Abstract
Objective
This study was designed to determine the histopathologic correlation at surgery of residual mammographic calcifications in patients after neoadjuvant chemotherapy (NAC) for locally advanced breast cancer (LABC).
Methods
This single-institution, retrospective study was approved by the Institutional Review Board and was Health Insurance Portability and Accountability act compliant. Women with LABC who underwent NAC between January 1, 2004 and December 31, 2008 and had mammography performed before and after NAC available for review were included in this study. The extent of microcalcifications associated with cancer before and after the completion of NAC was correlated with histopathology and biomarker status.
Results
Of 494 patients who met the inclusion criteria, 106 demonstrated microcalcifications on pre-, post-chemotherapy, or both sets of mammograms and were included in this study. Of 106 women, 31 (29 %) had invasive ductal carcinoma (IDC) and 60 (57 %) had both IDC and ductal carcinoma in situ (DCIS). Microcalcifications decreased or remained stable in 76 (72 %) patients after completion of NAC. Correlation of microcalcifications with histopathology after NAC showed that 43 (40.6 %) patients had tumors associated with benign pathology. Of 32 patients with pathologic complete response, calcifications were associated with DCIS in 9 (9 %) and benign findings in 21 (22 %). The proportion of residual malignant calcifications was higher in ER+ versus ER− patients after NAC.
Conclusions
The extent of calcifications on mammography following NAC does not correlate with the extent of residual disease in up to 22 % of women; this information may impact surgical planning in subsets of women with breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The treatment of locally advanced breast cancer has evolved over the past decade and currently includes a multidisciplinary approach that is directed both toward locoregional control of disease and the treatment of micrometastases.1 , 2 In this setting, neoadjuvant chemotherapy has several advantages. First, accurate staging of locally advanced breast cancer is critical for locoregional planning. Second, neoadjuvant chemotherapy may reduce tumor size and increase the likelihood of breast conservation surgery without increasing the rate of local recurrence. Third, the possibility of evaluating tumor response to medical therapy in situ allows for potential modification of the therapeutic regimen and prediction of long-term disease control and outcome. Studies have been conducted to determine the role of different imaging modalities in monitoring tumor response to neoadjuvant chemotherapy.3 , 4 Despite the emergence of novel breast imaging techniques, such as conventional dynamic contrast-enhanced and functional MR, and nuclear medicine imaging methods, mammography remains the “gold standard” for the evaluation of patients with breast cancer due to its wide availability.5 – 7 The reliability of mammography for monitoring tumor response, however, has been shown to vary. Previous reports have shown that mammography is comparable with and often complementary to physical examination for monitoring tumor response.8 – 10 These investigators found that although evaluation of tumor size on mammography is concordant with clinical evaluation of response, change in the number of microcalcifications observed is an unreliable indicator of response as not all residual calcifications represent carcinoma.
Data on the histopathologic correlation of mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer remain sparse. Whether these calcifications reflect residual disease is uncertain. The purpose of this retrospective study was to review the mammographic changes in patients who underwent neoadjuvant chemotherapy for locally advanced breast cancer and to correlate residual mammographic microcalcifications posttherapy with histopathologic findings of the tumor at diagnosis and at surgery. We hypothesized that the persistence of calcifications following neoadjuvant chemotherapy may not necessarily indicate residual malignant disease. On the contrary, a percentage of these calcifications may reflect a benign pathology.
Materials and Methods
Study Population
We searched the surgical database at a single institution to identify all patients with invasive carcinoma who underwent neoadjuvant chemotherapy before surgery for breast cancer from January 1, 2004, to December 31, 2008. Institutional review board approval was obtained for this Health Insurance Portability and Accountability Act (HIPAA)-compliant study, and informed consent was waived. Patients who had pre- and/or posttreatment mammograms that demonstrated calcifications within the tumor bed available for review and received neoadjuvant chemotherapy followed by segmentectomy or mastectomy based on the response to neoadjuvant chemotherapy were study eligible.
Imaging
Mammography was performed using one of two units (Lorad M3, Hologic, Bedford, MA; DMR, GE Healthcare, Milwaukee, WI). Standard three-view diagnostic mammographic examinations were performed, and additional views were acquired as deemed necessary. For the current study, the mammographically detected lesions were reviewed by three radiologists (B.A., E.A., and D.L.) with 5, 11, and 7 years of experience in breast imaging, respectively according to the American College of Radiology BI-RADS mammography lexicon,11 which describes the presence, shape, margins, density and location of masses; the presence, morphologic characteristics, and distribution of calcifications.
Assessment of Mammographic Features
The morphology and distribution of microcalcifications was tabulated, and correlated with molecular markers of each tumor. Pre- and posttreatment mammograms were evaluated retrospectively for mammographic assessment of tumor response using the following features: mass shape, margins, and size were recorded. The dimensions of the mass, asymmetry, or distortion in orthogonal axes were obtained in the craniocaudal and lateromedial views. The morphology and distribution of calcifications associated with the tumor were recorded. The extent of calcifications was measured in centimeters in three dimensions (anteroposterior, transverse, and superior-inferior). To assess change in size of tumor mass, asymmetry, distortion, and calcifications before and after neoadjuvant chemotherapy, the greatest diameter of the mass, asymmetry, distortion, and calcifications at each study was compared and categorized as follows: Increase in size was defined as an increase of more than 25 % in the greatest diameter on the posttreatment mammogram over that on the pretreatment mammogram; decrease in size was defined as a decrease of more than 25 % in the greatest diameter on the posttreatment mammogram from that on the pretreatment mammogram; stable in size was defined as increase or decrease ≤25 % in the greatest diameter on the posttreatment mammogram from those on the pretreatment mammogram; new mass, asymmetry, distortion, or calcifications were defined as those seen only on the posttreatment (and not the pretreatment) mammograms.
Pathologic Evaluation
Core biopsies were performed before neoadjuvant chemotherapy and confirmed the diagnosis of invasive and/or in situ breast cancer at histopathological evaluation in all patients. Information on the three main molecular markers for breast cancer was recorded: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). For hormone status, >1 % staining of cells by immunochemical (IHC) was considered receptor-positive. Tumors were considered HER2+ if they were 3+ by IHC or if they demonstrated gene amplification by fluorescence in situ hybridization (FISH) [HER2-chromosome 17 centromere ratio > 2.0]. Two breast pathologists (L.H. and E.R.) reevaluated pathology reports and slides from core biopsy specimens to determine that at least one core biopsy showed carcinoma (IDC and DCIS) associated with calcifications. Tumor histopathology subtype, molecular subtype and axillary nodal status were documented.
Routine specimen radiography was performed on women undergoing segmentectomy and mastectomy for radiologic-histopathologic correlation. Using specimen radiography, histopathologic correlation of the tumor bed containing calcifications was documented in all patients. Final histopathology was obtained following surgery at the conclusion of neoadjuvant chemotherapy. The same pathologists also reviewed the mastectomy specimens to estimate the histopathologic response of the tumor to therapy by examining the tumor bed and to determine that at least one section of the mastectomy specimen was evaluated for calcifications associated with carcinoma (invasive, or in situ) or benign findings. Pathologic complete response (pCR) was defined as the absence of invasive tumor in the breast after neoadjuvant chemotherapy. Patients with residual carcinoma in situ were not considered as having pCR. Moreover, a pathologic partial response (pPR) indicated residual invasive tumor.
Statistical Methods
Patient characteristics were summarized using mean, standard deviation, and range for continuous variables and by frequency tables and percentages for categorical variables. The morphology and distribution of calcifications was correlated with tumor molecular subtypes using Fisher’s exact test. The correlation between tumor size (mass, asymmetry, or distortion) and the maximum size of calcifications was assessed using the Spearman’s correlation test. P values ≤ 0.05 were considered statistically significant. The change in the tumor mass (asymmetry or distortion) size, and the size of calcifications on the pre- and posttreatment mammograms was correlated with pathologic complete response (pCR) using Fisher’s exact test. Statistical analysis was performed using SAS version 7 (SAS Institute, Cary, NC). The relationship between tumor response and molecular markers was tabulated.
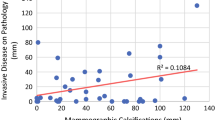
Results
Of 494 patients with invasive carcinoma who underwent neoadjuvant chemotherapy (12 or 24 cycles), 106 had calcifications visible on pre-neoadjuvant chemotherapy, post-neoadjuvant chemotherapy, or both pre- and post-neoadjuvant chemotherapy mammograms and were included in this study. Of the 106 patients, 31 (29 %) had invasive ductal carcinoma (IDC), 60 (57 %) had IDC and ductal carcinoma in situ (DCIS), and 15 (15 %) had a combination of IDC, invasive lobular carcinoma (ILC), and/or DCIS (Table 1). The median age of patients at diagnosis was 49 years (range, 24–80). The median size of the tumor mass, asymmetry, or distortion at presentation was 3.6 cm (range, 1–10). The median size of calcifications on the pre-treatment mammograms was 4 cm (range, 0.0–12); the median size of calcifications on the post-treatment mammograms was 3.5 cm (range, 0.2–12). There was no correlation between radiographic tumor size at presentation and the extent of calcifications. Regarding calcifications, pleomorphic morphology was most frequently noted and described in 53.8 % (57/106) of tumors; clustered distribution was most common and described in 65/106 (61 %) tumors (Table 2).
Seventy-one tumors (67 %) were ER+, 13 (12 %) were ER-HER2+, and 20 (19 %) triple-negative. In our study, 32 of 106 patients (30 %) achieved pCR after neoadjuvant chemotherapy. Among these, 12 of 71 (17 %) were ER+ tumors and 19 of 33 (57.6 %) were ER−. Partial response was observed in 74 (70 %) patients (Table 3).
Overall, tumor (mass, asymmetry, or distortion) size decreased after neoadjuvant chemotherapy in 71 of 106 (67 %) of tumors (Table 4). Patients with pCR demonstrated a significantly higher proportion of decrease in tumor (mass, asymmetry, or distortion) size compared with patients without pCR (p = 0.02). In contrast, extent of calcifications decreased in 35 (33 %) patients, remained stable in 41 (39 %), and increased or were new in 30 (28 %; Table 4). There was no correlation between change in the extent of calcifications before and after neoadjuvant chemotherapy and pCR (p = 0.11).
Calcifications were correlated with histopathology and were associated with carcinoma before neoadjuvant chemotherapy in all patients. Of 106 patients, 31 (29.2 %) tumors had calcifications associated with IDC, 60 (56.6 %) with IDC and DCIS, and 15 (14.2 %) with ILC or mixed ILC and IDC. Mammographic calcifications post-neoadjuvant chemotherapy were correlated with histopathology at surgery and showed association with carcinoma in 55 (52 %) tumors and with benign findings in 43 (41 %; Table 5). Of 32 patients (30 %) who achieved pCR, calcifications were associated with DCIS in 9 patients (9 %) and with benign pathology in 21 patients (20 %) at final surgery. In 2 patients, the association was unknown. Of the 74 (70 %) patients who had partial response, calcifications were associated with invasive disease in 21 (20 %), DCIS in 22 (21 %), and benign pathology in 22 (21 %). In 9 patients, the association was unknown. Calcifications were associated with residual malignancy (invasive or in situ carcinoma) in approximately half of treated patients, and with benign changes, including benign breast epithelium, fibrocystic changes, adenosis, or stromal fibrosis in 43 (41 %) tumors (Fig. 1). Additionally, 15 of 106 (14 %) patients had DCIS in surgical specimens that was not associated with calcifications on their mammograms after neoadjuvant chemotherapy.
a CC mammogram in 60-year-old woman presenting with painful palpable left breast mass demonstrates coarse heterogeneous segmental calcifications (5 cm) associated with irregular mass (arrows). b Histopathology from ultrasound-core needle biopsy showed invasive ductal carcinoma and ductal carcinoma in situ. Photomicrograph (original magnification, ×100; hematoxylin-eosin, H&E stain) demonstrates microcalcifications associated with ductal carcinoma in situ in pre-treatment core biopsy (arrow). c CC mammogram postchemotherapy (6 months later) shows complete resolution of mass; decrease in size of calcifications (arrows, 4 cm). d Histopathology at surgery showed no residual invasive carcinoma; microcalcifications were associated with benign stroma. Photomicrograph (original magnification, ×100; hematoxylin-eosin, H&E stain) demonstrates microcalcifications associated with benign breast epithelium in fibrous tumor bed (arrow). No residual carcinoma was identified
Table 6 describes the distribution of malignant and benign residual calcifications by ER, HER2, and TRN status. ER+ patients had significantly higher proportion of residual malignant calcifications compared with ER− patients. TRN patients had significantly lower proportion of residual malignant calcifications compared with non-TRN patients.
Discussion
The goal of neoadjuvant chemotherapy is to eradicate microscopic evidence of invasive tumor in the breast and axillary lymph nodes as assessed by standard histologic examination. A secondary goal is to test rapidly the efficacy of systemic therapies in the elimination of occult micro metastases and improve overall survival. Additionally, tumor downstaging with neoadjuvant chemotherapy can convert inoperable to operable disease, thus enabling breast-conservation surgery in patients for whom mastectomy was the only initial option.12 – 14 Final preoperative physical and radiographic assessment for evidence of residual tumor in the breast helps to identify the region of the breast to be resected; thus facilitating breast-conservation surgery using needle-localized mammographic or ultrasound guidance.
In this study, although the proportion of decrease in tumor (mass, asymmetry, or distortion) size correlated with pCR, there was no correlation between change in the extent of the calcifications before and after neoadjuvant chemotherapy and pCR. Additionally, the presence of residual microcalcifications after neoadjuvant chemotherapy did not correlate with the presence or absence of pCR at histopathology of the surgical specimen. In this cohort of 106 patients with histopathological correlation at surgery, calcifications were associated with malignant histopathology in 49 % and with benign findings in 41 % of tumors, respectively, after chemotherapy. Interestingly, ER+ patients had a significantly higher proportion of residual malignant calcifications compared with ER− patients after neoadjuvant therapy. Triple-negative cancers had a significantly lower proportion of residual malignant calcifications compared with non-triple–negative tumors. To the best of our knowledge, this has not been previously reported and suggests that the proportion of residual malignant calcifications may reflect patterns of biologic response based on tumor immunophenotypes.
Studies have reported that decreases in size and density of the tumor mass on mammography were the most reliable and common indicators of response to the treatment, whereas calcifications associated with malignancy was misleading in evaluating treatment response using mammography.3 , 8 – 10
Vinnicombe et al.15 described the evolution of microcalcifications that were present in 44 (46 %) of 95 patients with breast cancer, who had postchemotherapy mammographic findings correlated with histopathology from surgical specimens. Following neoadjuvant chemotherapy, the calcifications decreased in 4 of 44 (9 %) tumors, were stable in 21 (48 %), became more conspicuous in 15 (34 %), and increased in 4 (9 %). IDC and DCIS were present in 59 (62 %) of 95 tumors following chemotherapy, and DCIS alone was present in 9 (9 %) tumors at histopathology evaluation. These investigators suggested that the persistence of calcifications does not necessarily indicate the presence of DCIS. Segel et al.16 found that calcifications after treatment may decrease but they rarely resolve. Moskovic et al.10 suggested that one of the greatest pitfalls in evaluating treatment response in mammography is the presence of residual calcifications, which may represent successfully treated cancer with calcified and necrotic material in the tumor bed.10
However, DCIS can be present in the surgical specimen without manifesting as calcifications on mammography. In our study, 15 (14 %) patients had residual DCIS in surgical specimens not associated with calcifications on the posttreatment mammograms. Similar changes in the evolution of microcalcifications on mammography were observed with primary radiation therapy. Libshitz et al.17 reported that calcifications can increase, decrease, or remain stable following primary irradiation. Because calcifications can develop secondary to necrotic tissue and sloughed cells, the authors postulated that residual calcifications do not necessarily represent failure of the treatment.
Conversely, Pierce et al.18 evaluated ten patients with locally advanced breast cancer that demonstrated mammographic calcifications pre- and post-chemotherapy and found that new or persistent calcifications were identified in all tumors, and that 90 % of the tumors revealed residual disease pathologically.
Regarding biological markers, 33 of 106 (30 %) tumors in this study were ER-negative, concordant with published data.19 In our patient population, 32 patients (30 %) achieved pCR after neoadjuvant chemotherapy. Rates of pCR with neoadjuvant chemotherapy vary between 25 and 66.7 % depending on tumor type and treatment.20 Seventeen percent (12/71) were ER+ and 58 % (19/33) were ER−. These findings are consistent with published results reporting higher pCR for ER− than for ER+ tumors.20 Our study showed that patients with ER+/HER2+ (5.6 %) cancer demonstrated a lower rate of pCR than did patient with ER−/HER2+ (8.4 %) cancer, concordant with findings by Esserman et al.21
The limitations of this study include its retrospective nature and small sample size. Despite these limitations, this study provides the first data set that describes radiologic-pathologic correlation of microcalcifications associated with primary breast cancer before and after chemotherapy, as well as correlation with biologic subtypes.
In summary, evaluating microcalcifications remains an area of difficulty in the interpretation of mammograms when evaluating response in patients who have undergone chemotherapy for locally advanced breast cancer. Microcalcifications frequently persist after chemotherapy, which contributes to disagreement between the clinical and radiological response. Our findings suggest that the presence of persistent calcifications, some of which are benign, may contribute to overestimation of the extent of malignant disease in approximately 40 % of patients and that these events may have implications for surgical planning. Furthermore, ER+ patients had a significantly higher proportion of residual malignant calcifications compared with ER− patients after neoadjuvant therapy. It is possible that patients with ER− breast cancers that have extensive residual calcifications after neoadjuvant therapy may be considered candidates for lumpectomy. Residual calcifications that are not completely excised in specific cohorts of patients (ER− disease) who have negative surgical margins may be regarded differently from patients with ER+ disease. Concerns about incomplete excision related to residual calcifications may be less of an issue with certain subtypes of breast cancer (e.g. ER−). These observations may inform future clinical practice if validated in prospective trials.
References
Redden MH, Fuhrman GM. Neoadjuvant chemotherapy in the treatment of breast cancer. Surg Clin North Am. 2013;93(2):493–9.
McLaughlin SA. Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am. 2013;93(2):411–28.
Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, Zuiani C. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14(8):1371–9.
De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer: Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119(10):1776–83.
O’Sullivan TD, Leproux A, Chen JH, et al. Optical imaging correlates with magnetic resonance imaging breast density and reveals composition changes during neoadjuvant chemotherapy. Breast Cancer Res. 2013;15:R14. doi:10.1186/bcr3389.
Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–72.
Tateishi U, Miyake M, Nagaoka T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging–prospective assessment. Radiology. 2012;263(1):53–63.
Dershaw DD, Drossman S, Liberman L, Abramson A. Assessment of response to therapy of primary breast cancer by mammography and physical examination. Cancer. 1995;75(8):2093–8.
Helvie MA, Joynt LK, Cody RL, Pierce LJ, Adler DD, Merajver SD. Locally advanced breast carcinoma: accuracy of mammography versus clinical examination in the prediction of residual disease after chemotherapy. Radiology. 1996;198(2):327–32.
Moskovic EC. Mammography in the assesment of response to medical treatment of large primary breast cancer. Clin Radiol. 1993;47:339–44.
American College of Radiology (ACR). ACR breast imaging reporting and data system (BI-RADS). 4th edn. Reston, VA: American College of Radiology, 2003.
Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73(2):362–9.
Singletary SE, McNeese MD, Hortobagyi GN. Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer. 1992;69(11):2849–52.
Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16(1):93–100.
Vinnicombe SJ, MacVicar AD, Guy RL, Sloane JP, Powles TJ, Knee G, Husband JE. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. 1996;198(2):333–40.
Segel MC, Paulus DD, Hortobagyi GN. Advanced primary breast cancer: assessment at mammography of response to induction chemotherapy. Radiology. 1988;169(1):49–54.
Libshitz HI, Montague ED, Paulus DD. Calcifications and the therapeutically irradiated breast. AJR Am J Roentgenol. 1977;128(6):1021–5.
Pierce L, Adler D, Helvie M, Lichter A, Merajver S. The use of mammography in breast preservation in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 1996;34(3):571–7.
Wang Y, Ikeda DM, Narasimhan B, et al. Estrogen receptor-negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology. 2008;246(2):367–75.
Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–9.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9.
Disclosures
No disclosures are declared by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adrada, B.E., Huo, L., Lane, D.L. et al. Histopathologic Correlation of Residual Mammographic Microcalcifications After Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer. Ann Surg Oncol 22, 1111–1117 (2015). https://doi.org/10.1245/s10434-014-4113-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4113-8