Abstract
Background
Neoadjuvant chemotherapy (NAC) is an important treatment strategy for cervical cancer; however, few predictive markers of the response to NAC exist. Aldehyde dehydrogenase 1 (ALDH1), a cancer stem cell marker, is associated with chemoresistance in a variety of cancers. This study attempted to investigate the value of ALDH1 as a predictive marker of chemosensitivity and its prognostic value in cervical cancer patients treated with NAC.
Methods
Immunohistochemistry was used to evaluate ALDH1 expression in matched pre- and post-NAC tumor samples from 52 patients with cervical cancer. Kaplan–Meier analysis and a Cox proportional hazards regression model were applied to determine overall survival (OS) and disease-free survival (DFS).
Results
Fourteen patients (26.9 %) had ALDH1-positive tumors pre-NAC, and ALDH1 expression pre-NAC was significantly associated with a low clinical chemotherapy response rate and clinical non-response. Twenty-two patients (42.3 %) had ALDH1-positive tumors post-NAC, and ALDH1 expression post-NAC was associated with poor DFS and OS (both p = 0.004). Multivariate analysis revealed that ALDH1 expression post-NAC was an independent prognostic factor for OS (hazard ratio 3.513; p = 0.033). Moreover, we observed that ALDH1 expression was increased after NAC in 18 patients (36.7 %). Increased levels of ALDH1 expression after NAC predicted poor DFS and OS (p = 0.013 and p = 0.08, respectively).
Conclusions
Our findings suggest that ALDH1 expression pre-NAC may be a predictive marker for response to NAC, and ALDH1 expression post-NAC could be a prognostic marker for cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cervical cancer is the second most frequently diagnosed malignancy in women in developing countries1. According to the International Federation of Gynecology and Obstetrics (FIGO) guidelines, concurrent chemoradiotherapy (CCRT) is the primary standard treatment for locally advanced cervical cancer 2 but is associated with a high incidence of long-term complications such as sexual dysfunction and ovarian dysfunction.3,4 Neoadjuvant chemotherapy (NAC) followed by radical surgery has been proposed as a valid alternative to CCRT; 5,6 however, NAC is ineffective in approximately1 0–50 % of patients with locally advanced cervical cancer.7–9 Therefore, to avoid potential therapy-related complications and inappropriate delays in surgical treatment, it would be advantageous to identify chemosensitive tumors before initiating NAC. To date, only a few markers have been described that can predict response to NAC.
Aldehyde dehydrogenase 1 (ALDH1), one of 19 human ALDH isoforms, is responsible for catalyzing the conversion of retinol to retinoic acid.10 Recently, ALDH1 was recognized as a reliable marker of cancer stem cells (CSCs),11–14 and ALDH1-positive cancer cells have been demonstrated to be chemoresistant from a variety of tumors, including breast, rectal, and esophageal carcinomas.15–17 These results suggest that the expression of ALDH1 by tumor cells may predict a poor response to chemotherapy; however, the potential of ALDH1 as a predictive marker of the response to NAC has not been investigated in patients with cervical cancer.
Hererin, we evaluated the potential of ALDH1 as a predictive marker of chemoresistance in patients with locally advanced cervical cancer by investigating the association between ALDH1 expression pre-NAC and the response to NAC. We also assessed the prognostic value of ALDH1 expression in patients with locally advanced cervical cancer treated with NAC.
Patients and Methods
Patients and Tissue Samples
From January 2003 to June 2008, patients diagnosed with cervical cancer and registered at Sun Yat-sen Memorial Hospital, Sun Yat-sen University, were considered for the study; 52 patients with stage IB2–IIB disease for whom matching biopsies (taken pre- and post-NAC following surgery) were available for pathological and immunohistochemical analysis were included. Their clinicopathological characteristics are summarized in Table 1. All specimens were anonymously coded in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki). The study protocol was approved by the University Review Board. Follow-up (median 71 months; range 3–123 months) was as previously described.8,9
Treatment and Response
Patients received two or three courses of cisplatin-based chemotherapy. During the inclusion period, patients were enrolled on one of two common regimens: (i) PF: 75 mg/m2 cisplatin or carboplatin area under concentration-time curve (AUC)4–5 intravenously on day 1, 750 mg/m2 fluorouracil on days 1–5 with an interval of 21 days; and (ii) TP: 135 mg/m2 paclitaxel plus 75 mg/m2 cisplatin or carboplatin AUC4–5 intravenously with an interval of 21 days. All patients subsequently underwent a type III radical hysterectomy with systematic pelvic lymphadenectomy plus para-aortic lymphadenectomy, if indicated, within 3 weeks of finishing chemotherapy. Postoperative adjuvant therapy was administered to patients with risk factors for recurrence, according to the FIGO guidelines. Chemotherapeutic response was assessed before chemotherapy and 2 weeks after the last cycle of chemotherapy by bimanual gynecological examination, colposcopy, transvaginal ultrasound and/or magnetic resonance imaging (MRI). Clinical response to NAC was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria,18 as follows: complete resolution of the tumor (CR); partial response (PR), i.e. >50 % decrease in the tumor volume; stable disease (SD), i.e. <50 % decrease or a <25 % increase in the tumor volume; and progressive disease (PD), i.e. >25 % increase in the tumor volume. Pathological response was determined by the final postoperative pathological analysis. Patients were classified as responders (CR or PR) and non-responders (SD or PD).19
Immunohistochemistry and Evaluation
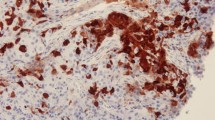
Immunohistochemistry was performed using an anti-ALDH1 antibody (BD Biosciences, Franklin Lakes, NJ, USA) following a previously described standard method.20 Paraffin sections of normal human liver tissue were used as a positive control, and the primary antibody was replaced with phosphate buffered saline (PBS) for the negative control. Cytoplasmic staining of tumor cells was considered when scoring ALDH1-positive cells; stromal and vascular staining was not evaluated. Immunostaining was evaluated using a scoring system for ALDH1 as follows:21 0, negative staining in all tumor cells; 1+, weak positive or focal positive staining of ≤10 % cells; 2+, moderate positive staining of >10 to ≤50 % cells; 3+, strong positive staining of >50 % cells; ALDH1 expression was considered positive if the score was ≥2 (electronic supplementary Fig. S1).
Statistical Analysis
Statistical analysis was performed using SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA). Associations between the clinicopathologic characteristics and the pattern of ALDH1 expression pre- and post-NAC were examined using the Pearson’s χ 2 test. Multiple logistic regression models were used to identify predictors of response to NAC. Survival rates were calculated using the Kaplan–Meier method and compared using the log-rank test. Changes in the ALDH1 immunohistochemical score after NAC were assessed using the Wilcoxon signed-rank test. Univariate and multivariate survival analyses were performed using the Cox regression model for disease-free survival (DFS) and overall survival (OS). A forward stepwise procedure was used to identify independent variables in the multivariate analysis. p values ≤0.05 indicated statistical significance.
Results
Clinical Response of Patients with Cervical Cancer to Neoadjuvant Chemotherapy (NAC)
Of the 52 patients, 16 (30.8 %) received the TP regimen and 36 (69.2 %) received the PF regimen (Table 1). Overall, 34 patients (65.4 %) responded to NAC, including 5 CR and 29 PR, and 18 patients (34.6 %) were non-responders, all of whom had SD. Samples from the five patients with CR were evaluated by postoperative pathological examination: three patients were confirmed to have a pathologically complete response (pCR), whereas the other two had residual tumors <3 mm.
Aldehyde Dehydrogenase 1 (ALDH1) Expression Pre- and Post-NAC and its Association with the Clinicopathologic Features of Cervical Cancer
Immunohistochemical analysis was performed on 52 paired samples collected pre- and post-NAC. ALDH1 staining was mainly localized to the cytoplasm of the tumor cells, with faint expression observed in the surrounding stromal and vascular areas (electronic supplementary Fig. S1). The associations between the expression of ALDH1 pre- and post-NAC and the clinicopathologic features of the patients are listed in Table 1. Of the pre-NAC biopsies, 14 (26.9 %) were ALDH1-positive and 38 (73.1 %) were ALDH1-negative. Positive ALDH1 staining pre-NAC was associated with a higher rate of lymphovascular space invasion (p = 0.006), a poor clinical response to NAC, and a lower reduction in tumor size after NAC (p = 0.006 and p = 0.008, respectively). The post-NAC biopsies of 22/49 (42.3 %) patients who did not achieve pCR had positive ALDH1 expression. Moreover, post-NAC expression of ALDH1 was associated with lymph node metastasis and parametrial invasion (p = 0.003 and p = 0.043, respectively).
ALDH1 Expression Pre-NAC is Predictive of Response to NAC
To determine pretreatment predictors of the response to NAC, we assessed pretreatment clinical features (tumor stage, tumor grade, histologic subtype, and tumor size pretreatment) and ALDH1 expression pre-NAC. Patients with negative ALDH1 expression pre-NAC and those with ‘earlier’ tumor stage (IB2 vs. IIA/IIB) were significantly more responsive to NAC (p = 0.006 and p = 0.031, respectively). Logistic regression analysis showed that both ALDH1 expression pre-NAC [p = 0.017; odds ratio (OR) 6.264; 95 % CI 1.385–28.322] and tumor stage (p = 0.041; OR 4.193; 95 % CI 1.063–16.539) were independent predictors of response to NAC (Table 2).
Association Between ALDH1 Expression Pre- and Post-NAC
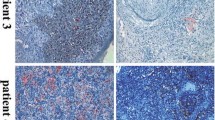
An increase in ALDH1 expression after NAC has been reported in patients with breast and rectal cancer.15,16 To investigate whether expression of ALDH1 is affected by NAC in cervical cancer, we assessed ALDH1 expression pre- and post-NAC in the 49 patients who did not achieve pCR (among 52 patients, three with pCR were not included). Images of ALDH1 staining for representative cases are shown in Fig. 1a. The grade of ALDH1 expression increased after NAC in 18/49 (36.7 %) patients (p = 0.037; Fig. 1b) [including 13 with negative pre-NAC staining who had positive staining post-NAC, and five with positive pre-NAC who had increase in tumor grade], remained stable in 22 (44.9 %) patients, and decreased in nine (18.4 %) patients. Among the 13 patients with negative pre-NAC staining and positive post-NAC staining, nine were clinical responders and four were non-responders. Increased ALDH1 expression after NAC was associated with a higher rate of lymph node metastasis and parametrial invasion (p = 0.002 and p = 0.011, respectively; electronic supplementary Table S1).
Comparison of ALDH1 expression pre- and post-NAC. a ALDH1 immunostaining pre- and post-NAC for three representative cases: Case 22, indicating increased staining from 1+ to 2+; Case 17, which shows stable ALDH1 immunostaining at 1+; and Case 40, indicating decreased staining from 3+ to 1+ (original magnification ×400). b The Wilcoxon signed-rank test was used to analyze the changes in ALDH1 expression after NAC. Of the 49 paired samples not achieving pCR before or after NAC, ALDH1 expression significantly increased after NAC in 18 patients (p = 0.037), remained stable in 22 patients, and decreased in 9 patients. ALDH1 aldehyde dehydrogenase 1, NAC neoadjuvant chemotherapy, pCR pathologically complete response
Prognostic Value of ALDH1 Expression Pre- and Post-NAC
During the median follow-up of 71 months (range 3–123), 18/52 (34.6 %) patients relapsed, of whom 16 (88.9 %) died due to their disease. Two patients with local recurrent vaginal cervical cancer were alive after surgery and adjuvant CCRT. The 5-year OS and DFS rates were 69.2 and 65.4 %, respectively. Although not significant, patients with positive ALDH1 expression pre-NAC had poorer 5-year DFS (57.1 vs. 68.4 %; p = 0.343) [electronic supplementary Fig. S2a] and 5-year OS (57.1 vs. 73.7 %; p = 0.186) [electronic supplementary Fig. S2b] than patients with negative ALDH1 expression pre-NAC. Responders to NAC had longer 5-year DFS (p = 0.001) [electronic supplementary Fig. S2c] and OS (p = 0.015) [electronic supplementary Fig. S2d] than non-responders. Positive ALDH1 expression post-NAC was associated with poorer 5-year DFS (p = 0.004) [electronic supplementary Fig. S2e], and OS (p = 0.004) [electronic supplementary Fig. S2f] than negative ALDH1 expression post-NAC. Increased expression of ALDH1 after NAC was associated with poorer 5-year DFS (p = 0.013) [electronic supplementary Fig. S2g] and OS, although this effect was not significant for OS (50.0 % vs. 77.4 %; p = 0.08) [electronic supplementary Fig. S2h].
In the univariate analysis, tumor size pre-NAC (>5 cm), non-response to NAC, lymph node metastasis, positive ALDH1 expression post-NAC, and increased ALDH1 expression after NAC correlated with poor 5-year DFS. Non-response to NAC emerged as an independent negative prognostic factor for 5-year DFS in the multivariate analysis [p = 0.003; hazard ratio (HR) 5.072; 95 % CI 1.781–14.976]. For 5-year OS, tumor size pre-NAC, response to NAC, and ALDH1 expression post-NAC were entered into the multivariate analysis; ALDH1 expression post-NAC was the only significant independent variable (p = 0.033; HR 3.513; 95 % CI 1.109–11.250) [Table 3].
Discussion
NAC is widely used in the treatment of locally advanced cervical cancer in many developing countries, including China; however, few predictors of the response to NAC exist.19,22–25 The present study demonstrates that ALDH1 expression pre-NAC is an independent predictor of clinical response to NAC in patients with locally advanced cervical cancer (FIGO stages IB2–IIB). In addition, ALDH1 expression significantly increased after NAC, and ALDH1 expression post-NAC and an increase in ALDH1 expression after NAC were associated with a poorer outcome in patients with cervical cancer. These results provide new evidence of a correlation between ALDH1 positivity and chemoresistance in cervical cancer.
In the current study, patients with a high proportion of ALDH1-positive tumor cells had a poorer response to NAC. This observation suggests that pretreatment screening of ALDH1 expression may provide helpful information for decision making as patients with ALDH1-positive tumors may be less likely to benefit from NAC. High ALDH1 expression has been associated with resistance to chemotherapy in breast, rectal and esophageal carcinomas.15–17 Moreover, recent reports showed that ALDH1 was expressed at high levels in cisplatin-resistant cervical cancer cell lines.12,14Therefore, ALDH1-positive tumor cells in pre-NAC biopsies may be predictive of chemoresistance to NAC. Pre-NAC ALDH1 expression could be used as a reliable marker to identify patients who could benefit most from NAC.
The biochemical link between ALDH1-positive cells and resistance to chemotherapy is not clearly understood.26 As a cytosolic enzyme, ALDH1 plays a significant role in oxidizing toxic aldehydes and other potentially harmful chemical components and drugs, such as cyclophosphamide in hematopoietic cell lines.27 Furthermore, ALDH1 is a marker of CSCs, and ALDH1-positive cells are thought to either be inherently chemoresistant or acquire chemoresistance via clonal evolution during chemotherapy.26,28 Based on our evaluation of paired cervical cancer tissues obtained pre- and post-chemotherapy, we propose that both intrinsic and acquired characteristics are involved in the refractory behavior of ALDH1-positive tumor cells to NAC.
In pre-NAC specimens, positive ALDH1 expression was significantly associated with lymphovascular space invasion and, more importantly, was significantly associated with non-response to NAC. This may indicate that ALDH1-positive tumor cells are intrinsically chemoresistant. Previously, we and other researchers demonstrated that ALDH1 is a reliable marker of cervical CSCs.11,13,14 Although present in very small numbers, CSCs are thought to be inherently chemoresistant.29 We speculate that ALDH1-positive tumors are chemoresistant as they may contain a higher proportion of CSCs.
In the post-NAC samples, positive ALDH1 expression was significantly associated with lymph node metastasis and parametrial invasion. Only positive ALDH1 expression post-NAC remained an independent prognostic indicator in the multivariate survival analysis. Similar to this study, Sakakibara et al. found that breast cancer patients with residual tumors containing ALDH1-positive cells post-NAC had a poorer prognosis than patients with ALDH1-negative cells or no residual tumor.30 This indicates that it may be useful to evaluate ALDH1 expression post-NAC in patients with cervical cancer, even in patients whose tumors are ALDH1-negative pre-NAC. For those patients with positive ALDH1 staining post-NAC, closer follow-up is needed. We speculate that anti-ALDH1 treatments may bring some benefits;26 however, more evidence is needed from further preclinical and clinical studies.
Furthermore, when we compared ALDH1 expression pre-NAC and post-NAC, we found that ALDH1 expression significantly increased after NAC, and 13 patients with negative ALDH1 staining pre-NAC had positive ALDH1 staining post-NAC. ALDH1-positive cells after NAC may, to some extent, imply acquired chemoresistance. Chemotherapy can selectively enrich ALDH1-positive CSCs in breast and colorectal cancers.31,32 Similarly, the upregulation of multidrug resistance protein 1 (MDR1/P-gp/ABCB1) and ATP-binding cassette sub-family G member 2 (BCRP/ABCG2) can occur after chemotherapy for breast cancer.31 Increased ALDH1 expression after NAC was associated with poorer clinical outcomes and significantly higher rates of tumor-related death and recurrence in this study. As a result, NAC should not be recommended for those patients with positive pre-NAC ALDH1 staining.
Although ALDH1-positive tumor cells are chemoresistant, 5 of the 14 patients with positive pre-NAC markers were clinical responders in our study. Furthermore, nine patients with ALDH1-positive tumor cells had a reduced grade of ALDH1 staining after NAC, which showed that they were sensitive to chemotherapy.30 This discrepancy may be partially explained by the specificity of ALDH1 in marking CSCs, since CSC markers may detect not only stem cells exclusively but also a larger tumor cell population with similar expression of stem cell markers.16
There were several limitations to this present study. As a result of the limited number of samples, we failed to observe any significant effect of pre-NAC ALDH1 expression on DFS or OS. Moreover, the potential bias of adjuvant postoperative treatment on DFS and OS may have been present in our research.
Conclusions
This study demonstrates that assessment of the CSC marker ALDH1 prior to treatment could provide valuable information to help identify patients with cervical cancer who are likely to respond to NAC. Expression of ALDH1 significantly increased after NAC, and ALDH1 expression post-NAC, and increased expression of ALDH1 after NAC, were associated with poorer clinical outcomes. A prospective multicenter study with a larger sample size is warranted to further confirm these findings.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Wiebe E, Denny L, Thomas G. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2012;119:S100–09.
Pareja R, Rendon GJ, Sanz-Lomana CM, Monzon O, Ramirez PT. Surgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy: a systematic literature review. Gynecol Oncol. 2013;131(1):77–82.
Marchiole P, Tigaud JD, Costantini S, et al. Neoadjuvant chemotherapy and vaginal radical trachelectomy for fertility-sparing treatment in women affected by cervical cancer (FIGO stage IB-IIA1). Gynecol Oncol. 2011;122(3):484–90.
Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecol Oncol. 2008;110(3):308–15.
Buda A, Fossati R, Colombo N, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol. 2005;23(18):4137–45.
Ye Q, Yuan HX, Chen HL. Responsiveness of neoadjuvant chemotherapy before surgery predicts favorable prognosis for cervical cancer patients: a meta-analysis. J Cancer Res Clin Oncol. 2013;139(11):1887–98.
Wen H, Wu X, Li Z, et al. A prospective randomized controlled study on multiple neoadjuvant treatments for patients with stage IB2 to IIA cervical cancer. Int J Gynecol Cancer. 2012;22(2):296–302.
Gong L, Lou JY, Wang P, Zhang JW, Liu H, Peng ZL. Clinical evaluation of neoadjuvant chemotherapy followed by radical surgery in the management of stage IB2-IIB cervical cancer. Int J Gynaecol Obstet. 2012;117(1):23–6.
Alison MR, Guppy NJ, Lim SM, Nicholson LJ. Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol. 2010;222(4):335–44.
Bortolomai I, Canevari S, Facetti I, et al. Tumor initiating cells: development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension. Cell Cycle. 2010;9(6):1194–206.
Casagrande N, De Paoli M, Celegato M, et al. Preclinical evaluation of a new liposomal formulation of cisplatin, lipoplatin, to treat cisplatin-resistant cervical cancer. Gynecol Oncol. 2013;131(3):744–52.
Rao QX, Yao TT, Zhang BZ, et al. Expression and functional role of ALDH1 in cervical carcinoma cells. Asian Pac J Cancer Prev. 2012;13(4):1325–31.
Liu SY, Zheng PS. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget. 2013;4(12):2462–75.
Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–41.
Avoranta ST, Korkeila EA, Ristamaki RH, et al. ALDH1 expression indicates chemotherapy resistance and poor outcome in node-negative rectal cancer. Hum Pathol. 2013;44(6):966–74.
Minato T, Yamamoto Y, Seike J, et al. Aldehyde dehydrogenase 1 expression is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2013;20(1):209–17.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Jin L, Shen Q, Ding S, Jiang W, Jiang L, Zhu X. Immunohistochemical expression of Annexin A2 and S100A proteins in patients with bulky stage IB-IIA cervical cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;126(1):140–46.
Lax S, Schauer G, Prein K, et al. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer. 2012;130(10):2232–39.
Aomatsu N, Yashiro M, Kashiwagi S, et al. CD133 is a useful surrogate marker for predicting chemosensitivity to neoadjuvant chemotherapy in breast cancer. PloS One. 2012;7(9):e45865.
Choi CH, Song SY, Choi JJ, et al. Prognostic significance of VEGF expression in patients with bulky cervical carcinoma undergoing neoadjuvant chemotherapy. BMC Cancer. 2008;8:295.
Costa S, Terzano P, Bovicelli A, et al. CD44 isoform 6 (CD44v6) is a prognostic indicator of the response to neoadjuvant chemotherapy in cervical carcinoma. Gynecol Oncol. 2001;80(1):67–73.
Park JS, Jeon EK, Chun SH, et al. ERCC1 (excision repair cross-complementation group 1) expression as a predictor for response of neoadjuvant chemotherapy for FIGO stage 2B uterine cervix cancer. Gynecol Oncol. 2011;120(2):275–79.
Watari H, Kanuma T, Ohta Y, et al. Clusterin expression inversely correlates with chemosensitivity and predicts poor survival in patients with locally advanced cervical cancer treated with cisplatin-based neoadjuvant chemotherapy and radical hysterectomy. Pathol Oncol Res. 2010;16(3):345–52.
Januchowski R, Wojtowicz K, Zabel M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed Pharmacother. 2013;67(7):669–80.
Magni M, Shammah S, Schiro R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87(3):1097–103.
Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61.
Sakakibara M, Fujimori T, Miyoshi T, et al. Aldehyde dehydrogenase 1-positive cells in axillary lymph node metastases after chemotherapy as a prognostic factor in patients with lymph node-positive breast cancer. Cancer. 2012;118(16):3899–910.
Gong C, Yao H, Liu Q, et al. Markers of tumor-initiating cells predict chemoresistance in breast cancer. PloS One. 2010;5(12):e15630.
Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PloS One. 2008;3(6):e2428.
Acknowledgment
The authors would like to thank Dr. Bo Wang for his technical support. This work was supported by the National Natural Science Foundation of China (30672221, 30872743).
Conflict of interest
Qingsheng Xie, Jinxiao Liang, Qunxian Rao, Xiaofei Xie, Ruixin Li, Yunyun Liu, Hui Zhou, Jingjing Han, Tingting Yao, and Zhongqiu Lin declare that they have no actual or potential competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Identification and grading of ALDH1-positive tumor cells using immunohistochemistry. Representative images of (A) cervical cancer tissues with an ALDH1 score of 0; (B) 1+; (C) 2+; (D) and 3+; original magnification ×400. ALDH1 staining was mainly localized to the cytoplasm of the tumor cells, though faint expression was also observed in the surrounding stromal and vascular areas of the tumour tissues. Supplementary material 1 (TIFF 1985 kb)
Fig. S2
Kaplan-Meier 5-year disease-free survival (DFS) and 5-year overall survival (OS) curves for patients with cervical cancer. (A and B) Patients with ALDH1-positive tumors pre-NAC tended to have poorer DFS and OS, though this difference was not significant. (C and D) Patients who responded to NAC had significantly better DFS and OS. (E and F) Patients with ALDH1-positive tumors post-NAC had poorer 5-year DFS and 5-year OS. (G and H) Patients with increased ALDH1 expression after NAC had poorer DFS and OS than non-increased ones; however, this effect was not significant for DFS. Supplementary material 2 (TIFF 984 kb)
Rights and permissions
About this article
Cite this article
Xie, Q., Liang, J., Rao, Q. et al. Aldehyde Dehydrogenase 1 Expression Predicts Chemoresistance and Poor Clinical Outcomes in Patients with Locally Advanced Cervical Cancer Treated with Neoadjuvant Chemotherapy Prior to Radical Hysterectomy. Ann Surg Oncol 23, 163–170 (2016). https://doi.org/10.1245/s10434-015-4555-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4555-7