Abstract
Purpose
This study was designed to determine the relationship of microcalcification morphology and distribution with clinical, histopathologic, biologic features, and local recurrence (LR) in patients with pure ductal carcinoma in situ (DCIS) of the breast.
Methods
All patients with pure DCIS who underwent preoperative mammography at our institution from 1996 through 2009 were identified. Mammographic findings were classified according to the ACR BI-RADS lexicon. Associations between mammographic findings and clinical, histopathologic, biologic characteristics, and LR were analyzed. Statistical inference used multiple logistic regression and Cox proportional hazards regression adjusted for age and confounding due to bias from nonrandomized selection of radiation therapy.
Results
We identified 1657 patients with microcalcifications visualized on mammography. The mean age at diagnosis was 55 years (SD, 11). The mean follow-up was 7 years (range 1–16). Ipsilateral LR was 4 % in segmentectomy (987) and 1.5 % in mastectomy (670) patients. Increased LR risk was seen in patients with dense breast tissue (p < 0.05) and larger DCIS size (p < 0.01). Radiation therapy was associated with a 2.8-fold decrease in the LR risk. Fine linear (branching) microcalcifications were associated with 5.2-fold increase in LR. Extremely dense breast tissue was associated with positive/close margins (p = 0.04) and multicentricity (p < 0.01). Younger women were more likely to have extremely dense breast tissue (p < 0.0001), multicentric disease (p < 0.0004), and undergo mastectomy (p < 0.0001).
Conclusions
Dense breast tissue, large DCIS size, and fine linear (branching) microcalcifications were associated with increased LR, yet overall LR rates remained low. Extremely dense breast tissue was a risk factor for multicentricity and positive margins in DCIS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The introduction of screening mammography has led to a significant increase in the number of patients diagnosed with pure ductal carcinoma in situ (DCIS). Before the widespread use of screening mammography, DCIS accounted for fewer than 5 % of breast cancers. Currently, DCIS accounts for 20–30 % of all breast cancers and 30–50 % of all mammographically detected breast cancers.1–5
There is a general consensus that DCIS represents a noninvasive nonobligate precursor of invasive breast cancer.6–8 Therefore, the early diagnosis and management of DCIS are critical to prevent the development of invasive cancer.9–12 Mammographic detection accounts for 90–95 % of DCIS, which presents as suspicious microcalcifications in 60–90 % of lesions.13–16
The survival of women with a diagnosis of DCIS is excellent, with no difference in long-term survival between patients who undergo mastectomy or breast-conserving surgery (BCS) with postoperative radiation therapy (XRT). Therefore, despite the low rates of treatment failure seen with mastectomy (1–2 % at 10 years), the standard management option is BCS with or without sentinel node biopsy and XRT, which has been reported to result in local treatment failure rates of 5–10 % at 10 years.17–20
It has been proposed that the biological aggressiveness of breast cancer also can be predicted by mammographic characteristics. Fine linear (branching) calcifications have been suggested to indicate higher risk of LR and poor long-term outcomes. However, these studies have been based mainly on reports of invasive breast cancer or DCIS with an invasive component.6,7,21–24
The purpose of this study was to determine the relationship between the morphology and distribution of microcalcifications on mammography and clinical, histopathologic, biologic features, and LR in patients with pure DCIS.
Materials and Methods
Patient Selection, Treatment, Clinical Assessment, and Follow-Up
Following institutional review board approval, our Breast Cancer Management System database was searched to identify all patients with a diagnosis of pure DCIS who underwent mammography and were treated at our institution during the period from January 1, 1996 through July 31, 2009. This is an expansion of our previous study, which included only patients with known ER status; in the current study, we included 692 additional patients who did not have ER status determined.25
Radiation therapy following BCS was routinely discussed with every patient who had DCIS and underwent surgery at our institution. For patients with lesions <1 cm and margins >3 mm and for postmenopausal women with low-grade disease, the possibility of observation instead of XRT, with or without tamoxifen, was discussed. In general, the decision whether or not to deliver XRT was made by consensus on the basis of a multidisciplinary conference input together with patient preference. After the diagnosis and treatment of DCIS, patients had yearly clinical examinations and yearly mammography with or without sonography.
Histopathologic Assessment of Microcalcifications
The original pathology slides from biopsy and/or surgery were reviewed by 1 of 11 dedicated breast pathologists with 5–25 years of experience and re-reviewed by a dedicated breast pathologist with 20 years of experience. When the original slides were not available for review, data were obtained through review of pathology reports. The following parameters were recorded: nuclear grade; presence of comedonecrosis; architectural pattern; DCIS size; and ER status, defined as positive if nuclear staining was present in at least 1 % of cells.
Imaging Assessment of Microcalcifications
Each mammogram (standard two-view with additional views as necessary) was originally read by 1 of 14 dedicated breast radiologists with 6–21 years of experience and was re-read for this study by 1 of 4 dedicated breast radiologists with 6–12 years of experience. When the original images were not available for review, data were obtained through review of mammography reports.
The American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS) lexicon, fifth edition, was used to classify all mammographic findings with respect to breast tissue density, masses, microcalcifications, architectural distortion, and focal asymmetry.26 Only patients with microcalcifications demonstrated on mammograms were included in this study. The DCIS size was defined as the maximum length of microcalcifications on mammography. Microcalcification morphology was classified as punctate/amorphous, coarse heterogeneous/fine pleomorphic, or fine linear (branching). Microcalcification distribution was classified as clustered/grouped, linear/segmental, or regional/diffuse. Multifocality was defined as two or more foci of disease in the same breast quadrant within 5 cm of one another. Multicentricity was defined as disease in multiple breast quadrants or disease foci separated by more than 5 cm. For tumors with multifocality or multicentricity, the size of the largest lesion was recorded.
Statistical Methods
Pearson’s Chi squared test for marginal homogeneity was used to test for association between two categorical variables. Pearson’s product-moment correlation was used to evaluate the extent of linear dependence between two continuous variables. Multiple logistic regression analyses were used to evaluate relationships between mammographic features and several clinicopathologic endpoints. Partial effects were evaluated for significance (p < 0.05) using two-sided Wald tests. Multiple Cox proportional hazard regression analysis was used to evaluate the extent to which mammographic features were associated with time from diagnosis of DCIS to diagnosis of LR. Patient follow-up was censored at the end of the study or death if no LR had occurred previously. Death without recurrence was considered a competing event. Cumulative incidence for each type of microcalcifications morphology was estimated according to the method proposed by Fine and Gray.27 The reported hazard ratios were adjusted for the partial effect of age. Propensity scores were used to adjust for confounding due to nonrandomized selection of XRT.28 Specifically, a propensity score characterizing the probability of undergoing XRT was estimated for each patient using stepwise logistic regression with the following clinical predictors: age, surgical margin status, breast tissue density, and tumor grade. Analyses were performed using the statistical software SAS 9.3 and R (R Development Core Team, http://www.r-project.org), version 3.1.2.
Results
Of 1911 patients with pure DCIS who underwent mammography and were treated at our institution during the study period, 254 patients were excluded because they had no suspicious findings on mammography (n = 99) or had noncalcified lesions (n = 155), leaving 1657 patients in the final analysis.
Association of Mammographic Findings with Clinical, Histopathologic, and Biologic Features
The imaging, clinical, and histopathologic features are summarized in Table 1. The mean age at diagnosis was 55 years (standard deviation, 11). A total of 987 patients (60 %) had BCS, and 670 patients (40 %) had mastectomy. At pathologic examination, positive/close surgical margins were seen in 82 patients (8 %) who underwent BCS and 18 patients (3 %) who underwent mastectomy. Of the patients with known ER status, most (n = 867; 82 %) had ER-positive disease. Of the patients who had BCS 82 % (n = 807) received adjuvant XRT.
Separate analysis by age (Table 2) showed that women aged 50 years or older were more likely to have ER-negative disease (p < 0.0045) and undergo BCS (p < 0.0001), whereas women younger than 50 years were more likely to have extremely dense breast tissue (p < 0.0001) and multicentric disease (p < 0.0004).
Mastectomy was strongly associated with multicentricity (p < 0.0001) and large lesion size (p < 0.0001). Extremely dense breast tissue was associated with positive/close margins (p = 0.04) and multicentricity (p < 0.01).
Results of multiple regression analysis are shown in Table 3. Grade 3 DCIS and comedonecrosis were associated with fine linear (branching) microcalcifications (p < 0.0001 and p < 0.05, respectively), coarse heterogeneous/fine pleomorphic microcalcifications (p < 0.0001 and p = 0.08, respectively), larger mammographic DCIS size (p = 0.02 and p < 0.0001, respectively), and ER-negative disease (p < 0.0001 for both).
Association of Mammographic Findings with LR
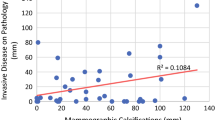
Ipsilateral LR occurred in 38 (4 %) of the 987 women who underwent BCS. Of these recurrences, 25 (66 %) were DCIS, and 12 (32 %) were invasive cancer; the pathology subtype was not recorded in 1 patient (2 %). LR occurred in 10 (1.5 %) of the 670 women who underwent mastectomy. Of these recurrences, 2 (20 %) were DCIS, and 8 (80 %) were invasive cancer. The LR rate after BCS without XRT was 5.3 % [95 % confidence interval (CI) 2.67–10.32 %] at 5 years and 7.3 % (95 % CI 3.93–13.3 %) at 10 years. The LR rate after BCS with XRT was 2.6 % (95 % CI 1.59–4.13 %) at 5 years and 6.2 % (95 % CI 1.59–4.13 %) at 10 years. The mean time to LR after surgery was 4.5 (range 1–13) years. Dense (heterogeneously dense and extremely dense) breast tissue (p < 0.05) and larger DCIS size (p < 0.01) were associated with increased rate of LR. Multivariate analysis in patients who underwent BCS (Table 4) showed that XRT was associated with an estimated 2.78-fold reduction in the rate of LR. In addition, the rate of recurrence was 5.13 (p = 0.023) times as high for women with fine linear (branching) calcifications and 3.41 (p = 0.052) times as high for women with coarse heterogeneous/fine pleomorphic calcifications as it was for women with punctate/amorphous microcalcifications (Fig. 1). Among patients who presented with coarse heterogeneous/fine pleomorphic microcalcifications and underwent BCS, patients with postoperative XRT were four times less likely to have LR than patients without XRT.
Discussion
This study validates previously reported descriptions of the mammographic appearance of microcalcifications in pure DCIS. Most patients had microcalcifications as a presenting finding, in agreement with previous reports.29–31 Furthermore, coarse heterogeneous/fine pleomorphic was the most common morphologic type, and clustered/grouped was the most common distribution, in agreement with previous findings in patients with DCIS of all types (pure DCIS and DCIS with microinvasion or invasion).13–15,22,23,32
This study also provides clinically relevant information regarding the relationship between microcalcification morphology and distribution and clinical and histopathologic features. We found that high grade and comedonecrosis were associated with fine linear (branching) and coarse heterogeneous/fine pleomorphic microcalcifications, larger mammographic DCIS size; linear/segmental distribution was associated with comedonecrosis, as previously reported for DCIS patients.13,15,30,33 Our data confirm the clinical significance of these mammographic features as indicators of more aggressive disease.
Because reports indicate that presentation and biology of DCIS may differ by patient age we examined imaging, clinical, and histopathologic features in patients 50 years of age or older and younger than 50 years.34,35 We found no difference in microcalcification morphology or distribution between these two groups. However, younger patients were more likely to have dense breast tissue and multicentric/multifocal disease and undergo mastectomy, while older patients were more likely to present with ER-negative disease.
There is increased interest in the evaluation of patients with dense breast tissue, because multiple states have passed legislation regarding supplemental screening in this subgroup. Our analyses of patients with pure DCIS showed associations between young age, dense breast tissue, multicentricity/multifocality, larger size, and positive surgical margins. Fitz Sullivan et al. reported that multicentricity, pathologic lesion larger than 1.5 cm, and necrosis were independent risk factors for close surgical margins.36 Recommending supplementary staging with an additional imaging tool such as MRI may be appropriate for patients with pure DCIS and dense breast tissue.37–39
The significance of ER expression in terms of the biologic behavior of DCIS has been reported in the literature.40–42 There was no significant association between imaging features of microcalcifications and ER status; however, ER-negative pure DCIS was associated with high nuclear grade, comedonecrosis, and older age.25 Therefore, ER-negative pure DCIS is more likely to be aggressive; this needs to be taken into consideration when management options are selected, especially in older women.
The relationship between mammographic, clinical, and histopathologic features of DCIS and LR was studied. We found that age, smaller pathologic DCIS size, and XRT decrease the risk of ipsilateral recurrence, as previously established in the literature.20,43–45 We also found that dense breast tissue (heterogeneously dense or extremely dense) was a significant risk factor for ipsilateral recurrence in patients with pure DCIS, which is a novel finding. Patients with extremely dense breast tissue had an increased incidence of positive surgical margins and multicentricity/multifocality; we hypothesize that these factors may contribute to the increased risk of LR for these patients. There are prior reports of increased rate of LR in patients with multicentric/multifocal DCIS treated with BCS, supporting our hypothesis.46,47
Fine linear (branching) microcalcifications were associated with a fivefold increase and coarse heterogeneous/fine pleomorphic microcalcifications with a 3.4-fold increase in the rate of LR. These types of microcalcifications were also significantly associated with nuclear grade 3 and comedonecrosis. These associations remained after correction for histopathologic variables. Holmberg et al. studied the association between mammographic microcalcifications and LR in the ipsilateral breast in a case-cohort study within a randomized trial of breast conservation for DCIS (SweDCIS).48 They found an increased risk of ipsilateral breast cancer recurrence in patients with coarse heterogeneous/fine pleomorphic and fine linear (branching) microcalcifications, similar to our findings. The most common type of recurrence was in situ carcinoma, as was the case in our patient population. Therefore, certain mammographic appearances of microcalcifications in patients with pure DCIS may predict higher risk of LR and may need to be taken into account in treatment planning and surveillance. However, it should be kept in mind that the overall recurrence rate in our study was very low: only 2.5 % of patients who underwent BCS had a recurrence in the form of in situ carcinoma, and only 1.2 % had an invasive cancer recurrence. Currently, there are multiple discussions about “overtreatment” and “overdiagnosis” of DCIS, and clinical trials addressing this question are underway in Europe and under development in the United States.49 This question is beyond the scope of our study; however, our results confirming a low recurrence rate in patients with pure DCIS, with the majority of recurrences being in situ carcinomas, support the need for such trials. Also, the increased LR rate in patients with extremely dense breast tissue and larger DCIS size seen in our study may indicate that special consideration should be given regarding enrollment of patients with these features in such clinical trials.
Our study had some limitations. The major limitation was the retrospective design. Also, not all variables were available for the entire set of patients, further limiting analysis.
This study is one of the largest published to date systematically analyzing mammographic, clinical, histopathologic, biologic findings, and LR in patients with pure DCIS. We found that grade 3 DCIS and comedonecrosis were associated with fine linear (branching) microcalcifications, linear/segmental distribution, large DCIS size, and ER-negative lesions. Young patients with dense breast tissue are at increased risk for multicentricity and close surgical margins. Dense breast tissue, larger DCIS size, and fine linear (branching) calcifications were associated with increased rate of ipsilateral LR in patients who underwent BCS for pure DCIS. Therefore, patients with these features undergoing BCS may be candidates for exploration of supplemental imaging at staging and for alternate treatment and surveillance options.
References
Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–54.
Parker J, Dance DR, Davies DH, Yeoman LJ, Michell MJ, Humphreys S. Classification of ductal carcinoma in situ by image analysis of calcifications from digital mammograms. Br J Radiol. 1995;68(806):150–9.
Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853–a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–7.
Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer. 1999;85(3):616–28.
Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005;11(6):382–90.
Sue GR, Lannin DR, Killelea B, Chagpar AB. Predictors of microinvasion and its prognostic role in ductal carcinoma in situ. Am J Surg. 2013; 206: 478–81.
Zunzunegui RG, Chung MA, Oruwari J, Golding D, Marchant DJ, Cady B. Casting-type calcifications with invasion and high-grade ductal carcinoma in situ: a more aggressive disease? Arch Surg. 2003;138(5):537–40.
Stomper PC, Geradts J, Edge SB, Levine EG. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am J Roentgenol. 2003;181(6):1679–84.
Roses RE, Arun BK, Lari SA, et al. Ductal carcinoma-in situ of the breast with subsequent distant metastasis and death. Ann Surg Oncol. 2011;18(10):2873–8.
Boughey JC, Gonzalez RJ, Bonner E, Kuerer HM. Current treatment and clinical trial developments for ductal carcinoma in situ of the breast. Oncologist. 2007;12(11):1276–87.
MacDonald HR, Silverstein MJ, Mabry H, et al. Local control in ductal carcinoma in situ treated by excision alone: incremental benefit of larger margins. Am J Surg. 2005;190(4):521–5.
Yi M, Krishnamurthy S, Kuerer HM, et al. Role of primary tumor characteristics in predicting positive sentinel lymph nodes in patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg. 2008;196(1):81-7.
Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Sem Diagn Pathol. 1994;11(3):181–92.
Hofvind S, Iversen BF, Eriksen L, Styr BM, Kjellevold K, Kurz KD. Mammographic morphology and distribution of calcifications in ductal carcinoma in situ diagnosed in organized screening. Acta Radiol. 2011;52(5):481–7.
Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol. 2005;54(1):55–61.
Poplack SP, Wells WA. Ductal carcinoma in situ of the breast: mammographic-pathologic correlation. AJR Am J Roentgenol. 1998;170(6):1543–9.
Bai HX, Motwani SB, Higgins SA, et al. Breast conservation therapy for ductal carcinoma in situ (DCIS): does presentation of disease affect long-term outcomes? Int J Clin Oncol. 2013; 19:460–66
Shaitelman SF, Wilkinson JB, Kestin LL, et al. Long-term outcome in patients with ductal carcinoma in situ treated with breast-conserving therapy: implications for optimal follow-up strategies. Int J Radiat Oncol Biol Phys. 2012;83(3):e305–12.
Vargas C, Kestin L, Go N, et al. Factors associated with local recurrence and cause-specific survival in patients with ductal carcinoma in situ of the breast treated with breast-conserving therapy or mastectomy. Int J Radiat Oncol Biol Phys. 2005;63(5):1514–21.
Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30(6):600–7.
Tse G, Tan PH, Pang AL, Tang AP, Cheung HS. Calcification in breast lesions: pathologists’ perspective. J Clin Pathol. 2008;61(2):145–51.
Tabar L, Chen HH, Duffy SW, et al. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10-14 mm invasive breast cancers: a prospective study. Lancet. 2000;355 (9202):429–33.
Tabar L, Tony Chen HH, Amy Yen MF, et al. Mammographic tumor features can predict long-term outcomes reliably in women with 1-14-mm invasive breast carcinoma. Cancer. 2004;101(8):1745–59.
Palka I, Ormandi K, Gaal S, Boda K, Kahan Z. Casting-type calcifications on the mammogram suggest a higher probability of early relapse and death among high-risk breast cancer patients. Acta Oncol. 2007;46(8):1178–83.
Rauch GM, Kuerer HM, Scoggins ME, et al. Clinicopathologic, mammographic, and sonographic features in 1,187 patients with pure ductal carcinoma in situ of the breast by estrogen receptor status. Breast Cancer Res Treat. 2013;139(3):639–47.
D’Orsi C, Mendelson E, Ikeda D. Breast imaging reporting and data system: ACR BI-RADS—Breast Imaging Atlas. Reston, VA: American College of Radiology; 2003.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424.
Stomper PC, Connolly J. Ductal carcinoma in situ of the breast: correlation between mammographic calcification and tumor subtype. AJR Am J Roentgenol. 1992;159(3):483–85.
Evans AJ, Pinder S, Ellis IO, et al. Screening-detected and symptomatic ductal carcinoma in situ: mammographic features with pathologic correlation. Radiology. 1994;191(1):237–40.
Thomson JZ, Evans AJ, Pinder SE, Burrell HC, Wilson AR, Ellis IO. Growth pattern of ductal carcinoma in situ (DCIS): a retrospective analysis based on mammographic findings. Br J Cancer. 2001;85(2):225–7.
Tabár L, Tot T, Dean PB. Casting type calcifications: sign of a subtype with deceptive features. Thieme; 2007.
Stomper PC, Margolin FR. Ductal carcinoma in situ: the mammographer’s perspective. AJR Am J Roentgenol. 1994;162(3):585–91.
Alvarado R, Lari SA, Roses RE, et al. Biology, treatment, and outcome in very young and older women with DCIS. Ann Surg Oncol. 2012;19(12):3777–84.
Ho A, Goenka A, Ishill N, et al. The effect of age in the outcome and treatment of older women with ductal carcinoma in situ. Breast. 2011;20(1):71–7.
Fitzsullivan E, Lari SA, Smith B, et al. Incidence and consequence of close margins in patients with ductal carcinoma-in situ treated with mastectomy: is further therapy warranted? Ann Surg Oncol. 2013;20(13):4103–12.
Biglia N, Bounous VE, Martincich L, et al. Role of MRI (magnetic resonance imaging) versus conventional imaging for breast cancer presurgical staging in young women or with dense breast. Eur J Surg Oncol. 2011;37(3):199–204.
Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485–92.
Lehman CD. Magnetic resonance imaging in the evaluation of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):150–1.
Kuerer HM, Albarracin CT, Yang WT, et al. Ductal carcinoma in situ: state of the science and roadmap to advance the field. J Clin Oncol. 2009;27(2):279–88.
Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–61.
Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: diagnosis and management of ductal carcinoma in situ, September 22-24, 2009. J Natl Cancer Inst. 2010;102(3):161–9.
Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28(23):3762–9.
Wang F, Li H, Tan PH, et al. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin Oncol. 2014; 26: 684–91.
Pinsky RW, Rebner M, Pierce LJ, et al. Recurrent cancer after breast-conserving surgery with radiation therapy for ductal carcinoma in situ: mammographic features, method of detection, and stage of recurrence. AJR Am J Roentgenol. 2007;189(1):140–4.
Rakovitch E, Pignol JP, Hanna W, et al. Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol. 2007;25(35):5591–6.
Yerushalmi R, Tyldesley S, Woods R, Kennecke HF, Speers C, Gelmon KA. Is breast-conserving therapy a safe option for patients with tumor multicentricity and multifocality? Ann Oncol. 2011;23(4):876–81.
Holmberg L, Wong YN, Tabar L, et al. Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study. Br J Cancer. 2013;108(4):812–9.
Kuerer HM. Ductal carcinoma in situ: treatment or active surveillance? Expert Rev Anticancer Ther. 2015;15(7):777–85.
Acknowledgments
Supported by the NIH/NCI under Award Number P30CA016672 and used the Biostatistics Resource Group from the Department of Biostatistics. The authors thank Stephanie Deming for assistance in editing of this manuscript.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rauch, G.M., Hobbs, B.P., Kuerer, H.M. et al. Microcalcifications in 1657 Patients with Pure Ductal Carcinoma in Situ of the Breast: Correlation with Clinical, Histopathologic, Biologic Features, and Local Recurrence. Ann Surg Oncol 23, 482–489 (2016). https://doi.org/10.1245/s10434-015-4876-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4876-6