Abstract
Background
Atypical lobular hyperplasia (ALH) and classic lobular carcinoma in situ encompass a spectrum of proliferative lesions known as lobular neoplasia (LN). When imaging-concordant and found in isolation on core needle biopsy (CNB), LN infrequently upgrades to carcinoma on surgical excision, and routine excision is not indicated. Upgrade rates in the setting of synchronous carcinoma are not well studied.
Patients and Methods
Patients with radiology–pathology concordant synchronous LN and separately biopsied ipsilateral (n = 35) or contralateral (n = 15) carcinoma who underwent excision between 2010 and 2021 were retrospectively identified. Frequency of upgrade, to either invasive or in situ carcinoma, was quantified, and factors associated with upgrade were assessed using Fisher’s exact test.
Results
The median age was 55 (range 33–74) years. The upgrade rate of LN was 6% and not significantly different between ipsilateral (2.9%) and contralateral (13.3%) carcinoma (p = 0.15). All upgraded LN lesions were ALH on CNB and detected as non-mass enhancement on magnetic resonance imaging (MRI). No additional disease was demonstrated after excision at the site of the original LN CNB in 22.9% (8 out of 35) of ipsilateral and 13.3% (2 out of 15) of contralateral patients. Upgrade was not associated with family history, menopausal status, imaging modality used to detect LN, or extent of LN on CNB (p > 0.05).
Conclusions
Our results demonstrate a low upgrade rate (6%) in our study cohort of LN with synchronous ipsilateral or contralateral carcinoma, which suggests that not all LN mandates excision with synchronous carcinoma. Larger, multi-institution studies are needed to validate these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lobular neoplasia (LN) encompasses a spectrum of proliferative breast lesions including atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS). Nonvariant subtypes of ALH and LCIS are found incidentally in approximately 1–4% of core needle biopsy (CNB) specimens.1,2,3,4 In 1941, Foote and Stewart described LCIS as a noninvasive lesion arising from the lobules and terminal ducts, but believed it was premalignant and therefore recommended bilateral mastectomy as treatment.5 Further research has increased the understanding of the natural history and risk associated with LN, and what was previously considered an obligate precursor is now better understood as a high-risk lesion or nonobligate precursor. This has led to significant changes in management.
Excision is recommended when there is radiologic–pathologic discordance or variant subtypes of LN when found in isolation, as these lesions have upgrade rates ranging from 25% to 65%.6,7,8,9 However, as demonstrated in a recent meta-analysis, when isolated, classic LN is evaluated with imaging concordance, the risk of upgrade is relatively low: 2.5% for ALH and 5.8% for LCIS.10 These data support shifting management from routine to elective excision, with imaging surveillance and chemoprevention offered as alternatives to surgery. However, recommendations for nonsurgical management do not extend to individuals with synchronous invasive carcinoma or ductal carcinoma in situ (DCIS), as the frequency of LN upgrade is felt to be unacceptably high.11
Despite this concern, the upgrade rate of LN in the setting of synchronous carcinoma is not well defined. Several studies evaluating upgrade of isolated LN have included patients with either a history of, or a current, synchronous ipsilateral or contralateral breast cancer, but the inclusion criteria are not uniform and upgrade rates range from 0 to 44% (Table 1).12,13,14,15,16,17 Routine excision of LN when found with synchronous carcinoma may lead to more frequent mastectomy or unnecessary excisional biopsies that may compromise cosmetic outcomes. We hypothesized that the upgrade rate of radiology–pathology concordant LN in the setting of synchronous carcinoma would mirror the rate for LN found in isolation, which may impact recommendations for management of LN found with synchronous carcinoma.
Patients and Methods
We retrospectively queried the University of Washington pathology database for all core needle biopsies that contained LN, either ALH or classic LCIS, between 2010 and 2021. Cases were reviewed, and those with both LN and synchronous ipsilateral or contralateral DCIS or invasive carcinoma (hereafter, both are referred to as breast carcinoma) formed the study cohort. Surgery followed CNB for all patients within 3 months. For ipsilateral patients, at least two separate biopsies, one of which demonstrated isolated LN and the other carcinoma, were necessary for inclusion. Ipsilateral cases were not included if the LN lesion was contiguous with the carcinoma, either on imaging or pathology. There was not a specific distance separating two lesions on imaging that was required for inclusion. Instead, the assumption was made that if the lesions were far enough apart to recommend a second biopsy, the treating surgeon determined that a second biopsy may alter the surgical approach or clinical management. The mean imaging distance between lesions was 5.1 cm. Exclusion criteria included radiology–pathology discordance or inability to determine concordance, presence of atypical ductal hyperplasia, neoadjuvant chemotherapy, LN co-located with carcinoma on CNB specimen, unexcised LN, personal history of breast carcinoma, or if final upgrade could not be determined owing to lack of information in the pathology report and/or inability to obtain final surgical specimen for review.
Evaluation of Radiologic–Pathologic Concordance
Radiology–pathology concordance was reviewed by a fellowship-trained breast radiologist (K.P.L.) blinded to upgrade outcome. Cases were deemed concordant if the lesion was determined to be appropriately targeted on the basis of intra- and postprocedure imaging, had imaging features consistent with classic LN, or had other benign entities present on histopathology that accounted for the imaging features (including but not limited to fibrocystic changes, fibroadenomatoid change, and/or pseudoangiomatous stromal hyperplasia).
Pathology Review
All diagnostic core needle biopsy specimens were reviewed at the time of initial diagnosis by at least one breast fellowship-trained pathologist at our institution following the criteria of Page et al. for ALH and LCIS.18 Upgrade was determined by either review of pathology reports or, if the pathology report did not specify upgrade at the original LN CNB site, slides were rereviewed by a fellowship-trained breast pathologist (E.U.P.). For instance, if multiple lesions were targeted in one lumpectomy specimen but the area specific to the LCIS biopsy site was not documented as an upgrade/no upgrade in the pathology report, this case was re-reviewed matching the type of biopsy clip and imaging findings to determine upgrade at the site of LN biopsy. Similarly, mastectomy specimens were reviewed as most pathology reports did not comment specifically on upgrade at the site of the original LN biopsy. Cases were considered an upgrade if DCIS or invasive carcinoma was identified at or directly adjacent to CNB site changes.
Statistical Analysis
Demographic, clinical, radiographic, and pathologic variables were identified by retrospective chart review. Frequency of upgrade, to either invasive or in situ carcinoma, was quantified, and factors associated with upgrade were assessed using Fisher’s exact test. All statistical analyses were conducted using Stata version 15.0. This study was approved by the University of Washington Institutional Review Board as a minimal risk study with waiver of consent.
Results
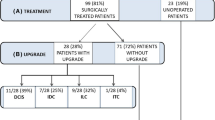
We identified 787 patients diagnosed with LN on CNB between 2010 and 2021; 698 were excluded because they had either CNB with LN but no synchronous carcinoma, or LN and carcinoma mixed within the same CNB (Fig. 1). A total of 89 patients met eligibility criteria of isolated LN on CNB and synchronous ipsilateral or contralateral breast carcinoma. Of these, 15 were excluded for radiology–pathology discordance or inability to assess concordance. An additional nine patients were excluded because the original biopsy site could not be evaluated, or patients did not consent to having pathology used for research. Patients were also excluded if they had a history of breast cancer (n = 5) or they underwent neoadjuvant chemotherapy (n = 6). Four patients were excluded because lobular neoplasia was not excised. One of these patients developed distant disease, but there were no local recurrence events with a median follow-up of 5.5 years. The final analytic cohort comprised 35 individuals with ipsilateral and 15 patients with contralateral carcinoma.
In the entire cohort, ALH was the most common LN lesion (37 of 50 patients, 74%) followed by LCIS (13 of 50 patients, 36%). The median age was 55 years. There were no significant differences among those who did or did not upgrade with regard to age, race, family history, menopausal status, imaging modality that led to CNB, or extent of LN on CNB. There was a significant association with type of synchronous carcinoma; the majority of synchronous carcinoma was invasive ductal carcinoma (46.8%), however, no upgrades were seen with this group (Table 2). When stratified by ipsilateral or contralateral cases, there was a significant association with race and type of synchronous carcinoma in the ipsilateral cases (Supplementary Table). Of the two Black patients with ipsilateral LN and carcinoma, one upgraded (50%) compared with 82.4% of white patients who did not upgrade.
LN was most commonly detected by magnetic resonance imaging (MRI) (39 of 50 cases, 78%), followed by mammography (10 of 50 cases, 20%) and then ultrasound (1 of 50 cases, 2%) (Table 2). Of the 39 LN lesions identified by MRI, the majority (22 of 39 cases, 56%) were defined as non-mass enhancement. None of the LN initially identified on MRI were seen on diagnostic mammogram, and 7.7% (3 of 39 cases) were seen on second look ultrasound. For the ipsilateral cases there were 10 masses seen on MRI that were considered concordant on the basis of pathology that demonstrated fibroadenomatoid changes, stromal fibrosis, and papillomas. In comparison with LN, a mammographic abnormality was apparent in 92% of patients with carcinoma. The majority of LN CNB (80%) took place after the biopsy for the primary carcinoma. In two cases, the lobular neoplasia was identified first, and in eight cases the CNB for both the LN and carcinoma occurred on the same date.
The upgrade rate for the entire cohort was 6%. Upgrade in the setting of ispsilateral carcinoma (1 of 35 cases, 2.9%) was lower than contralateral (2 of 15 cases, 13.3%), but this did not reach statistical significance (p = 0.15). All upgraded LN demonstrated ALH with < 4 terminal duct lobular units (TDLU) on the original CNB (Table 3). All cases were identified as non-mass enhancement on MRI, and not seen on any other imaging modality. The synchronous carcinoma histologic subtype matched the LN upgrade type for the contralateral cases. Among 15 patients who underwent excisional biopsy for contralateral ALH, there was one case of DCIS (6.6%) and one case of invasive lobular carcinoma (6.6%). For the single ipsilateral upgrade, the synchronous carcinoma was a mucinous carcinoma whereas the LN upgrade was invasive mammary carcinoma (mixed invasive ductal and lobular with heterogeneous loss of e-cadherin) (Table 3).
All upgraded LN lesions were pT1 with one contralateral case demonstrating multifocal invasive lobular carcinoma that was also pN0(i+). One of the ipsilateral cases also had axillary isolated tumor cells. A morphologic assessment of the isolated tumor cells was not performed to determine whether they represented the primary (mucinous) or the upgraded (mammary) carcinoma. In the second contralateral case, there was no lymph node assessment given that the upgrade was DCIS only. There was no additional disease demonstrated after excision at the site of the original LN CNB in 22.9% (8 of 35 cases) of ipsilateral and 13.3% (2 of 15 cases) of contralateral patients (Fig. 2). Atypical ductal hyperplasia was identified in 13.3% (2 of 15 cases) of the contralateral cases.
In patients with synchronous ipsilateral carcinoma, 15 (42.9%) underwent a mastectomy, 5 (14.3%) underwent a lumpectomy that included both the carcinoma and LN, and 15 (42.9%) underwent a lumpectomy and excisional biopsy. Among the contralateral patients with LN, four (26.7%) patients underwent mastectomy, and the remainder underwent excisional biopsy for the LN.
Discussion
Current research supports selective nonoperative management of imaging-concordant isolated LN with follow-up imaging surveillance and the option of chemoprevention, but there is limited information to guide management of LN that is found with synchronous carcinoma. To our knowledge, this is the largest and only study specifically attempting to understand the frequency of radiologic–pathologic concordant LN upgrade in the setting of synchronous ipsilateral and contralateral carcinoma. We found an overall upgrade rate of 6%, approximately 3% for ipsilateral and 13% for contralateral LN, which are similar to upgrade rates seen in isolated LN.
There are several recent studies with radiologic–pathologic concordance that have analyzed upgrade rate of LN with synchronous carcinoma as a subset of the larger cohort of LN (Table 1). We evaluated studies published within the past 10 years that included clear criteria for concordance evaluation and separately reported upgrade rates for those with synchronous carcinoma. For instance, Hwang et al. included synchronous carcinoma but did not include separate upgrade rates for these patients, and therefore this study was not included here.12 The studies that included ipsilateral synchronous carcinomas demonstrated upgrade rates ranging from 0 to 44%14,16,17 with similar upgrade rates in studies including contralateral cases (0–33%).3,14,15,16,17 Although the inclusion criteria for each of these studies are relatively similar, differences exist with respect to method of detection, radiology–pathology concordance assessment, and rates of surgical excision, which may account for this wide variability in upgrade rate.
For most of these studies, the imaging finding that initially detected the LN lesion was calcifications on mammogram (40–82%), which differs significantly from our study where 78% of LN was detected on MRI. Khoury et al. specifically evaluated LN detected on MRI and found a high rate of upgrade (44%) among ipsilateral lesions, but no upgrades in the contralateral cases. For the upgraded cases, three of four (75%) were detected as non-mass enhancement, which was also the most common imaging finding that led to the LN biopsy. All three of the upgraded cases in our study were initially detected as non-mass enhancement on MRI, but this was also the most common MRI finding, and therefore NME was not associated with increased risk of upgrade. Likely owing to the small numbers within each study, none were able to demonstrate demographic, radiologic, or pathologic factors associated with upgrade.
Interestingly, several studies did conclude that upgrades following excision of CNB were higher with a history of breast cancer or synchronous carcinoma. Pride et al. only evaluated upgrade of LCIS, demonstrating that patients with concurrent contralateral breast cancer were four times more likely to upgrade than those without contralateral breast cancer (odds ratio 4.41, 95% confidence interval: 1.06–17.38, p = 0.04).15 Muller et al. found a nonsignificant but higher likelihood of upgrade when there was a history of breast cancer and synchronous breast cancer compared with none (7.7% versus 1.6%, p = 0.20), but did not specify between history and concurrent diagnosis.14 In Chaudhary et al., two of three upgrades were in patients with concurrent breast carcinomas.16 With different LN detection methods, small numbers of qualifying patients (n = 12–28 inclusive of both ipsilateral and contralateral cases), including those with a history of carcinoma and synchronous carcinoma and often not distinguishing between the two, no commentary regarding neoadjuvant therapy, and limited upgrades (n = 1 in most subgroups), it is difficult to directly compare these data with ours.
Numerous previous studies have established that the upgrade risk is lower with ALH than with LCIS,10 and the majority of upgraded LN lesions in the aforementioned studies were diagnosed as LCIS on CNB.3,15,16,17 Our CNB cohort consisted largely of ALH (74%), and all three upgraded LN lesions were ALH on initial needle biopsy. Khoury et al. did have one upgrade associated with ALH, and Muller et al., whose study was restricted to only ALH, demonstrated an ALH upgrade in both an ipsilateral and contralateral case.14,17 Although guidelines are adhered to with respect to diagnostic criteria, there is interobserver variability in diagnosing ALH versus LCIS, which could account for some of these differences across institutions. It has also been demonstrated that extensive involvement of the terminal duct lobular unit (TDLU) is associated with higher risk of upgrade,19,20 but in the current study, all three upgraded ALH lesions showed limited involvement of < 4 TDLU. Similarly, Chaudhary et al. did not show a correlation between the extent of lobular neoplasia involvement and lesion upgrade, although this was reported for all cases in the study, not synchronous cases alone.16
In this cohort, 42.9% of women with LN on CNB and a synchronous ipsilateral carcinoma underwent mastectomy, which is slightly higher than a recent study from the National Cancer Database demonstrating that 37.8% of women in the USA underwent mastectomy in 2011.21 It is also substantially higher than the proportion of women (26.7%) who underwent mastectomy when their synchronous carcinoma was contralateral. Given the retrospective nature of this study, we do not have insight into the decision-making process between patient and provider, but it is reasonable to assume that there were concerns for cosmesis if both a lumpectomy and excisional biopsy needed to be performed, and that a patient’s anxiety may have been increased by the need for subsequent CNB after their cancer diagnosis. These factors in part may have contributed to a higher proportion of women undergoing mastectomy in the ipsilateral cases.
Although motivations for mastectomy are multifactorial, it is significant to note that in this study all patients received a breast MRI for extent of disease assessment, and upgraded cases were identified on MRI only. Primary breast lesions were either DCIS or T1c invasive carcinoma, but one of the contralateral cases was multifocal and associated with isolated tumor cells. These findings largely align with data from a systematic review and meta-analysis that found 35.1% of MRI-detected cancers were ductal carcinoma in situ, and 64.9% were invasive cancers, the majority of which were pT1 and node negative.22 Two subsequent meta-analyses have shown that preoperative MRI significantly increases the odds of receiving mastectomy for breast cancer treatment, and does not improve local recurrence or distant recurrence-free survival.23,24 Despite this, the use of preoperative MRI has increased,25,26 and there will be ongoing necessity to study whether there are any specific MRI characteristics that would allow for improved and more accurate classification of these MRI-detected LN lesions.
Limitations of this study include that it is a retrospective, single-institution study with a relatively limited number of patients. Many of the initial CNB for the LN lesions were collected outside of our institution, and we do not have information available for some of these patients on the number of cores sampled or the gauge of biopsy devices used. Increased number of samples and larger gauge needles are associated with lower upgrade rates on surgical excision.27,28 Similar to the other studies discussed above, the number of upgraded lesions is small and a single upgrade can dramatically alter the upgrade rate. It is difficult to draw definitive conclusions in the setting of a small cohort with limited upgrades.
Conclusions
The overall upgrade rate for this cohort was relatively low (6%), which is similar to LN identified in isolation. If these findings were validated in a larger, multicenter study, a more selective approach to surgical management may be considered. This may decrease the rate of unnecessary mastectomies and improve aesthetic outcomes for patients.
References
Li CI, Anderson BO, Daling JR, Moe RE. Changing incidence of lobular carcinoma in situ of the breast. Breast Cancer Res Treat. 2002;75:259–68. https://doi.org/10.1023/a:1019950918046.
Oppong BA, King TA. Recommendations for women with lobular carcinoma in situ (LCIS). J Oncol. 2011;25(11):1051–6.
Shah-Khan MG, Geiger XJ, Reynolds C, Jakub JW, DePeri ER, Glazebrook KN. Long-term follow-up of lobular neoplasia (atypical lobular hyperplasia/lobular carcinoma in situ) diagnosed on core needle biopsy. Ann Surg Oncol. 2012;19(10):3131–8. https://doi.org/10.1245/s10434-012-2534-9.
Sen LQC, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. Am J Roentgenol. 2016;207(5):1132–45. https://doi.org/10.2214/AJR.15.15425.
Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17(4):491–496.3. https://doi.org/10.3322/canjclin.32.4.234.
Downs-Kelly E, Bell D, Perkins GH, Sneige N, Middleton LP. Clinical implications of margin involvement by pleomorphic lobular carcinoma in situ. Arch Pathol Lab Med. 2011;135(6):737–43. https://doi.org/10.5858/2010-0204-OA.1.
Khoury T, Karabakhtsian RG, Mattson D, et al. Pleomorphic lobular carcinoma in situ of the breast: clinicopathological review of 47 cases. Histopathology. 2014;64(7):981–93. https://doi.org/10.1111/his.12353.
Flanagan MR, Rendi MH, Calhoun KE, Anderson BO, Javid SH. Pleomorphic lobular carcinoma in situ: radiologic–pathologic features and clinical management. Ann Surg Oncol. 2015;22(13):4263–9. https://doi.org/10.1245/s10434-015-4552-x.
Wazir U, Wazir A, Wells C, Mokbel K. Pleomorphic lobular carcinoma in situ: current evidence and a systemic review (review). Oncol Lett. 2016;12(6):4863–8. https://doi.org/10.3892/ol.2016.5331.
Shehata MN, Rahbar H, Flanagan MR, et al. Risk for upgrade to malignancy after breast core needle biopsy diagnosis of lobular neoplasia: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(10):1207–19. https://doi.org/10.1016/j.jacr.2020.07.036.
Degnim AC, King TA. Surgical management of high-risk breast lesions. Surg Clin North Am. 2013;93(2):329–40. https://doi.org/10.1016/j.suc.2012.12.005.
Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: Routine excision is not necessary. Mod Pathol. 2008;21(10):1208–16. https://doi.org/10.1038/modpathol.2008.134.
Susnik B, Day D, Abeln E, et al. Surgical outcomes of lobular neoplasia diagnosed in core biopsy: prospective study of 316 cases. Clin Breast Cancer. 2016;16(6):507–13. https://doi.org/10.1016/j.clbc.2016.06.003.
Muller KE, Roberts E, Zhao L, Jorns JM. Isolated atypical lobular hyperplasia diagnosed on breast biopsy low upgrade rate on subsequent excision with long-term follow-up. Arch Path Lab Med. 2018;142(3):391–5. https://doi.org/10.5858/arpa.2017-0155-OA.
Pride RM, Jimenez RE, Hoskin TL, Degnim AC, Hieken TJ. Upgrade at excisional biopsy after a core needle biopsy diagnosis of classic lobular carcinoma in situ. Surgery. 2021;169(3):644–8. https://doi.org/10.1016/j.surg.2020.07.025.
Chaudhary S, Lawrence L, McGinty G, Kostroff K, Bhuiya T. Classic lobular neoplasia on core biopsy: A clinical and radio-pathologic correlation study with follow-up excision biopsy. Mod Pathol. 2013;26(6):762–71. https://doi.org/10.1038/modpathol.2012.221.
Khoury T, Kumar PR, Li Z, et al. Lobular neoplasia detected in MRI-guided core biopsy carries a high risk for upgrade: a study of 63 cases from four different institutions. Mod Pathol. 2016;29(1):25–33. https://doi.org/10.1038/modpathol.2015.128.
Page DL, Kidd TE Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22(12):1232–9. https://doi.org/10.1016/0046-8177(91)90105-x.
Rendi MH, Dintzis SM, Lehman CD, Calhoun KE, Allison KH. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol. 2012;19(3):914–21. https://doi.org/10.1245/s10434-011-2034-3.
Esserman LE, Lamea L, Tanev S, Poppiti R. Should the extent of lobular neoplasia on core biopsy influence the decision for excision? Breast J. 2007;13(1):55–61. https://doi.org/10.1111/j.1524-4741.2006.00363.x.
Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. https://doi.org/10.1001/jamasurg.2014.2895.
Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27(33):5640–9. https://doi.org/10.1200/JCO.2008.21.5756.
Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257(2):249–55. https://doi.org/10.1097/SLA.0b013e31827a8d17.
Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32(5):392–401. https://doi.org/10.1200/JCO.2013.52.7515.
Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use trends from 2000 to 2011. JAMA Intern Med. 2014;174(1):114–21. https://doi.org/10.1001/jamainternmed.2013.11958.
Wernli KJ, DeMartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med. 2014;174(1):125–32. https://doi.org/10.1001/jamainternmed.2013.11963.
Green S, Khalkhali I, Azizollahi E, Venegas R, Jalil Y, Dauphine C. Excisional biopsy of borderline lesions after large bore vacuum-assisted core needle biopsy-is it necessary? Am Surg. 2011;77(10):1358–60. https://doi.org/10.1177/000313481107701019.
Houssami N, Ciatto S, Ellis I, Ambrogetti D. Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer. 2007;109(3):487–95. https://doi.org/10.1002/cncr.22435.
Acknowledgements
This work was funded by a grant from the Kuni Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
This work was funded by a foundation grant from Kuni Foundation. This study was presented in poster format at the 2022 American Society of Breast Surgeons Annual Meeting, 6–10 April 2022. K.P.L. reports receiving a teaching honorarium from the Radiological Society of North America unrelated to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crary, I.L., Parker, E.U., Lowry, K.P. et al. Risk of Lobular Neoplasia Upgrade with Synchronous Carcinoma. Ann Surg Oncol 29, 6350–6358 (2022). https://doi.org/10.1245/s10434-022-12129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12129-4