. 2014;9:121.
Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136:627–33.
Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013;269:701–12.
Acknowledgment
We are grateful for the funding support to the TBCRC from the AVON Foundation, the Breast Cancer Research Foundation, and Susan G. Komen.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Stuart J. Schnitt and Dr. Tari A. King share senior authorship of this article.
Rights and permissions
About this article
Cite this article
Nakhlis, F., Gilmore, L., Gelman, R. et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann Surg Oncol 23, 722–728 (2016). https://doi.org/10.1245/s10434-015-4922-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4922-4