Abstract
Overexpression of clusterin, an antiapoptotic molecule, has been reported to induce resistance to chemotherapy in a variety of cancer cell types. The aim of this study was to evaluate the significance of clusterin expression to predict response to platinum-based neoadjuvant chemotherapy and survival of patients with invasive cervical cancer who subsequently underwent radical hysterectomy. Biopsy specimens of invasive cervical cancer before neoadjuvant chemotherapy were obtained from 46 patients who subsequently underwent radical hysterectomy at Hokkaido University Hospital and Gunma University Hospital from 1994 to 2007. The expression of clusterin protein was analyzed by immunohistochemistry. Findings were evaluated in relation to several clinicopathological factors. Survival analyses were performed by the Kaplan-Meier curves and the log-rank test. Independent prognostic factors were determined by multivariate Cox regression analysis. Clusterin protein was mainly present in the cytoplasm of cervical cancer cells. The expression of clusterin protein in cervical cancer tissues before neoadjuvant chemotherapy was significantly related to poor response to chemotherapy among factors analyzed. Univariate analysis on prognostic factors showed that response to chemotherapy (p = 0.01), lymph node metastasis (p = 0.02), and clusterin expression (p = 0.02) were related to survival. Multivariate analysis revealed that lymph node metastasis (p = 0.03), and clusterin expression (p = 0.03) were independent prognostic factors for survival of cervical cancer patients. We conclude that clusterin expression could be a new molecular marker to predict response to platinum-based chemotherapy and survival of patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally accepted that radical surgery or radiotherapy can be curative for the majority of patients with early-stage cervical cancer. However, there is no agreement on the best approach to bulky or locally advanced disease, where the prognosis remain relatively poor. New treatment strategies such as concurrent chemoradiation (CCRT) or neoadjuvant chemotherapy (NAC) have been adopted to improve the prognosis for these cases. NAC has been advocated prior to radical hysterectomy in locally advanced cervical cancer since it may serve to control micrometastatic disease and so improve survival [1]. In addition, NAC offers the potential to reduce tumor volume and thus improve surgical resectability. Thus, there are theoretical advantages for the use of NAC. Chemotherapy may be most effective if treatment occurs before tumor blood flow is disturbed by surgery or radiotherapy. Furthermore, it may be less toxic if treatment is performed before the bone marrow is affected by radiotherapy. Patients with larger tumors are at risk for harboring micro-metastatic disease, and NAC may more effectively reduce this risk for distant failure. However, the implementation of a chemotherapeutic regimen delays surgery by 6 weeks, with the potential for transforming a surgically resectable tumors to unresectable ones. Many reports on NAC with cisplatin-based chemotherapeutic regimens have demonstrated high response rates. Nevertheless, those who have poor response to chemotherapy usually fail to respond to radiotherapy and have poor outcome. We, therefore, need to establish the new method to select patients who will respond to NAC, which might result in better outcome of locally advanced cases, and those who will not respond to NAC, which result in no delay of surgery for resectable cases.
Clusterin, also known as testosterone-repressed prostate message-2, sulphated glycoprotein-2 or apolipoprotein J, is a heterodimeric highly conserved disulfide-linked glycoprotein that is expressed in a wide variety of tissues and secreted in all human fluids and is implicated in various pathophysiological processes, including lipid transport, sperm maturation, complement inihibition, tissue remodeling, cell differentiation and transformation, and apoptotic cell death. It has been found to be overexpressed in several types of malignant tumors, and in these cancers, clusterin overexpression has been reported to be closely associated with cancer progression [2, 3]. Furthermore, the antiapoptotic action of clusterin has been reported to increase drug resistance [4] and radioresistance [5]. Clusterin expression has been associated with clinicopathologic parameters of aggressiveness and unfavorable prognosis [2, 6, 7]. Taken together, these reports indicate that clusterin may play an important role in chemoresistance, radioresistance, and may be a potent prognostic biomarker for different tumor types. Furthermore, we have recently reported that clusterin expression is a new prognosticator in cervical cancer patients who underwent radical hysterectomy followed by adjuvant therapy according to postoperative pathological risk factors [8].
The aim of this study was to examine the expression of clusterin by immunohistochemistry in invasive cervical cancer prior to surgery, its association with response to neoadjuvant platinum-based chemotherapy, and the prognostic significance of clusterin expression in cervical cancer to validate our previous observation in different patients’ cohort.
Materials and Methods
Tissue Specimens
Tumor biopsy samples were obtained from 46 invasive cervical cancer patients before chemotherapy. All patients were subsequently treated with neoadjuvant platinum-based chemotherapy followed by radical hysterectomy and lymphadenectomy at Hokkaido University Hospital and Gunma University Hospital from 1994 to 2007. All tissue samples were examined pathologically by two observers. The median follow-up of the patients was 49.5 months (range; 3–115 months). The median age of the patients was 46.5 years (range; 27–64).
Chemotherapy Regimen and Evaluation of Response
As the chemetherapy regimens used for NAC, BOMP (bleomycin (7 mg/body, day 1–5), vincristine (0.7 mg/m2, day 5), mitomycin C (7 mg/m2, day 5), and cisplatin (14 mg/m2, day 1–5)) was used for 19 patients, TC (paclitaxel (175 mg/m2, day 1) and carboplatin (AUC5, day 1)) or DC (docetaxel (70 mg/m2, day 1) and carboplatin (AUC 5, day 1)) for three, CAP (cyclophosphamide (500 mg/m2, day 1), adriamycin (50 mg/m2, day 1), cisplatin (50 mg/m2, day 1)) for four, TP (paclitaxel 135 mg/m2, day 1), and cisplatin (50 mg/m2, day 2) or ITP (ifosphamide 1.5 g/m2, day 1–3, paclitaxel 175 mg/m2, day 1, and cisplatin (14 mg/m2, day 1–5)) for ten, MEP (mitomycin C (10 mg/body, day 1), cisplatin (70 mg/m2, day 1), etoposide (100 mg/m2, day 1–3)) for seven, and intra-arterial infusion of cisplatin (100 mg/body) for three. Median number of cycles administered was two (range; 1–4). The response to NAC was evaluated by RECIST version 2.
Immunohistochemistry
Immunohistochemical staining of clusterin was performed as previously described [8]. Detection of clusterin was performed using a commercial polyclonal antibody (clusterin alpha/beta rabbit polyclonal antibody H330: Santa Cruz Biotechnology, Santa Cruz, CA, USA). The clusterin antibody was used at 1:200 dilution for overnight at 4 C. Negative control were obtained by omitting the primary antibody. All slides were blindly evaluated for clusterin immunoreactivity and protein localization, without knowledge of clinicopathological data. Staining intensity was scored as previously described(26). We divided all patients into two groups (negative/weak vs moderate/strong) based on staining intensity alone in this study, because positive staining was observed in >50% region of all cases with positive staining.
Statistics
Correlation between the variables and staining score of clusterin immunoreactivity was analyzed using chi square test. Patients survival was calculated using Kaplan–Meier method. The significance of the survival difference was examined by the log-rank test. Univariate and multivariate survival analyses were performed using the Cox regression model with disease-specific overall survival as the outcome measure. Forward stepwise procedure was used to select the independent variable in multivariate analysis. P < 0.05 was considered statistically significant. Statistical analyses were performed with the Statview software package (SAS Institute, Inc, Cary, NC).
Results
The Immunohistochemical Expression of Clusterin and Its Association with Clinocopathological Factors in Patients with Cervical Cancer Who Received NAC
The clinical characteristics of the patients are listed in Table 1. Six patients had stage Ib2 disease, four stage IIa and 36 stage IIb. The following histological tumor types were found; 25 squamous cell carcinomas, 15 adenosquamous carcinomas, three pure adenocarcinomas, and three other types (two; undifferentiated carcinoma, one; small cell carcinoma). Twenty-six patients had negative node group, and 20 had positive node groups. All patients received neoadjuvant platinum-based chemotherapy and adjuvant therapy (radiotherapy or chemotherapy or both). The regimen used are listed in Table 2. Overall response rate of NAC was 76.1% in this study.
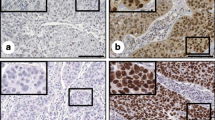
Immunolocalization with anti-clusterin antibody largely showed positive staining in the cytoplasm of cancer cells and occasionally positive in the nucleus. Representative results of staining intensity are shown in Fig. 1. The association between the staining of clusterin protein in invasive cervical cancer tissues and several clinicopathological factors revealed that higher expression of clusterin protein was significantly related to a poor response to NAC (p = 0.01). Age (p = 0.91), FIGO stage (p = 0.65), histologic subtype (p = 0.09), lymph vessel invasion (p = 0.09), blood vessel invasion (p = 0.46), lymph node metastasis (p = 0.96) were not related to clusterin expression (Table 3).
Typical outcomes of immunohistochemical staining of clusterin expression in cervical cancer tissues. a cervical cancer with strong clusterin expression (×100), b cervical cancer with moderate clusterin expression (×100), c cervical cancer with weak clusterin expression (×100), d cervical cancer with negative clusterin expression (×100)
Univariate and Multivariate Survival Analyses of the Patients with Invasive Cervical Cancer (Fig. 2, Table 4)
The estimated 5-year survival rate was 80.7% for patients with negative/weak clusterin expression (n = 32), 42.9% for those with moderate/high clusterin expression (n = 14; Fig. 2). There was a significant difference between the groups (p = 0.017). Since the clusterin expression was shown to have significant impact on the survival of patients with invasive cervical cancer treated with NAC, the clusterin expression was included in the multivariate analysis. The univariate analysis revealed that positive lymph node metastasis (p = 0.02), response to NAC (p = 0.01), and clusterin expression (p = 0.02) were shown to be related to survival. Age (p = 0.67), FIGO stage (p = 0.63) histological subtype (p = 0.68), lymph vessel invasion (p = 0.30), blood vessel invasion (p = 0.09), were not related to survival in this cohort. A multivariate analysis, including the prognostic factors determined by the univariate analysis to have statistical significance, was performed using a forward stepwise procedure, and it revealed a positive finding for lymph node metastasis (p = 0.03) and clusterin expression (p = 0.03) to be independent prognostic factors (Table 4).
Discussion
We firstly demonstrated a significant inverse correlation between expression of clusterin and chemotherapy response in cervical cancer patients. We also validated prognostic significance of clusterin expression for invasive cervical cancer of different patient cohort. This study, therefore, highlights the potential clinical role of clusterin as a predictor of chemotherapy response and survival in patients with cervical cancer.
Cervical cancer, especially squamous cell carcinoma, is known to show good response to platinum-based chemotherapy. However, the implementation of a chemotherapeutic regimen delays surgery with the potential for transforming a surgically resectable tumors to unresectable one, which might lead to worse outcome. Since patients will benefit the most when there is accurate information available about the effect of NAC before treatment is started, there have been some reports to find new molecular predictors to NAC in the literature. Konishi et al. reported that high MDR-1 expression and low PCNA labeling index were related to poor response to NAC [9]. Faried et al. reported that the expression of phosphorylated mTOR (target of rapamycin) has a role as a marker to predict response to chemotherapy and survival of cervical cancer patients who are treated with cisplatin-based NAC [10]. Saito et al correlated responsiveness of neoadjuvant chemotherapy and apoptosis-related proteins and found that overexpression of p53 was a factor to predict the chemotherapy resistance [11]. Costa et al. reported that CD44 isoform 6 (CD44v6) is involved in the response to NAC, and eventually in disease outcome, indicating that the assessment of CD44v6 expression might help in selecting patients who are likely to respond to NAC, i.e., women with significantly reduced CD44v6 expression in that tumors before treatment [12]. Ferrandina et al. reported that the assessment of COX-2 status could provide information to identify patients with cervical cancer with a poor chance of response to NAC and unfavorable outcome [13]. Chung et al. reported the genetic polymorphism of XRCC1 R399Q, a DNA repair gene, is associated with response to platinum-based NAC in bulky cervical cancer [14].
Clusterin has been shown to have antiapoptotic function in other cancer types. In those tumor types, knockdown of clusterin expression by antisense oligonucleotide or Si-RNA sensitized cancer cells to chemotherapeutic agents such as paclitaxel, cisplatin [4]. Furthemore, overexpression of clusterin is associated with resistance to docetaxel [15], gemcitabine [16], camptothecin [17], and doxorubicin [18]. Clusterin also has been implicated in resistance to radiotherapy in lung, prostate, and bladder cancer [5]. In gynecologic tumors, Park et al. showed a significant correlation between clusterin expression and paclitaxel IC50 in the cervical cancer cell lines and cervical cancer cells expressing high levels of clusterin became more sensitive to paclitaxel after silencing clusterin with Si-RNA, suggesting that clusterin may play a role in antagonizing the antitumor activity of paclitaxel in cervical cancer cells [19]. We also reported that stable overexpression of full-length clusterin induces resistance to paclitaxel in ovarian cancer cell model (data not shown). Lourda et al recently reported that clusterin upregulation in multi-drug resistant osteosarcoma cells related to enhanced drug resistance, and may represent a predictive marker, which correlates to response of cancer cells to chemotherapy [18]. We, therefore, speculate that clusterin is one of the key molecules to determine the sensitivity to any cytotoxic agents including platinum in cervical cancer cells. We also need to further examine the role of clusterin in resistance to radiotherapy, which is one of the primary therapy for cervical cancer, and in resistance to other chemotherapeutic agents such as platinum using cervical cancer cell lines.
In the present study, we have shown that clusterin expression is a new prognosticator for cervical cancer patients who were treated with NAC followed by radical hysterectomy as well as for those who underwent radical hysterectomy followed by adjuvant therapy as we previously reported [8]. Prognostic significance of clusterin expression have been reported in other cancer types. The level of expression of clusterin in renal cell cancer was found to be closely associated with pathological stage and grade of the tumor; and the overall and recurrence-free survival rate of patients with strong clusterin expression was significantly lower than that of patients with weak expression [6]. Clusterin expression levels correlated with tumor size, estrogen and progesterone receptor expression levels, and lymph node metastasis in breast carcinoma [7]. Clusterin has been proposed to be a potentially new prognostic and predictive marker for colon carcinoma aggressiveness, since overexpression of clusterin is observed in highly aggressive tumors as well as metastatic nodules [3].We have shown that clusterin expression and LNM are independent prognostic factors for cervical cancer patients (Table 4), which is consistent with our previous report in different patient cohort [8]. Adjuvant radiotherapy has been widely employed for node-positive cervical cancer patients. To improve their outcome, clinicians have searched for alternative adjuvant treatments such as chemotherapy. Lahousen et al. reported, in a randomized prospective multicenter trial, that neither adjuvant chemotherapy nor radiotherapy will improve the survival or recurrence rates in high-risk squamous cell cervical cancer patients, including positive pelvic nodes after radical hysterectomy [20]. We, therefore, might need to consider NAC for patients with apparently multiple positive nodes by CT before surgery for their better outcome. To enhance the efficacy of NAC in node-positive patients, it is necessary to identify the molecules which induce resistance to chemotherapy. Resistance to NAC and disease recurrences could reliably be predicted by assessing biochemical factors strictly related to tumor cell biology and tumor aggressiveness, such as oncogenes and tumor suppressor genes. The result obtained in this study suggests that clusterin might be one of the key molecules to induce resistance to NAC in cervical cancer cells, which may result in poor survival of patients with moderate/strong clusterin expression by immunohistochemistry.
Recent preclinical studies provide proof-of-principle evidence that targeting cell survival genes such as clusterin with antisense oligonucleotides enhances apoptosis induced by conventional chemotherapy [15, 16], and has led to clinical testing antisense oligonucleotide therapy in combination with chemotherapy. We, therefore, might combine the drug to knockdown clusterin expression such as OGX-011, the second generation of the clusterin antisense oligonucleotide, or Si-RNA, with cytotoxic agents in the neoadjuvant setting for cervical cancer patients to enhance the efficacy and to improve patients’ survival in the near future. OGX-011 is a promising drug to restore the response to chemotherapy in patients with refractory prostate cancer in phase II trial [21].
In conclusion, the present study showed that the assessment of clusterin expression status could provide additional information to identify patients with cervical cancer with poor chance of response to NAC and unfavorable prognosis and, therefore, potential candidates for more individualized treatments, and combination of chemotherapy with drugs targeting clusterin expression might be a new treatment strategy to enhance the response to chemotherapy for cervical cancer patients with high level of clusterin expression, which might result in better outcome.
References
Panici PB, Greggi S, Scambia G et al (1998) Long-term survival following neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Eur J Cancer 34:341–346
Redondo M, Villar E, Torres-Munoz J et al (2002) Overexpression of clusterin in human breast carcinoma. Am J Pathol 157:393–399
Pucci S, Bonanno E, Pichiorri F et al (2004) Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene 23:2298–2304
Chung J, Kwak C, Jin RJ et al (2004) Enhanced chemosensitivity of bladder cancer cells to cisplatin by suppression of clusterin in vitro. Cancer Lett 203:155–161
Zellweger T, Chi K, Miyake H et al (2002) Enhanced radiosensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res 8:3276–3284
Miyake H, Hara S, Arakawa S et al (2002) Overexpression of clusterin is an independent prognostic factor for nonpapillary renal cell carcinoma. J Urol 167:703–706
Kruger S, Ola V, Fisher D et al (2007) Prognostic significance of clusterin immunoreactivity in breast cancer. Neoplasma 54:46–50
Watari H, Ohta Y, Hassan MK et al (2008) Clusterin expression predicts survival of invasive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Gynecol Oncol 108:527–532
Konishi I, Nanbu K, Mandai M et al (1998) Tumor response to neoadjuvant chemotherapy correlates with the expression of P-glycoprotein and PCNA but not GST-pi in the tumor cells of cervical carcinoma. Gynecol Oncol 70:365–371
Faried LS, Faried A, Kanuma T et al (2006) Predictive and prognostic role of activated mammalian target of rapamycin in cervical cancer treated with cisplatin-based neoadjuvant chemotherapy. Oncol Rep 16:57–63
Saito T, Takehara M, Tanaka R et al (2004) Correlation between responsiveness of neoadjuvant chemotherapy and apoptosis-associated proteins for cervical adenocarcinoma. Gynecol Oncol 92:284–292
Costa S, Terzano P, Bovicelli A et al (2004) CD44 isoform 6 (CD44v6) is a prognostic indicator of the response to neoadjuvant chemotherapy in cervical carcinoma. Gynecol Oncol 80:67–73
Ferrandina G, Lauriola L, Distefano MG et al (2002) Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol 20:973–981
Chung HH, Kim MK, Kim JW et al (2006) XRCC1 R399Q polymorphism is associated with response to platinum-based neoadjuvant chemotherapy in bulhy cervical cancer. Gynecol Oncol 103:1031–1037
Sowery RD, Hadaschik BA, So AI et al (2008) Clusterin knockdown using the antisense oligonucleotide OGX-011 resensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int 102:389–397
Miyake H, Eto H, Hara I et al (2004) Synergistic antitumor activity by combined treatment with gemcitabine and antisense oligodeoxynucleotide targeting clusterin gene in an intravesical administration model against human bladder cancer kotcc-1 cells. J Urol 171:2477–2481
Mizutani K, Matsumoto K, Hasegawa N et al (2006) Expression of clusterin, XIAP and survivin, and their changes by camptothecin (CPT) treatment in CPT-resistant PC-3 and CPT-sensitive LNCaP cells. Exp Oncol 28:209–251
Lourda M, Trougakos P, Gonos ES (2006) Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. Int J Cancer 120:611–622
Park DC, Yeo SG, Shin EY et al (2006) Custerin confers paclitaxel resistance in cervical cancer. Gynecol Oncol 103:996–1000
Lahousen M, Haas J, Pockel H et al (1999) Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective multicenter trial. Gynecol Oncol 73:196–201
Saad F, Hotte SJ, North S, et al (2008) A phase II randomized study evaluating custirsen (OGX-011) in patients with hormone refractory prostate cancer (HRPC) who relapsed on or within 6 months of first-line docetaxel therapy. ASCO Genitourinary cancers symposium; abstract#151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watari, H., Kanuma, T., Ohta, Y. et al. Clusterin Expression Inversely Correlates with Chemosensitivity and Predicts Poor Survival in Patients with Locally Advanced Cervical Cancer Treated with Cisplatin-Based Neoadjuvant Chemotherapy and Radical Hysterectomy. Pathol. Oncol. Res. 16, 345–352 (2010). https://doi.org/10.1007/s12253-009-9235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-009-9235-0